Abstract
Although nanotechnology has provided a rich variety of nanomaterials (1–100 nm) for in vivo medical applications, the blood compatibility of all these nanobiomaterials is still largely unexamined. Here, we report the preparation of blood-compatible carbon nanotubes (CNTs) that potentially represent the building blocks for nanodevices having in vivo applications. Activated partial thromboplastin time (APTT) and thromboelastography (TEG) studies prove that heparinization can significantly enhance the blood compatibility of nanomaterials.
Introduction
Nanodevices, called “nanorobots” or “nanobots”, have been suggested for use in targeting disease sites, in controlled drug delivery,1–3 as enzyme supports,4 as biosensors,5 and for other medical applications.6,7 Nanotubes, fullerenes, dendrimers, and metallic nanoparticles are the most widely used building blocks for these nanodevices. Blood compatibility is an important property for most of these biomedical devices to render their intended functions in vivo.8 The lack of blood compatibility results in adsorption of plasma proteins, platelet adhesion, and activation, triggering the coagulation and complement cascade and clot formation often leading to device failure.9 The design of blood-compatible macroscopic devices has primarily relied on making their surfaces resemble the luminal surface of the endothelium through the immobilization of the glycosaminoglycan (GAG) heparin.10 Proteoglycans (PGs) are macromolecules that consist of a core protein to which multiple GAG chains are linked. Endothelial heparan sulfate PG regulates the coagulation cascade,11 while other PGs play essential physiological, biochemical, and structural roles within all animals and are involved in the compression resistance, arresting the movement of microorganisms, signal transduction, and cytoskeletal support. The GAG chains are primarily responsible for the functional aspects of PGs and are well studied and, like heparin, are widely used pharmaceuticals. Functional studies and applications of intact PGs are less common because of their limited availability and the susceptibility of their core proteins to proteolysis and denaturation.12 These problems have largely precluded the use of PGs as therapeutic agents and in biomaterials. We conceived of replacing the core protein of PGs with carbon nanotubes (CNTs) to afford highly stable neo-PGs for functional and structural studies. CNTs represent one of the most widely used building blocks for nanodevices and have also been successfully used as solid supports for biofunctionalization.13,14 CNTs, with their unique structural, electronic, and mechanical properties, have an enormous number of applications in making various materials, including nanotube polymeric composites, electronic and optical devices, and enzyme/catalytic supports.15,16 CNTs, because of their high surface volume ratio, are also useful in making enzyme-immobilized biosensors.17 Nanotubes are often preferred over metallic or nonmetallic nanoparticles for biomedical applications because of their larger inner volume, distinct inner and outer surfaces, and open mouths.18 These properties allow the filling of nanotubes with desired species (small molecule or macromolecule), differential modification of inner and outer surfaces, and access to inner surfaces for the incorporation of species. Multiwalled carbon nanotubes (MWNTs) with high stability and appropriate size (average diameter ~40 nm) were selected in the current study to replace vulnerable core proteins. It was anticipated that the heparinization of MWNTs would afford nanomaterials that could mimic endothelial heparan sulfate PGs.19,20 By following this approach, poly(ethyleneimine) (PEI)-functionalized single-walled carbon nanotubes, which showed potential as substrates for neuronal growth, 21 can also be made blood compatible.
Three steps were used to prepare nano-based neo-PGs: (a) coating of MWNTs with PEI; (b) activation of the tetrabutylammonium salt of heparin by cyanogen bromide;22 and (c) coupling of activated heparin to MWNTs. Heparinized MWNTs were then characterized structurally using atomic force microscopy (AFM) and biologically using activated partial thromboplastin time (APTT) and thromboelastography (TEG). The Materials and Methods section is presented in detail in the Supporting Information for this article.
Results and Discussion
While pristine MWNTs are hydrophobic, rapidly settling from water, coating with PEI made them more hydrophilic, affording solutions in water that are stable for more than two weeks. PEI also introduces free amino groups onto the MWNTs required for the immobilization of heparin. The amino groups that remain uncoupled after the immobilization of heparin were also used for the linking of a readily available fluorescent probe, fluorescein isothiocyanate (FITC), instead of employing expensive fluorescent probes such as 1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate or 3,3′-dihexyloxacarbocyanine iodide, or BODIPY naphthalimides.23,24 FITC remains stable on CNTs, even after repeated washings with water and physiological buffers.
After the immobilization of heparin, heparinized MWNTs were extensively washed with 2 M NaCl, followed by several water washes. All these washes were analyzed for heparin by carbazole assay.25 The assay confirmed the absence of heparin in the later washes and the test medium, and demonstrated the stability of the heparinized MWNTs.
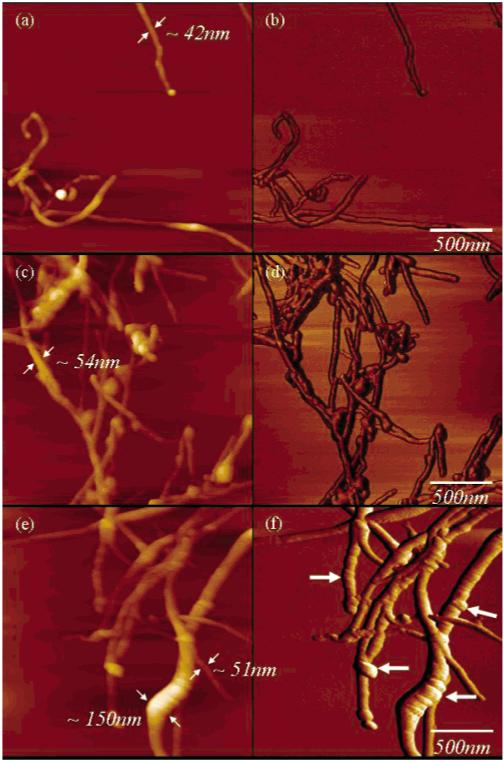
Tapping mode (TM)-AFM images of the pristine, PEI-coated and heparinized MWNTs are shown in Figure 1. Pristine nanotubes with an average diameter of 42 nm are shown in Figure 1a,b. Coating with PEI increased MWNT diameter by approximately 12 nm (Figure 1c,d), and the covalent attachment of heparin produced conspicuous bulges on the MWNT surface, increasing diameter by as much as 100 nm (Figure 1e,f), suggesting that these bulges correspond to domains of immobilized heparin on the nanotube surface. Further, the PEI coating provided a homogeneous increase in the nanotube diameter, while heparinization (a process of immobilization and not coating) led to a heterogeneous increase in the nanotube diameter. For instance, a PEI-coated nanotube without heparin immobilization (with a diameter of 51 nm) can also be seen along with the heparinized nanotubes in Figure 1e.
Figure 1.
TM-AFM topography (a,c,e) and phase (b,d,f) images of pristine (a,b), PEI-coated (c,d), and heparinized (e,f) MWNTs. Pristine and PEI-coated MWNTs show uniform diameters (a,c), while heparinized MWNTs showed variable diameters. The smooth surfaces of the pristine MWNTs and PEI-coated MWNTs are contrasted to the rough, uneven surface with bulges of the heparinized MWNTs associated with domains of immobilized heparin (bold arrows in panel f).
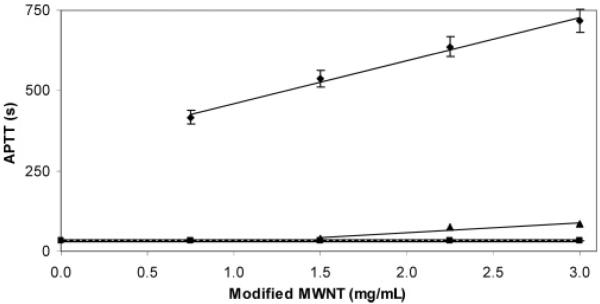
The amount of heparin present on the MWNTs was then determined chemically using carbazole assay.25 The heparin loading on the MWNTs was 30% (w/w). The biological activity of heparin is most commonly determined by coagulation-based assays such as APTT, which measures the abnormalities in the intrinsic coagulation cascade of blood, and TEG, which measures the abnormalities in the global coagulation cascade of blood.26 The details of these assays are presented in detail in the Supporting Information. The APTT assay (Figure 2) shows that pristine MWNTs did not prolong coagulation time, while PEI-coated MWNTs afforded only a small elongation of plasma-based clotting time and only at a concentration above 1.5 mg/mL. This slight improvement in blood compatibility can be explained by the transformation of the hydrophobic surface of pristine MWNTs to a hydrophilic PEI-coated surface. The APTT of heparinized MWNTs was significantly prolonged, providing a linear dose response curve over the range of 0.75–3.0 mg/mL. Thus, heparin retains its expected plasma-based anticoagulant activity when immobilized on MWNTs.
Figure 2.
APTT studies on modified MWNTs. The control (with no added MWNTs) is shown as a broad horizontal line at 32.4 ± 2.3 s: (■) pristine MWNTs, (▲) PEI MWNTs (3 mg/mL gave an APTT of 83.7 ± 4.2 s), and (◆) heparinized MWNTs. Heparinized MWNTs afforded a linear dose response curve with a substantial elongation of APTT of 717.9 ± 29.5 s at a 3 mg/mL amount.
Clotting kinetics experiments were then undertaken using whole human blood (Table 1). Again, pristine MWNTs showed a behavior similar to that of the control (no added MWNTs). PEI-coated MWNTs resulted in a slight prolongation of the clotting time (R) and a decrease in the clot strength (MA). Heparinized MWNTs (3 mg/mL) prolonged the clotting time by over 98 min, and additional heparinized MWNTs fully anticoagulated the blood, resulting in its failure to clot over a 2 h period. Both the maximum clot strength and the rate of clot formation (α) observed with heparinized MWNTs were much lower than those of the controls, demonstrating their blood compatibility. The heparinized MWNTs were stable, remaining active even after extensive washing with 2 M sodium chloride. Treatment of heparinized MWNTs with heparin lyase 1 (Flavobacterium heparinase) nearly eliminated the anticoagulant activity, demonstrating that heparin was responsible for the observed anticoagulant activity and that the immobilized heparin chains were accessible to heparinase.
Table 1.
TEG Clotting Kinetics of Modified MWNTsa
| MWNTs | R (min) | K (min) | α (deg) | MA (mm) |
|---|---|---|---|---|
| nil – control | 3.5 ± 0.3 | 3.1 ± 0.4 | 38.1 ± 2.2 | 39.5 ± 1.4 |
| pristine MWNTs (1.5 mg/mL) | 3.8 ± 0.8 | 3.9 ± 1.1 | 49.4 ± 6.2 | 39.5 ± 3.5 |
| PEI-MWNTs (3.0 mg/mL) | 10.5 ± 3.1 | 15.9 ± 2.9 | 12.8 ± 2.4 | 27.7 ± 4.7 |
| heparinized MWNTs (3.0 mg/mL) | 102.4 ± 10.1 | b | 0.5 ± 0.2 | 5.5 ± 2.3 |
| heparinized MWNTs (6.0 mg/mL) | 133.9 ± 17.2c | 0 | 0 | 0 |
| lyase-treated heparinized MWNTs (1.5 mg/mL) | 11.9 ± 3.8 | b | 12.0 ± 3.6 | 19.9 ± 3.2 |
The clotting kinetics of human whole blood was assessed in the presence of the heparinized nanotubes by using TEG. R corresponds to the time taken for the initiation of clotting (min), K is the time to reach a 20 mm clot amplitude, α is the rate of clot formation, and MA is the maximum clot strength. The results are taken as an average of three experiments.
No clot of 20 mm amplitude was observed.
No clot was observed.
It is expected that this enabling technology would facilitate the making of nanodevices using these blood-compatible nanomaterials as building blocks for biomedical applications8 such as artificial implants, including structural tissue replacements, that is, artificial blood vessels, or functional devices such as drug delivery matrixes. Other GAGs, such as chondroitin sulfate and hyaluronic acid, can be similarly immobilized on the MWNTs to afford an array of nano-based neo-PGs having a wide range of potential biomedical applications, including structural elements in load-bearing tissues such as cartilage.
Conclusions
This report, being the first of its kind, introduced ways of immobilizing heparin at nanoscopic dimensions. AFM studies showed an increase in the nanotube diameter, confirming the immobilizing of heparin on the nanotube surface. APTT and TEG studies proved that heparin stays bioactive, even in its immobilized form on the nanotube, rendering the nanotube blood compatibility.
Supplementary Material
Footnotes
Supporting Information Available: Preparation details, including the coating of MWNTs, the activation of heparin, and the coupling of activated heparin to MWNTs; characterization details, including AFM, carbazole assay, APTT, and clotting kinetics; and lyase digestion of immobilized heparin. This material is available free of charge via the Internet at http://pubs.acs.org
LA0534468
References
- (1).Koltover I, Salditt T, Radler JO, Safinya CR. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- (2).Potineni A, Lynn DM, Langer R, Amiji MM. J. Controlled Release. 2003;86:223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- (3).Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. Adv. Drug Delivery Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chang TMS, Prakash S. Mol. Biotechnol. 2001;17:249–260. doi: 10.1385/MB:17:3:249. [DOI] [PubMed] [Google Scholar]
- (5).Besteman K, Lee JO, Wiertz FG, Heering HA, Dekker C. Nano Lett. 2003;3:727–730. [Google Scholar]
- (6).Osafune T, Baba Y. Nippon Nogei Kagaku Kaishi. 2004;78:122–124. [Google Scholar]
- (7).Haberzettl CA. Nanotechnology. 2002;13:R9–R13. [Google Scholar]
- (8).Buxton DB, Lee SC, Wickline SA, Ferrari M. Circulation. 2003;108:2737–2742. doi: 10.1161/01.CIR.0000096493.93058.E8. [DOI] [PubMed] [Google Scholar]
- (9).Ratner BD. J. Biomater. Sci., Polym. Ed. 2000;11:1107–1119. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- (10).Munoz EM, Linhardt RJ. Arterioscler., Thromb., Vasc. Biol. 2004;24:1549–1557. doi: 10.1161/01.ATV.0000137189.22999.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Colburn P, Buonassisi V. Biochem. Biophys. Res. Commun. 1982;104:220–227. doi: 10.1016/0006-291x(82)91962-3. [DOI] [PubMed] [Google Scholar]
- (12).Matouschek A. Curr. Opin. Struct. Biol. 2003;13:98–109. doi: 10.1016/s0959-440x(03)00010-1. [DOI] [PubMed] [Google Scholar]
- (13).Lin Y, Taylor S, Li H, Fernando KAS, Qu L, Wang W, Gu L, Zhou B, Sun Y-P. J. Mater. Chem. 2004;14:527. [Google Scholar]
- (14).Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V. J. Biomed. Nanotechnol. 2005;1:3–17. doi: 10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ajayan PM. Chem. Rev. 1999;99:1787–1799. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- (16).Baughman RH, Zakhidov AA, de Heer WA. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- (17).Pan D, Chen J, Yao S, Tao W, Nie L. Anal. Sci. 2005;21:367–371. doi: 10.2116/analsci.21.367. [DOI] [PubMed] [Google Scholar]
- (18).Martin CR, Kohli P. Nature. 2003;2:29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- (19).Palmer RE, Pratontep S, Boyen H-G. Nat. Mater. 2003;2:443–448. doi: 10.1038/nmat897. [DOI] [PubMed] [Google Scholar]
- (20).Solomon DE. Int. J. Exp. Pathol. 2002;83:209–216. doi: 10.1046/j.1365-2613.2002.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. J. Phys. Chem. B. 2005;109:4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- (22).Yafuso M, Linhardt RJ. U.S. Patent 5583213. 1996.
- (23).Prakash R, Washburn S, Superfine R, Cheney RE, Falvo MR. Appl. Phys. Lett. 2003;83:1219–1221. [Google Scholar]
- (24).Zhu W, Minami N, Kazaoui S, Kim Y. J. Mater. Chem. 2003;13:2196–2201. [Google Scholar]
- (25).Bitter T, Muir HM. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- (26).Vance NG. Anesth. Analg. 2002;95:1503–1506. doi: 10.1097/00000539-200212000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.