Abstract
Summary
Low-molecular-weight heparins (LMWH) exhibit potent anti-coagulant efficacy via their plasmatic effects on thrombin and factor Xa.These agents are also effective in releasing endothelial tissue factor pathway inhibitor (TFPI), the natural inhibitor of tissue factor, and exhibit significant anti-metastatic effects in experimental animal models. However, the potential for bleeding complications has slowed down the more widespread adoption of LMWH therapy in cancer patients. In this study, the effect of a non-anticoagulant form of LMWH (NA-LMWH) on experimental lung metastasis and tumor cell-induced platelet aggregation in vivo was compared to the LMWH enoxaparin. Using the B16 melanoma mouse model of metastasis, subcutaneous (s.c.) injection of NA-LMWH or enoxaparin (10 mg/kg), three hours before intravenous (i.v.) injection of metastatic melanoma cells, followed by daily doses for 14 days, reduced lung tumor formation by 70% (P<0.001). l.v. injection of tumor cells resulted in a significant (50–62%, P<0.01) fall in platelet counts. Pre-injection (i.v.) of enoxaparin completely abolished the tumor cell-induced thrombocytopenia, whereas NA-LMWH had no effect. Four hours after a single s.c. dose, enoxaparin but not NA-LMWH prolonged the clotting time three-fold and delayed the time to clot initiation more than 10-fold as measured by a Sonoclot analyzer and by thromboelastography, respectively. Enoxaparin but not NA-LMWH demonstrated a significant anti-coagulant effect in mice. Both NA-LMWH and enoxaparin caused similar TFPI release from endothelial cells in vitro. These data provide evidence to support the potential of NA-LMWH as an anti-metastatic agent without any significant impact on coagulation.
Keywords: Heparin, low-molecular-weight heparin, metastasis, anticoagulant
Introduction
The link between coagulation and malignancy has been recognized for over a century and is now well accepted (1, 2). Coagulation and platelet activation in cancer may not only lead to thromboembolic complications but also may enhance tumor growth and promote the metastatic process (3). Hypercoagulability in cancer patients is often observed as chronic low-grade intravascular coagulation associated with a variety of laboratory abnormalities (2, 4). It is now well established that rather than being an epiphenomenon, coagulation activation in cancer promotes blood-borne tumor dissemination by various and not fully understood mechanisms. Hemostatic activation has also been shown to influence tumor growth. For example, a number of factors that positively or negatively regulate tumor angiogenesis are related to the hemostatic system (5). These include platelet-derived products (such as vascular endothelial growth factor [VEGF], platelet factor 4 and thrombospondin), fibrin, fibrin-degradation products, and tissue factor (TF); TF is present on the surface of many tumor cell types and is responsible for the tumor cell procoagulant activity (6). With regard to hematogeneous metastasis, thrombin generation via tumor cell TF and subsequent platelet activation have been shown to enhance this process in experimental animal models (6, 7). In addition, TF has been shown to promote metastasis via mechanisms independent of its procoagulant function (8, 9).
Retrospective analyses of clinical trials in which low-molecular-weight heparins (LMWH) have been used to treat cancer patients with established thrombosis have suggested a survival advantage for the treated groups (10–15). A recent prospective, randomized, double-blind study designed to determine the potential value of long-term LMWH therapy to improve survival in cancer patients suggested a striking survival advantage for heparin treatment in a subgroup of patients with good-prognosis tumors (14). More recently, a second clinical trial in patients with small-cell lung carcinoma showed advantages in terms of progression-free and overall survival for patients who received LMWH for 18 weeks (13). Other studies have demonstrated survival advantages in patients without evidence of metastatic disease (10), including a subgroup of patients with various tumor types (15). In the latter study, the benefits of LMWH therapy were seen for months and years after the period of active administration (15). The improved outcome in the LMWH group compared to those treated with unfractionated heparin (UFH) could not be attributed to better prevention of venous thromboembolism, leading to the hypothesis that LMWH might inhibit cancer proliferation and/or metastasis independent of the coagulation system. Currently, the use of heparins as an anti-cancer agent is limited due to their potent anticoagulant effects. A recent review supports the use of non-anticoagulant heparins as a potentially promising approach for prevention of tumor metastasis, suggesting the possibility of separating the anti-metastatic and anticoagulant effects of heparins (16). A potential anti-metastatic non-anticoagulant heparin with low- to no-systemic anticoagulant activity could be given safely to any patient and, importantly, in higher doses (if necessary), thereby fully exploiting the anti-metastatic activity of the heparin-derived compounds. The aim of the present study was to compare the effect of subcutaneously administered non-anticoagulant (NA)-LMWH versus LMWH enoxaparin on coagulation and experimental metastasis in mice.
Materials and methods
Non-anticoagulant heparin
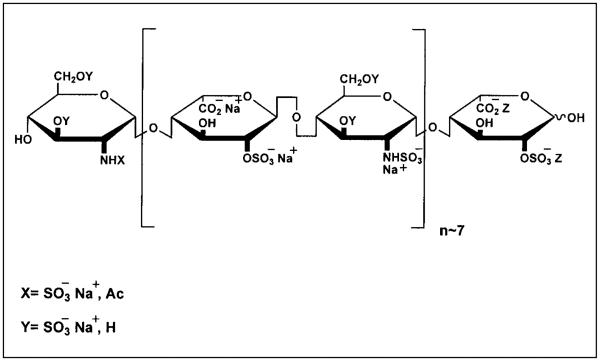
Non-anticoagulant heparin was prepared by fragmenting porcine mucosa) heparin into LMWH. This was done by treatment of periodate-oxidized heparin with sodium hydroxide, followed by reduction with sodium borohydride and acid hydrolysis. Gradient polyacrylamide gel electrophoresis analysis showed a mixture of heparin fragments with an average size of eight disaccharide units (17; see Fig. 1). In addition, it was revealed by nuclear magnetic resonance (NMR) that two-thirds of the N-acetyl groups were lost on periodate cleavage and that such cleavage had occurred at the glucopyranosyluronic acid (GlcpA) and idopyranosyluronic acid (IdopA) residues located within and adjacent to the antithrombin (AT) binding site.
Figure 1.
A representative structure for non-anticoagulant oxidized ultra-LMWH (NA-LMWH), which is devoid of anti-thrombin and heparin co-factor II binding but has optimal releasing capacity of endothelial TFPI.
Tumor cells
B16F10 murine malignant melanoma cells (National Cancer Institute, Fredrick, MD, USA) were cultured in RPMI 1640 media (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum, penicillin and streptomycin (Sigma, St Louis, MO, USA). Tumor cells were cultured to 70% confluency and harvested by a non-enzyrnatic cell-dissociation solution (Sigma), washed twice with and suspended in phosphate-buffered saline (PBS) without Ca2+/Mg2+. Cells were prepared at a concentration of 1 × 106 cells/ml for experimental metastasis experiments or at 1 × 107 for experiments involving the assessment of tumor cell-induced thrombocytopenia.
Animals
Male or female C57/BL6 mice (Harlan, Indianapolis, IN, USA) weighing 18–21 g were used for this study. All procedures were carried out in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines.
Measurement of anticoagulant activity of enoxaparin and NA-LMWH
Anticoagulant effect of enoxaparin and NA-LMWH (both at 10 mg/kg) was measured 3 hours (h) after subcutaneous or 15 minutes (min) after intravenous (tail vein) injection of either drug in experimental mice. Blood samples (0.5–1 ml) were collected by cardiac puncture into microtainer tubes containing 50–100 μl of 3.8% trisodium citrate. Whole blood coagulation was assessed using a Sonoclot Analyzer (Sienco, Morrison, CO, USA) and by thromboelastography. For Sonoclot analysis, whole blood (400 μl) from control or heparin-treated animals was added to Sonoclot plain cuvettes and incubated at 37°C for 1 min. The blood was then recalcified by the addition of CaCl2 (40 μl, 0.1 M). In addition, time to clot initiation (R-time) was measured in whole blood using a Thromboelastograph (Hemoscope, Niles, IL, USA) according to the basic manufacturer's instructions. Activated partial thromboplastin times (APTT), anti-Xa, and anti-IIa in mouse platelet-poor plasmas were measured using the AMAX Coagulation Analyzer (Sigma).
Assessment of tumor cell-induced thrombocytopenia
Mice (n=5 per group) were injected via the lateral tail vein with a 200-μl bolus (10 mg/kg) of NA-LMWH, or enoxaparin or PBS (as control). Five min thereafter, all mice were injected via the tail vein with 2 × 106 B16F10 cells (in a 200-μl bolus). Fifteen min after tumor cell injection, exactly 450 μl of whole blood were collected by cardiac puncture into microtainer tubes containing 50 μl of 3.8% trisodium citrate. Platelet counts were measured within 5 min after collection using an electronic Coulter AcT dif Analyzer (Beckman Coulter; Miami, FL, USA).
Effect of NA-LMWH and enoxaparin on experimental metastasis
Mice (10 in each group) were injected subcutaneously with a 200-μl bolus of PBS or enoxaparin or NA-LMWH (200 μg/mouse for both drugs). Four hours thereafter all mice were injected via the lateral tail vein with a 200-μl bolus of 2 × 105 B16F10 melanoma cells. After cell injections, PBS or enoxaparin or NA-LMWH (in the above-mentioned quantities) were given subcutaneously to respective groups once a day for 14 days. On day 15, all mice were euthanized by carbon dioxide inhalation. Lungs were dissected en bloc, rinsed in PBS and placed in Bouin's fixative solution (Sigma). Lung seeding was quantified independently by two laboratory personnel blinded to the treatment groups. Tumor nodules appeared as black or grey-white foci against a yellow background and were counted on the entire lung surface.
The effect of NA-LMWH on tissue factor pathway inhibitor (TFPI) release from endothelial cells
Confluent (1 × 107/ml) human umbilical vein endothelial cells (HUVEC, ATCC, Manassas, VA, USA) were harvested and 2 × 106 cells (200 μl) were plated in fibronectin-coated 24-well plates (Neuro Probe Inc, Gaithersburg, MD, USA). Cells were allowed to attach to fibronectin polymer for 3 h at 37°C (5% CO2). After washing, fresh culture medium with and without enoxaparin or NA-LMWH at various concentrations was added to the endothelial cells, and the final volume in each well was adjusted to 1 ml. After a 24-h incubation period, TFPI released in the medium was measured using a commercially available ELISA kit for total TFPI antigen (IMUBIND; American Diagnostica Inc., Greenwich, CT, USA) according to the manufacturer's instructions. The linear range of this assay is between 0.1 – 5 ng of total TFPI antigen (r2 = 0.99) according to the calibration curve and the manufacturer.
Statistical analysis
All data were normally distributed as assessed by normal probability plots (Statistica™ for Windows). Summary statistics were therefore presented as means and standard deviations, and differences between groups were analyzed using Student's t-test. Throughout the study, statistical significance was assumed when P <0.05.
Results
Anticoagulant effect of NA-LMWH versus enoxaparin
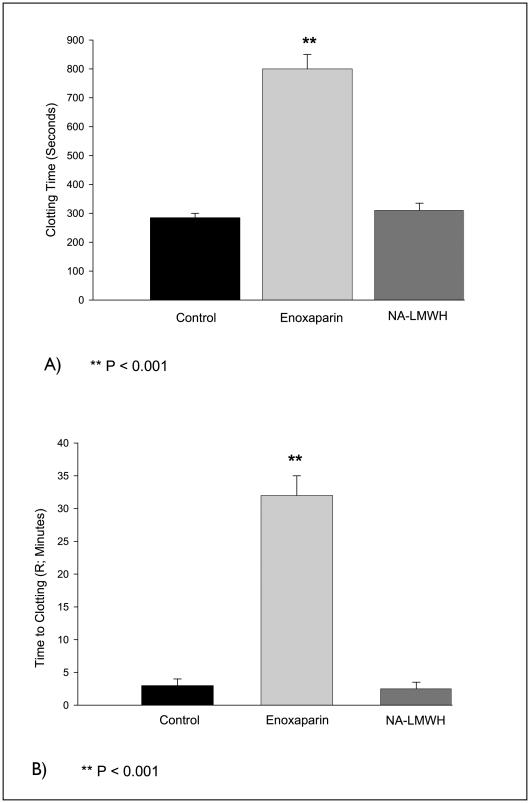
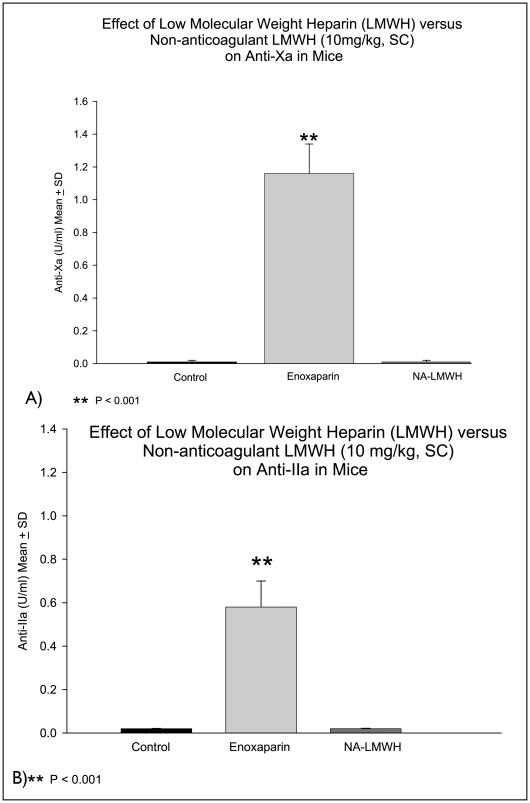
Four hours after subcutaneous injection of NA-LMWH or enoxaparin (10 mg/kg), the whole blood recalcification time measured by the Sonoclot Analyzer was prolonged three- to four-fold (P<0.001) in the enoxaparin group, but not in the NA-LMWH group (Fig. 2A). APTT was significantly prolonged (>200 seconds) in the enoxaparin-treated group, but not in the NA-LMWH group. Similarly, thromboelastography showed that the time to clot initiation (R-time) was prolonged 10-fold in the enoxaparin group, but not in animals treated with NA-LMWH (Fig. 2B). Finally, enoxaparin, but not NA-LMWH, exhibited significant anti-Xa and anti-IIa activities compared to controls (Fig. 3A, B).
Figure 2.
Clotting time (A) and time to clot initiation (B). Whole blood clotting times were measured by the Sonoclot analyzer (A) and by thromboelastography (B); n = 5. While enoxaparin significantly prolonged the clot times, NA-LMWH effects were not different from control.
Figure 3.
Anti-Xa (A) and anti-IIa (B) for enoxaparin versus NA-LMWH in mice.
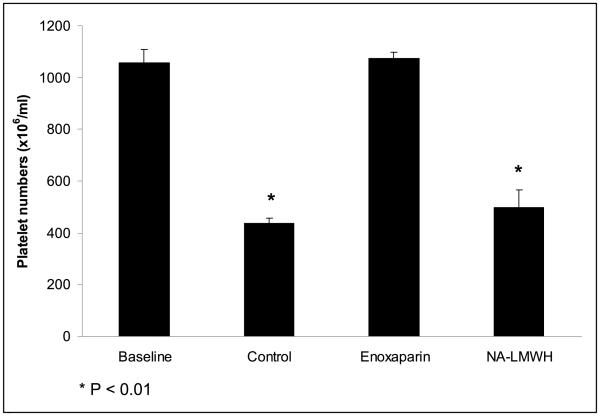
Effect of NA-LMWH versus enoxaparin on tumor cell-induced thrombocytopenia
Intravenous injection of B16F10 cells (2 × 106) resulted in a significant and rapid reduction (>50%) in the platelet counts of control mice previously injected with PBS (P<0.01, Fig. 4). This fall in platelet count was completely abolished in mice treated with enoxaparin prior to tumor cell injection. In contrast, the NA-LMWH had no effect on B16F10-induced thrombocytopenia.
Figure 4.
The effect of enoxaparin versus NA-LMWH on tumor cell-induced thrombocytopenia.
Effect of NA-LMWH versus enoxaparin on experimental metastasis
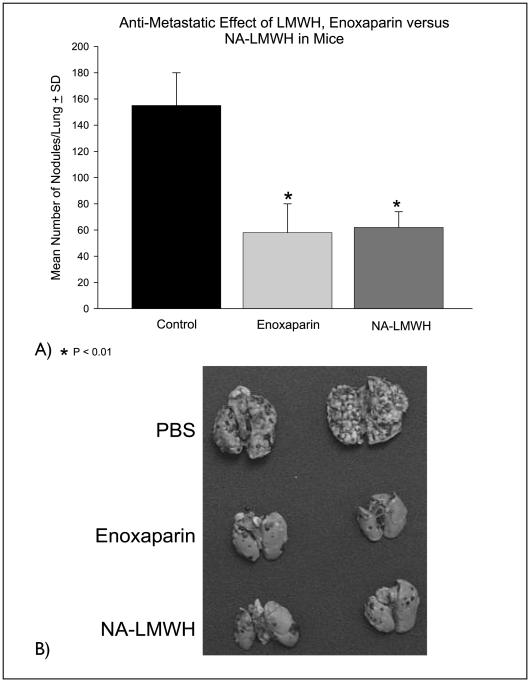
A pre-tumor cell injection dose of NA-LMWH or enoxaparin followed by daily doses (for 14 days) reduced lung tumor formation by 70% (P<0.01). There was no statistically significant difference in lung seeding between enoxaparin- and NA-LMWH-treated animals (Fig. 5A, B).
Figure 5.
A) The effect of enoxaparin versus NA-LMWH (10 mg/kg) on experimental lung metastasis. Control animals (n = 10) were injected with PBS. B) Representative lungs of control and heparin-treated mice 15 days after injection of 2 × 105 B16F10 cells.
The effect of NA-LMWH on TFPI release from endothelial cells
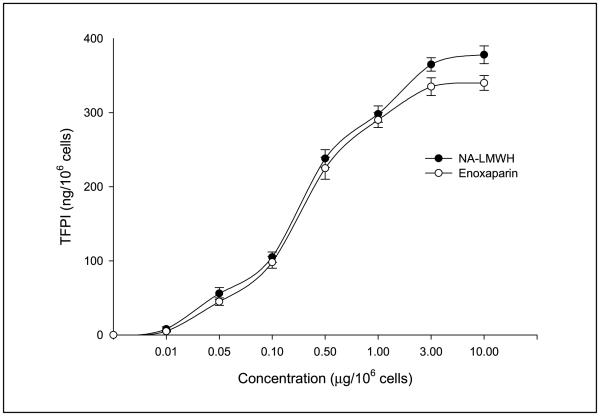
Enoxaparin and NA-LMWH were equally capable of causing the release of TFPI from HUVEC cells in vitro (Fig. 6).
Figure 6.
The effect of enoxaparin versus NA-LMWH on endothelial cell TFPI release in vitro.
Discussion
Tumor cells may activate coagulation reactions directly, through contact with coagulation factors, or indirectly, through formation of cytokines capable of activating certain host cells such as macrophages or endothelial cells (1). Current evidence suggests that the TF/factor VIIa pathway mediates the most abundant procoagulant stimulus in malignancy by promoting thrombin generation.
Injectable murine models of experimental metastasis have been used frequently to investigate the effect of anti-hemostatic agents on blood-borne metastasis. Although such artificial models do not encompass the entire metastatic process, they remain useful for “proof-of-concept” experiments, focusing on the hematogenous phase of tumor cell dissemination. During experimental lung metastasis, the injected tumor cells become trapped in the pulmonary microvasculature and adhere to the vascular endothelium. Coagulation is then activated locally via surface expressed TF, leading to thrombin generation, fibrin deposition and platelet aggregation. Several interventional studies have demonstrated significant efficacy of anticoagulant and anti-platelet agents in inhibiting experimental metastasis. These agents include UFH and LMWH (7, 18), coumadin (7), hirudin (19), recombinant TFPI (20), anti-TF antibodies (6), and glycoprotein IIb/IIIa inhibitors (21, 22). In addition, injection of tumor cells in thrombocytopenic or fibrinogen-deficient animals results in a significant inhibition of metastasis (23, 24). Together, these studies strongly suggest a requirement for coagulation and platelet activation for optimum lung seeding in murine models of metastasis. We have shown previously that delaying anticoagulation (i.e. administering hours after tumor cell injection) significantly reduces the antimetastatic effect of those agents (18). These observations suggest that in the injectable model of metastasis used in the present study, maximal antimetastatic effect is achieved if the host animal is treated with either LMWH or NA-LMWH shortly before the time of tumor cell injection. The 10 mg/kg dose of enoxaparin or NA-LMWH used in our study would be considered high in relation to therapeutic doses given to humans. However, we did not observe any bleeding in any of the mice in our study. Since NA-LMWH does not have any anti-coagulant effect and exhibited a 70% reduction in experimental metastasis (similar to enoxaparin), we did not perform a dose-response experiment to determine the doses at which enoxaparin would cause bleeding in mice. Such doses would be very high and certainly not relevant to the doses used in humans. However, it is reasonable to suggest that even higher (>10 mg/kg) doses of NA-LMWH will not have a significant impact on coagulation in either mice or humans.
Platelet activation and fibrin deposition occur either directly on the surface or in close vicinity of the trapped tumor cells. Fate studies have shown that tumor cells are retained in the pulmonary circulation for shorter periods in anticoagulated or fibrinogen-deficient animals (18, 24), suggesting that tumor cell-induced hemostatic activation prolongs the retention of tumor cells in the pulmonary microvasculature. The prolonged microvascular presence of tumor cells may increase the possibility of extravasation and successful metastasis.
In the present study, however, we have demonstrated that a NA-LMWH with no anti-thrombin activity significantly inhibits B16F10 melanoma experimental metastasis. In addition, we showed that in contrast to enoxaparin and other anti-metastatic anticoagulant agents, NA-LMWH is not able to prevent tumor cell-induced platelet aggregation in the blood vessels of the lung. In the context of other interventional studies mentioned above, the significant anti-metastatic effect of NA-LMWH is intriguing and points to anti-metastatic mechanisms that are independent of coagulation and platelet activation pathways.
The anionic properties of heparin and its fractionated derivatives are thought to be responsible for heparin's anti-cancer effects, including angiogenesis, tumor cell adhesion, and malignant cell transformations. Possible coagulation-independent mechanisms for inhibition might include binding of heparin to angiogenic growth factors (such as basic fibroblast growth factor and VEGF) and modulation of TF (25–27). A key component of metastasis is the adhesion of cells to the vascular endothelium of organs distant from the primary tumor site. P-selectin-mediated tumor cell interactions have been shown to promote metastasis (28, 29). Expression of carbohydrate moieties required for P-selectin binding is associated with increased metastasis and poor survival in various tumor cell types (30–32). In a recent study using a B16 melanoma model of metastasis, Ludwig et al. (29) demonstrated that heparins (unfractionated and low-molecular-weight) and derivatives (fondaparinux) exert differential anti-metastatic effects at comparable anticoagulant activities in vivo. They further showed significantly diverse inhibitory effects on P-selectin-mediated interactions by the variousheparins and derivatives. It was thereforesuggested that the degree of inhibition of P-selectin function of a given anticoagulant may provide a parameter to indicate its anti-metastatic potential. Investigating the effect of NA-LMWH on P-selectin function is therefore warranted.
Enoxaparin and NA-LMWH were equally effective at inducing the release of TFPI from vascular endothelial cells. This could represent a key mechanism by which LMWH or NA-LMWH exert their antimetastatic effect (3, 25, 26). Previously, we have shown a reduction in experimental metastasis by TFPI by three different approaches (20). First we demonstrated that intravenous injection of recombinant TFPI significantly reduced the metastasis of B16 melanoma. Second, stably transfected TFPI (+) B16 melanoma cells produced significantly fewer lung metastases compared to wild-type and vector-control cells. Third, mice receiving intravenous somatic gene transfer of TFPI expression vector developed significantly fewer lung nodules than controls.
Heparin has also been shown to exert an inhibitory effect on cell adhesion by interfering with the functions of cell receptor CD44, which is enhanced in a variety of malignant tumors. The principal binding target of this receptor is hyaluronate (HA), and this interaction is associated with tumor development. It has been suggested that HA may be a molecular link between the tumor cell and the host tissue (33). HA is a negatively charged polysaccharide (34), and heparin could competitively interfere with CD44-HA interactions, thus reducing tumor cell adhesion.
In addition to their anticoagulant properties, the anti-metastatic potential of heparins in clinical settings has been considered based on recent positive results from randomized prospective clinical trials (13, 14). Interestingly, the clinical study by Lee et al. (14) supports the hypothesis that anti-metastatic effects of heparins are mediated through mechanisms that are independent from their anti-coagulant function. In that study, the use of dalteparin compared to coumarin was associated with significant survival benefit in patients with solid tumors, and this finding was not related to the changes in the incidence of thromboembolic events. In conclusion, the non-anticoagulant nature of the heparin described in our study, along with its significant anti-metastatic effect, may represent a novel anti-cancer agent, particularly in patients with an increased risk of bleeding complications.
References
- 1.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–48. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 2.Bick RL. Coagulation abnormalities in malignancy – a review. Semin Thromb Hemost. 1992;18:353–72. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 3.Mousa SA. Antithrombotics in thrombosis and cancer. Future Oncol. 2005;1:395–403. doi: 10.1517/14796694.1.3.395. [DOI] [PubMed] [Google Scholar]
- 4.Sun NCJ, McAfee WM, Hum GJ, et al. Hemostatic abnormalities in malignancy, a prospective study of one hundred eight patients. Part I. Coagulation studies. Am J Clin Pathol. 1979;71:10–6. doi: 10.1093/ajcp/71.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273:H2091–H2104. doi: 10.1152/ajpheart.1997.273.5.H2091. [DOI] [PubMed] [Google Scholar]
- 6.Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost. 2002;28:29–38. doi: 10.1055/s-2002-20562. [DOI] [PubMed] [Google Scholar]
- 7.Amirkhosravi M, Francis JL. Coagulation activation by MC28 fibrosarcoma cells facilitates lung tumor formation. Thromb Haemost. 1995;73:59–65. [PubMed] [Google Scholar]
- 8.Bromberg ME, Konigsberg WH, Madison JF, et al. Tissue factor promotes melanoma metastasis by a path way independent of blood coagulation. Proc Natl Acad Sci USA. 1995;92:8205–9. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic do main signaling. Nanire Med. 2004;10:502–9. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 10.Alifano M, Benedetti G, Trisolini R. Can low-molecular weight heparin improve the outcome of patients with operable non-small cell lung cancer? An urgent call for research. Chest. 2004;126:601–17. doi: 10.1378/chest.126.2.601. [DOI] [PubMed] [Google Scholar]
- 11.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low molecular weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–71. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin therapy with Dalteparin, and survival in advanced cancer: the fragmin advanced ma lignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–8. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Klerk CPW, Smorenbury SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–5. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 14.Lee AYY, Rickles FR, Julian JA, et al. Randomized comparison oflow molecular weight heparin and coumarin derivates on the survival of patients with cancer and ve nous thromboembolism. J Clin Oncol. 2005;23:2123–9. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 15.Loynes JT, Zacharski LR, Rigas JR. Regression of metastatic non-small cell lung cancer with low molecular weight heparin. Thromb Haemost. 2002;88:686. [PubMed] [Google Scholar]
- 16.Kragh M, Loechel F. Non-anticoagulant heparins: a promising approach for prevention of tumor metastasis [review] Int J Oncol. 2005;27:1159–67. [PubMed] [Google Scholar]
- 17.Islam T, Butler M, Sikkander SA, et al. Further evidence that periodate cleavage of heparin occurs pri marily through the antithrombin binding site. Carbohydr Res. 2002;337:2239–43. doi: 10.1016/s0008-6215(02)00229-x. [DOI] [PubMed] [Google Scholar]
- 18.Amirkhosravi A, Mousa SA, Amaya M, et al. Antimetastatic effect of Tinzaparin, a low-molecular weight heparin. J Thromb Haemost. 2003;1:1972–6. doi: 10.1046/j.1538-7836.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 19.Langer F, Amirkhosravi A, Ingersoll SB, Walker JM, et al. Experimental metastasis and primary tumor growth in mice with hemophilia A. J Thromb Haemost. 2006;4:1056–62. doi: 10.1111/j.1538-7836.2006.01883.x. [DOI] [PubMed] [Google Scholar]
- 20.Amirkhosravi A, Meyer T, Chang JY, et al. Tissue factor pathway inhibitor reduces experimental lung metastasis of B16 melanoma. Thromb Haemost. 2002;87:930–6. [PubMed] [Google Scholar]
- 21.Arnirkhosravi A, Amaya M, Siddiqui F, et al. Blockade of GpIIb/IIIa inhibits the release of vascular en dothelial growth factor (VEGF) from tumor cell-acti vated platelets and experimental metastasis. Platelets. 1999;10:285–92. doi: 10.1080/09537109975915. [DOI] [PubMed] [Google Scholar]
- 22.Amirkhosravi A, Mousa SA, Amaya M, et al. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost. 2003;90:549–54. doi: 10.1160/TH03-02-0102. [DOI] [PubMed] [Google Scholar]
- 23.Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–90. [PubMed] [Google Scholar]
- 24.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tmnor cells. Blood. 2000;96:3302–9. [PubMed] [Google Scholar]
- 25.Mousa SA, Mohamed S. Anti-angiogenic mechanisms and efficacy of the low molecular weight heparin, Tinzaparin: anti-cancer efficacy. Oncol Rep. 2004;12:683–8. [PubMed] [Google Scholar]
- 26.Mousa SA, Mohamed S. Inhibition of endothelial cell tube formation by the low molecular weight hepa rin, Tinzaparin, is mediated by tissue factor pathway in hibitor. Thromb Haemost. 2004;92:627–33. doi: 10.1160/TH04-02-0069. [DOI] [PubMed] [Google Scholar]
- 27.Lupu C, Poulson E, Toquefeuil S, et al. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vase Biol. 1999;19:2251–62. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Borsig L, Varki NM, et al. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig RJ, Boehme B, Podda M, et al. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–50. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- 30.Nakamori S, Kameyama M, Imaoka S, et al. Increased expression of Sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:36–32. [PubMed] [Google Scholar]
- 31.Nakayama T, Watanabe M, Katsumata T, et al. Expression of sialyl Lewis(a) as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer. 1995;75:2051–6. doi: 10.1002/1097-0142(19950415)75:8<2051::aid-cncr2820750804>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen T, Berner A, Kaalhus O, et al. Upregulation of the oligosaccharide sialyl Lewis X: a new prognostic parameter in metastatic prostate cancer. Cancer Res. 1995;55:1817–9. [PubMed] [Google Scholar]
- 33.Bartolazzi A, Peach R, Aruffo A, et al. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–404. [PubMed] [Google Scholar]