Abstract
Heparin is a glycosaminoglycan mixture currently used in prophylaxis and treatment of thrombosis. Heparin possesses non-anticoagulant properties, including modulation of various proteases, interactions with fibroblast growth factors, and anti-inflammatory actions. Senile dementia of Alzheimer’s type is accompanied by inflammatory responses contributing to irreversible changes in neuronal viability and brain function. Vascular factors are also involved in the pathogenesis of senile dementia. Inflammation, endogenous proteoglycans, and assembly of senile plagues and neurofibrillary tangles contribute directly and indirectly to further neuronal damage. Neuron salvage can be achieved by anti-inflammation and the competitive inhibition of proteoglycans accumulation. The complexity of the pathology of senile dementia provides numerous potential targets for therapeutic interventions designed to modulate inflammation and proteoglycan assembly. Heparin and related oligosaccharides are known to exhibit anti-inflammatory effects as well as inhibitory effects on proteoglycan assembly and may prove useful as neuroprotective agents.
INTRODUCTION
The history of heparin began in 1916 when McLean discovered a substance derived from canine liver that prevented blood from clotting [1]. The new anticoagulant was named ‘heparin’, reflecting the compound’s abundance in liver. Heparin was not practically applied by doctors until the early 1930s when large-scale isolation procedures on beef lung and porcine intestinal mucosa were developed to make heparin available in a purified, plentiful and inexpensive supply safe for human use. An early documentation of the clinical trials of heparin was published in 1939 and the effectiveness of heparin treatment in the prevention of postoperative thrombosis was quickly established [2]. The use of heparin also became essential for cardiovascular surgery to maintain extracorporeal circulation of blood through the heart-lung machine. Even today, though challenged by low molecular weight heparins and synthetic anticoagulants, heparin plays a pivotal role in the treatment and prophylaxis of multiple thrombosis-related diseases, such as venous thromboembolism, myocardial infarction, and unstable angina [3].
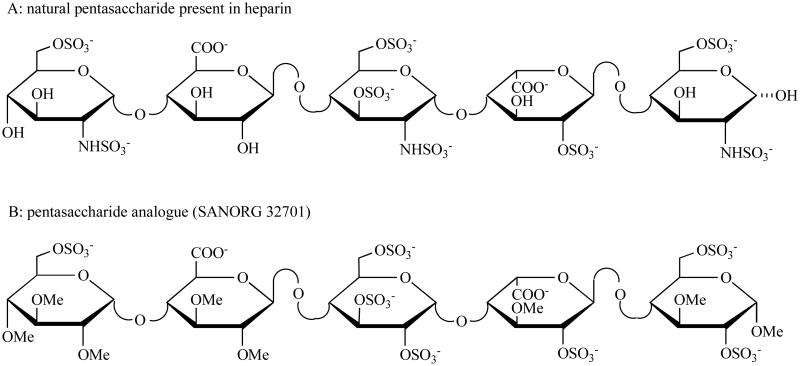
Heparin owes its popularity and importance to the remarkable array of its poly-component properties and accordingly, its various biological activities. Investigations have characterized heparin as a polysaccharide mixture, which is naturally present in the granules of mast cells of several tissues such as lung, skin, ileum, lymph nodes, thymus and liver. Most of the studies performed on structure and function of heparin have concentrated on understanding its ability to inhibit blood coagulation. In the 1970s, the mechanism of heparin-antithrombin interaction was first elucidated, which is responsible for the major anticoagulant activity of heparin [4]. Subsequently, it was discovered that heparin binds to and potentiates the activity of antithrombin through a unique pentasaccharide sequence [5]. Fig. (1) illustrates the chemical structures of natural pentasaccharide and its synthetic analogue. In recent years, the research focus on heparin has broadened to include a range of non-anticoagulant applications, such as anti-inflammatory properties in asthma, modulation of neovascularization and the control of tumor angiogenesis.
Fig. (1). Molecular structures of the pentasaccharide sequence present in heparin and its synthetic derivative.
Heparin is composed of alternating hexosamine (α-D-glucosamine) and hexuronic acid (α-L-iduronic acid or β-D-glucuronic acid) residues, joined as a disaccharide unit by (1,4)-glycosidic linkages. The natural pentasaccharide binds to antithrombin with a high affinity. A synthetic form of pentasaccharide (SR 90107A/Org 31540, fondaparinux) is the alpha methyl glycoside of the natural pentasaccharide, which exactly reproduces this unique sequence. SANORG 32701 is the synthetic analogue of natural pentasaccharide with the similar biologic effects.
Low molecular weight heparins, also referred to as low molecular mass heparins, are a group of heparin-derived fragments that emerged during the last quarter of the 20th century [6]. In the mid-1970s, several standard procedures were developed for controlled heparin depolymerization to prepare low molecular weight heparins. The discovery that heparin fragments maintained a similar antithrombotic activity while exhibiting reduced anticoagulant effects as compared to unfractionated heparin prompted extensive research on the lower molecular weight fragments. Numerous preclinical and clinical trials examining the prevention and treatment of venous thrombo-embolism followed in the 1980s and continue today. Low molecular weight heparins are now being used widely as antithrombotic agents, either prophylactically after surgery or therapeutically for deep vein thrombosis [7]. The first approval of a low molecular weight heparin from the U.S. Food and Drug Administration was granted in early 1993 for enoxaparin. Currently, there are three low molecular weight heparins, namely dalteparin, enoxaparin and tinzaparin, available in the United States.
The widespread use of low molecular weight heparins has been prompted by three main advantages over heparin, namely reduced anticoagulant activity relative to antithrombotic activity, more favorable benefit-risk ratios (antithrombotic effects versus bleeding potential) and superior pharmacokinetic properties [8]. With an increased clinical importance of low molecular weight heparins, more attention has also been paid in recent years to their non-anticoagulant effects, e.g. anti-cancer activity.
Parallel to the development of low molecular weight heparins, the introduction of heparin oligosaccharides has resulted from an improved understanding of the molecular basis of the coagulation cascade and non-anticoagulant effects associated with heparin [5, 9]. Based upon the discovery of the specific binding sequence in heparin to antithrombin, the Choay group succeeded in synthesizing the first chemically defined heparin oligosaccharides - pentasaccharide as a potent antithrombotic agent in 1980s [5, 10]. The majority of heparin oligosaccharides, however, is produced by chemical or enzymatic cleavage of heparin and, therefore, represents polysaccharide mixtures [11]. Even though some new investigations on heparin oligosaccharides have moved to their anti-inflammatory and neuroprotective actions, which may lead to the development of these agents for the treatment of Alzheimer’s disease [12], most of their biological functions and clinical implications are not fully known. The era of heparin oligosaccharides is just beginning.
NON-ANTICOAGULANT EFFECTS OF HEPARINS
The non-anticoagulant actions of heparins primarily include anti-inflammatory effects and interactions with growth factors. These effects may provide rationales for expanded use of heparins as well as the further development of heparin oligosaccharides and heparinmimetics in the neurological areas.
Anti-Inflammatory Effects of Heparins
Inflammation is mostly defined in terms of its processes [13]. It is initiated through immune recognition by T lymphocytes or antibodies, or by non-immune tissue injury. The effectors lead to multiple reactions through triggering, activation and extravasation of leukocytes and humoral cascade systems, which are collectively called inflammation. The physiological functions of this powerful response are to combat microbial invasion, to eliminate damaged tissue and eventually to repair damaged tissue. The self-limiting control mechanisms normally lead to health. However, pathological inflammation is often the result of inadequate control within the immune system leading to allergies or autoimmune diseases. Inflammation also contributes to morbidity in chronic infections (e.g. tuberculosis), chronic tissue injury (e.g. atherosclerosis) or acute tissue injury (e.g. acute myocardial infarction).
The role for endogenous heparin in inflammation is suggested by the fact that heparin is stored in mast cell granules and is released by several inflammatory effectors [14]. It is also well known that shortly after administration of heparin to patients with deep vein thrombosis, amelioration of pain and swelling occurs, indicating an in vivo anti-inflammatory effect of heparin [15]. Recent experimental studies have provided several mechanisms through which heparin may inhibit inflammatory reactions. These mechanisms include modulation of selectin-ligand interactions and integrindependent adhesion, interference of leukocyte extravasation and migration into tissues, inhibition of complement activation, interference with leukocyte chemotaxis and haptotaxis, and inhibition of platelet activation and aggregation [15]. In addition, it is becoming increasingly clear that coagulation augments inflammation and natural anticoagulants, in particular antithrombin, protein C and TFPI can limit the coagulation induced increases in inflammatory response [16]. Therefore, heparin may also exert its anti-inflammatory effects through interactions with these proteins. The main reason why heparin has not been used clinically for control of pathological inflammation is the high risk for bleeding.
Progress in the understanding of structure–activity relationships regarding anticoagulant effects and anti-inflammatory mechanisms of heparin have resulted in increased interest in heparin and its derivatives as a new treatment of inflammatory diseases. Two important findings have provided a basis for the development of heparin oligosaccharides in this area. Firstly, heparin’s inhibition of inflammatory responses is independent of its anticoagulant activity [17-19]. Secondly, heparin oligosaccharides exhibit similar or even better anti-inflammatory effects in comparison to heparin [20].
Since anticoagulant activity of heparin can be distinguished from its anti-inflammatory properties, many efforts have also been made in the development of heparin oligosaccharides in the treatment of inflammation-associated diseases, such as asthma and ischemia-reperfusion injury. Using a sheep asthma model, Ahmed and coworkers have demonstrated that inhaled heparin oligosaccharides can inhibit the antigen-induced airway responses and post antigen airway hyper-responses that lead to asthma attack [20]. This antiallergic activity of oligosaccharides is mediated by nonanticoagulant fractions and resides in chains with molecular weight of 2,500 Da. In a model of cerebral ischemia in rodents in which the animals are subjected to 1 h of ischemia and 48 h of reperfusion, the neural protective effects of heparin and low molecular weight heparins have been evaluated [21-22]. The treatment groups receiving heparin and enoxaparin showed a significant reduction in neutrophil accumulation, infarct size and neurological dysfunction 48 h after reperfusion. Although the authors concluded that neural protection was dependent upon the anti-leukocyte properties rather than the anticoagulant activity of heparins, the underlying mechanisms, in particular the structure-activity relationship, were not fully clear yet.
Heparins and Fibroblast Growth Factors
The interactions between heparins and fibroblast growth factors (FGFs) have been extensively studied for over two decades, and are still a subject of controversy. The potentiation of angiogenesis, i.e. growth of new blood vessels, by heparin was first reported by Folkman and colleagues, which eventually led to the discovery of modulatory effects of heparin on the mitogenicity and stability of FGFs [23].
FGFs comprise a family of at 22 structurally related proteins. The biological functions of FGFs are primarily associated with cell migration and differentiation; though also include diverse cellular and developmental processes, such as limb, inner ear and brain development, wound healing, early embryogenesis, skeletal growth and differentiation [24-27]. Additionally, FGFs play an important role in the neuronal responses to central nervous system injury. It has been demonstrated that many of the neurons that transport FGFs are affected in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [28]. Hence, FGFs may be a potential neurotrophic factor for the slowing of cell death related to various neurodegenerative diseases.
The activity of FGFs is regulated at multiple levels. There are four fibroblast growth factor receptors (FGFRs) with an additional three splice variants, which are transmembrane tyrosine kinases displaying unique expression patterns and specific binding interactions [29]. Additionally, GAGs such as heparin or heparan sulfate are required for FGFs to bind to high affinity receptors and constitute another level at which FGF biologic activity may be modulated [30].
The mechanism by which heparin is involved in interacting with FGFs is largely unknown and several models have been proposed. The well-accepted ‘dynamic’ theory is that FGFs bind to heparan sulfate on the endothelial cell surface of the extracellular matrix (ECM), which acts as a reservoir for FGFs. Exogenous heparins compete with heparan sulfates for binding of FGFs, and may release these proteins from the ECM [23,31], as well as promote the activation of their receptors [32,33]. In man, therapeutic dosages of heparin can cause an increase in plasma levels of FGFs [34-36].
The molecular weight dependence of the heparin/FGFs interaction is reviewed by D’Amore [34] and Conrad [37]. It has been proposed that heparin oligosaccharides inducing mitogenic activities of the FGFs must be (a) saccharides longer than the smallest oligosaccharides required to bind to the FGFs, and (b) highly sulfated. Barzu et al. described the fractionation of heparin fragments on a column of immobilized FGFs; the smallest fragment, which bound to the column, was a hexasaccharide [38]. The bound fragments from 6 to 12 saccharides (hexa- to decasaccharide) were all capable of potentiating the mitogenic activity of FGF under the appropriate conditions. However, in a recent study on the interaction between various heparin oligosaccharides and FGF2, Ornitz et al. have reported that several synthetic unsulfated heparin diand trisaccharides are capable of stimulating FGF2-mediated DNA synthesis in a lymphoid cell line. These results suggest that unique oligosaccharide structure rather than molecular weight is a more important determinant in the heparin/FGFs interactions [39].
Despite the uncertainty about the interactions between heparin and FGFs, both heparin and FGFs are associated with Alzheimer’s disease. In a recent study, it has been demonstrated that β-amyloid fibrils and FGF-2 have common binding sites in heparan sulfate. β-amyloid fibrils and FGF-2 represent neurotoxic and neuroprotective pathways, respectively. Thus, heparin oligosaccharides might enhance the neuroprotective effects of FGF-2 by blocking the binding of β-amyloid fibrils with heparan sulfate [40].
HEPARIN OLIGOSACCHARIDES AND ALZHEIMER’S DISEASE
Alzheimer’s Disease
Dementia is characterized by ‘a decline in intellectual function severe enough to interfere with a person's normal daily activity and social relationships [41]. Alzheimer's disease (AD) is the most common form of dementing illness in the elderly and remains one of the major causes of death in the US. It is currently estimated that approximately 4 million people in the US have AD. This number is expected to rise to 14 million by the year 2040, as the elderly population continues to increase, with the so-called ‘baby-boomers’ aging. Thus, the potential impact of AD is enormous, and projected costs for health care could rise dramatically if AD cannot be prevented or managed better than it is today. Currently, the national cost of AD is placed at $50-100 billion per year [42].
Alzheimer's disease (AD) is characterized by three histopathological markers: amyloid deposition (senile plaques), neurofibrillary tangles (NFT), and neuronal cell loss in several cortical and subcortical regions. The main component of senile plaques is the β-amyloid peptide [43]. It is derived from a large trans-membrane protein, the amyloid precursor protein (APP) [44] and is thought to play an important role in the pathogenesis of AD. Mutations in the APP gene located on chromosome 21 are associated with familial AD [45]. The main component of the NFT is a paired helical filament, which consists largely of the microtubule associated protein tau in an abnormal state of hyper-phosphorylation [46]. More recent studies have indicated that mutations in a complex, 8-transmembrane-domain protein called presenilin (PS), at the PS1 and PS2 loci on chromosomes 14 and 1, respectively, PS1 exon 9 deletions, as well as changes in intronic polymorphisms at the PS loci account for a growing number of early and late-onset AD cases [104]. As with most APP mutations, the mutations in PS lead to increased generation of the Aβ42 peptide and elevated oligomer levels.
Although the exact pathogenesis of AD remains to be fully defined, several pharmacological strategies for preventing and treating AD are under active investigation. These strategies include cholinesterase inhibition [47], M1 agonist administration [48], administration of nerve growth factor [49], estrogen replacement therapy [50], antioxidants, and anti-inflammatory treatment [51-52]. More recently, new drug design has targeted molecular events involved in the pathogenesis of AD including β-amyloid and NFT formation [53-55].
Low molecular weight heparins and heparin oligosaccharides have been proposed as promising agents in the management of AD due to their multiple interventions on the pathogenesis of AD. These interventions are summarized in the following sections including potential competitive interactions with proteoglycans, vascular effects, interactions with serpins and anti-inflammatory actions.
Proteoglycans and Vascular Risk Factors
Proteoglycan Hypothesis
Antiamyloid strategies may theoretically fall into several categories: reducing Aβ overproduction, inhibiting Aβ aggregation, and favoring its clearance. Reduction of Aβ production and enhancement of its clearance have been limited due to certain factors such as lack of knowledge in general. The antiaggregation approach is primarily based upon proteoglycan hypothesis, which is represented by commercial efforts at blocking the promotion of amyloidogenesis.
Heparan sulfate and related proteoglycans (PGs) have been implicated in the progressive development of polymerized amyloid-β (Aβ) deposits and other pathophysiological effects in AD [56]. This hypothesis is based on the fact that PGs and glycosaminoglycans (GAGs) are found in a number of peripheral amyloidoses including reactive systemic amyloidoses, which are secondary to chronic inflammatory conditions. Although AD amyloid plaques are different from peripheral amyloidoses, they share certain characteristics, such as β-pleated secondary structures that associated with PGs. Studies have shown that the association between PGs and amyloid plaques in AD may be due to specific binding of Aβ sequence and APP to PGs as well as to GAGs [56-58]. Functional GAG mimics, including heparins, which have been shown to block the binding of heparan sulfate PGs to the APP molecule could theoretically be beneficial in preventing plaque deposition with aging.
Moreover, PGs have been shown in both in vitro and in vivo models to increase the aggregation rate of Aβ into β-pleated fibrils [57-59]. Snow et al have shown that after Aβ along with heparan sulfate PG was infused into the lateral ventricles of the rat, there was an increased fibrillary Aβ deposition in the neuropil [60]. Interestingly, in the same study when the Aβ and GAGs were infused together, no deposition was detected. However, they did not test whether the effects of heparan sulfate PG could be blocked by coinfusion with GAGs. PGs have also been shown to increase the polymerization of tau proteins into paired-helical filaments, which make up the neurofibrillary tangles of AD [61-63]. Theoretically, GAGs could also decrease the polymerization of tau and block the formation of neurofibrillary tangles by competing with PGs. Based on this theory, a small synthetic molecule, namely tramiprosate (Alzhemed™), which shares certain functional properties of GAGs, has been designed in attempt to modify the course of AD by binding to the soluble amyloid β peptide and interfere with the amyloid cascade that is associated with amyloid deposition and the toxic effects of Aβ peptide in the brain [105]. In a recently completed phase II clinical trial for Alzhemed™ in patients with mild-to-moderate Alzheimer’s disease, promising results were revealed [106]. For example, a significant decrease in amyloid protein was observed in patients’ cerebrospinal fluid after three months of treatment. Furthermore, the majority of patients on the highest dose of Alzhemed™ showed, at the 20 month time point of the open-label extension study, stable results on cognitive functioning as measured by the Alzheimer’s Disease Assessment Scale, Cognitive Subpart, especially in mild AD patients [107].
Vascular Risk Factors
Alzheimer's disease (AD) is a primary degenerative dementia and is not considered to be of vascular origin. Indeed, stroke and severe cerebrovascular diseases are generally exclusionary for the clinical diagnosis of AD. However, both epidemiological and neuropathological studies have recently suggested an association between AD and several vascular risk factors, such as thrombosis and atherosclerosis [64].
One of the milestone studies carried out to determine the risk factors associated with AD is the Rotterdam Study. More than 7000 elderly subjects have been studied since 1990 in a series of reports consisting of demented subjects and non-demented, age-matched controls [65]. On the basis of the data gathered in this study, it was concluded that vascular risk factors and indicators of vascular disease, particularly in elderly subjects, have an association with AD [66]. The risk factors for AD reported in the Rotterdam Study include the following: diabetes mellitus, thrombosis, high fibrinogen concentrations, high serum homocysteine, atrial fibrillation, smoking, and atherosclerosis. All these conditions have a vascular involvement and are known to reduce cerebral perfusion [67]. Since heparins are extensively used in the modulation of the vascular functions, their effects on the brain and peripheral vasculatures may theoretically benefit AD patients.
Serine Proteases and Serine Protease Inhibitors
Serine Proteases
The serine proteases are a family of enzymes that catalyze the hydrolysis of covalent peptide bonds of other proteins [68]. This activity depends on a set of amino acid residues in the active site of the enzyme, one of which is always a serine, thus accounting for their name. In mammals, serine proteases perform many important functions, especially in digestion, blood clotting, and the complement system [69].
Three protein-digesting enzymes secreted by the pancreas are serine proteases including chymotrypsin, trypsin and elastase. These three enzymes share similar tertiary as well as primary structures. In fact, the active serine residue is at the same position (Ser-195) in all three. Despite their similarities, they have different substrate specificities; that is, they cleave different peptide bonds during protein digestion. Several activated clotting factors and fibrinolytic proteins are serine proteases as well, such as factors VIIa, IXa, Xa, XIa, XIIa, prekallikrein, thrombin, plasmin, and tissue plasminogen activator. The control of blood clotting by the cascade of protease activation has been reviewed in the previous sections. In addition to protein digesting enzymes and clotting factors, several proteins involved in the complement cascade are serine proteases, including C1r and C1s, the C3 convertases, C4b,2a, and C3b,Bb, which possess potent pro-inflammatory effects. The role of serine proteases in the pathogenesis of AD is not fully understood. Table 1 summarizes the recent studies on several serine proteases and their association with AD.
Table 1.
Serine Proteases Possibly Involved in the Pathogenesis of Alzheimer’s Disease (AD)
| Serine proteases | Functions | Potential Role in the Pathogenesis of Alzheimer’s Disease | Reference |
|---|---|---|---|
| Trypsin | Protein digestion | Present in senile plaques | [91] |
| Factor IIa (thrombin) | Coagulation protease | Inducing APP secretion, cleaving APP into amyloidogenic fragments, increased in senile plaques |
[92] |
| Factor Xa | Coagulation protease | Cleaving APP into amyloidogenic fragments | [93] |
| Factor XIa | Coagulation protease | Cleaving APP and modulating APP-mediated cell adhesion | [94] |
| Factor XIIa | Coagulation protease | Present in senile plaques | [95] |
| Kallikrein 8 | Coagulation protease | High mRNA levels in the hippocampus | [96] |
| Plasmin | Fibrinolysis protease | Cleaving APP, reduced in AD brains | [97] |
| uPA | Fibrinolysis protease | Present in senile plaques | [98] |
| tPA | Fibrinolysis protease | Present in senile plaques | [98] |
| C1q, C3, C4, C5 C6, C7, C8, C9 | Complement proteins | Highly co-localized with amyloid deposits and neurofibrillary tangles | [99] |
APP, amyloid precursor protein.
Serine Protease Inhibitors and Alzheimer’s Disease
Serpins is an acronym given to a large family of serine protease inhibitors that share a complex, but well conserved, tertiary structure. Members of the family are diversely present in viruses, insects, plants and higher organisms, but not in bacteria or yeast [70]. Serpins share about 30% homology and have similar tertiary structure and inhibition mechanisms [71]. Each serpin plays a role in inhibiting excessive action of its specific target proteases. The serpins combine with proteases to form inactive complexes, which are then cleared from the circulation. The serpins are, in fact, suicide substrates for their target proteases in that they interact with the active sites on the proteases and are cleaved by proteases [72].
Although serpins regulate the activity of proteases involved in such diverse processes as coagulation, fibrinolysis, inflammation, cell migration, and tumor-genesis as depicted in Table 2, the physiological role of serpins remains largely unknown. For example, in vitro studies have demonstrated trypsin and plasmin as target proteases of antithrombin (AT), but the reaction of trypsin and plasmin with AT in vivo is too slow to be of any physiological importance [73]. In addition, the expression of AT has been recently detected in the cerebral cortex and brain microvessels [74], suggesting its role in the maintenance of neuronal integrity. Thus, the tissue distribution patterns of serpins can provide clues about their physiological function.
Table 2.
Serine Protease Inhibitors Possibly Involved in the Pathogenesis of Alzheimer’s Disease
| Serpins | Functions | Expression in Alzheimer’s Disease | Reference |
|---|---|---|---|
| Alfa1-antichymotrypsin | Acute-phase protein in inflammation | Increase in senile plaques | [100] |
| Alfa1-antitrypsin | Acute-phase protein in inflammation | Increase in senile plaques | [101] |
| Antithrombin | Destruction of released coagulation proteases, natural anticoagulant | Increase in senile plaques | [74] |
| Plasminogen activator inhibitor | Inhibition of plasminogen activator; regulation of fibrinolysis | Increase in senile plaques | [102] |
| Protease nexin I | Natural anticoagulant in the central nervous system decrease in the brain | Increase in senile plaques, | [103] |
| Tissue factor pathway inhibitor | Inhibition of tissue factor and factor Xa; natural anticoagulant | Elevation in frontal cortex | [75] |
Recently, a number of serpins have been linked with AD as summarized in Table 2, including AT, α1-antichymotrypsin, α1-antitrypsin, neuroserpin, protease nexin I, and tissue factor pathway inhibitor [72,75,76]. The pathological mechanisms behind this linkage are not fully understood. Carrell and Lomas have proposed a ‘serpinopathies’ model to describe the pathogenesis of common neurodegenerative diseases using structural biology, in which point mutations of serpins eventually lead to conformational abnormities, such as the amyloid aggregation, and AD [77]. On the other hand, Janciauskiene et al. reviewed various neuroinflammatory factors involved in AD and proposed serpins as a part of anti-inflammatory mechanism. More interestingly, PGs and GAGs were also involved in neuroinflammation and AD, even though their functions, in particular interactions with serpins were not clear.
Tissue Factor Pathway Inhibitor and Alzheimer’s Disease
Tissue factor pathway inhibitor (TFPI) is a member of the serpin superfamily. Like several other serpins, a strong expression and significantly elevated amount of TFPI in frontal cortex in AD patients were demonstrated by Hollister et al. using immuno-histochemical localization. It is still unclear whether the increase in TFPI is due to a compensatory mechanism in which the functionality of TFPI decreases, or is the etiology in AD. Since TFPI is localized to microglia in both AD and non-AD individuals and to senile plaques, Hollister et al. suggested that TFPI might play a cell specific role in protease regulation in the brain [75].
Tissue factor pathway inhibitor (TFPI) is also involved in the regulation of atherosclerosis, which is one of the vascular risk factors of AD. Westrick et al. evaluated the effect of heterozygous TFPI deficiency on the development of atherosclerosis and thrombosis in atherosclerosis-prone mice [78]. Compared with mice with a normal TFPI genotype, mice with a TFPI deficiency exhibited a greater atherosclerotic burden and more tissue factor activity within the plaques involving the carotid and common iliac arteries. These observations indicated that TFPI protects from atherosclerosis and is an important regulator of the thrombosis that occurs in the setting of atherosclerosis.
Heparin Oligosaccharides and Alzheimer’s Disease
Heparins are considered conventionally as cardiovascular medicines. The introduction of heparins in the treatment of AD was based on the hypothesis that heparins might improve the cerebral microcirculation through its antithrombotic effects [79]. However, the hemorrhage potential and adverse effects of heparin hindered its neurological applications. With the introduction of non-anticoagulant heparin oligosaccharides and a better understanding of the vascular risk factors associated with AD, the re-evaluation of heparins as a potential therapy for Alzheimer’s patient has been carried out in both pre-clinical and clinical studies.
In a study by Lorens et al., aged rats showed a significant partial reversal of age related behavioral deficits following a high molecular weight GAG polysulfate (Ateroid™) administration [80]. Biochemical observations showed that Ateroid™ counteracted the age-related reduction in dopamine metabolites and also aided in the normalization of stress induced corticosterone secretion seen in the aged rats. Conti et al. prescribed Ateroid™ for primary dementia patients. These patients showed significant improvements in objective performance, psychology, and social behavior over placebo groups [81]. Parnetti et al. showed that prescribed doses of Ateroid™ could significantly improve certain biochemical abnormalities found in primary dementia [82]. In recent studies, Walzer et al. have tested the neuroprotective effects of a LMWH-certoparin in an animal model mimicking the pathology of AD. A significant decrease of tau toxicity by certoparin was observed among treated animals [83]. Using the same animal model, Dudas et al. demonstrated the protective effects of a heparin oligosaccharide mixture on the brain after the injection of amyloid peptide into the amygdale [84].
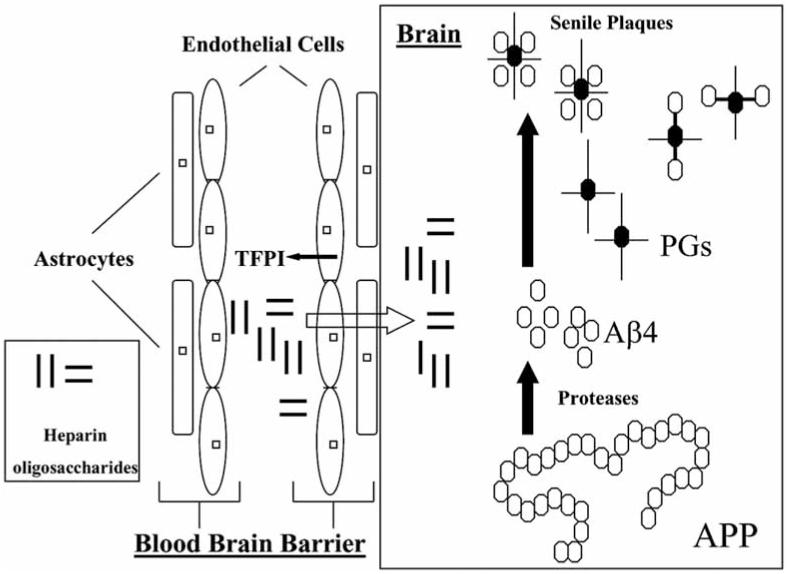
Despite the positive results obtained from these studies, the underlying mechanisms behind the effects of heparins on AD are not clear. Snow and Wight have proposed that heparin GAGs might interfere directly with endogenous PGs [85]. Both in vitro and in vivo studies have provided experimental evidence to support this hypothesis that heparin oligosaccharides pass through the blood brain barrier [86-87]. In addition, heparins might also participate in the regulation of cerebral vascular functions by interactions with endogenous serpins, such as tissue factor pathway inhibitor [88]. Since both PGs and serpins are associated with neuro-inflammation [72], it is likely that the anti-inflammatory effects in the central nervous system also contribute to the overall mechanism of heparins in AD as illustrated in Fig. (2).
Fig. (2). Potential mechanisms of heparin oligosaccharides in the treatment and prevention of Alzheimer’s disease.
Heparin oligosaccharides may act at multiple sites. They are capable of releasing tissue factor pathway inhibitors (TFPI) from endothelial cell that exert anti-inflammatory and anti-atherosclerosis effects. After passing through the blood-brain barrier, they may interact with proteoglycans (PGs) to block the assembling of beta amyloid peptide (Aβ4) and PGs and senile plaque formation (APP: amyloid precursor protein).
CONCLUSION
The rationales behind development of heparin oligosaccharides for the treatment of senile dementia are rather complicated. They might be mainly associated with three factors involved in the etiology of AD, namely proteoglycans, inflammation and vascular deficits. It is hypothesized that these agents may exert their therapeutic effects by interfering with the proteoglycan-induced amyloid and tau abnormality in AD. Recent studies have supported this hypothesis showing that low molecular weight heparins and related oligosaccharides are capable of attenuating proteoglycan-associated tau toxicity in rat brain [83-84].
Inflammation is known to play an important role in the pathogenesis of AD [72]. Even though heparin and low molecular weight heparins have potent inhibitory effects on inflammation, it is unknown whether lower molecular weight glycosaminoglycans also possess this property. In our previous studies, oligosaccharides were demonstrated to release TFPI from endothelium, which is considered to be part of the vascular control of inflammation [16]. It has not been definitively proven that the anti-inflammatory effects of heparin oligosaccharides are associated with their beneficial effects in models of AD. A recent study has shown that heparin and related oligosaccharides exhibit remarkable anti-inflammatory effects and attenuate beta amyloid mediated neurotoxicity in the PC12 cells, suggesting a correlation between anti-inflammation and therapeutic potential in the treatment of AD [89]. The underlying mechanisms of these effects have been proposed to be related to the inhibitory effects of heparin oligosaccharides on inflammatory mediators, such as the complement system, L-selectin, P-selectin and phospholipase A2 [14, 89, 90].
To date, several vascular risk factors such as arteriosclerosis and thrombosis have been suggested to play a role in the etiology of senile dementia. Even though heparin is well known for its vascular effects, it is not clear whether heparin oligosaccharides possess the same effects as heparin. In our previous studies, heparin oligosaccharides showed relatively weak antithrombotic effects in comparison to heparin and low molecular weight heparins. Furthermore, the treatment of senile dementia is a rather long-term management; there is no data available regarding the long-term vascular effects of heparin or its derivatives. It is also unclear whether maintaining a slight antithrombotic effect through long-term treatment with oligosaccharides would decrease vascular risks in AD patients. If this were the case, this treatment would be suitable as a preventive means for AD.
In summary, the development of heparin oligosaccharides has progressed such that two glycosaminoglycan agents are in the different phases of clinical development. In addition, several other ultra low molecular weight heparins and heparinomimetics are in the various phases of pre-clinical development (Table 3). The clinical spectrum of these agents may not only include the management of AD, but also encompass a wide array of diseases, such as thrombosis, cancer, inflammatory disorders, sepsis, hypertension and diabetes. Despite this remarkable progress in this area, there still remains a considerable amount of research work unexplored to understand that chemistry and biology of heparin oligosaccharides. It is therefore projected that with advanced technology and structure-activity relationship studies, many newer agents may be designed based on heparin oligosaccharides or their combinatory components. Thus, heparin oligosaccharides may have major impact on drug development in the coming years.
Table 3.
Glycosaminoglycans and Mimetics Under Development for the Treatment of Alzheimer’s Disease
| HF0402 (C3) | Ateroid® | AlzhemedTM | |
|---|---|---|---|
| Chemistry | Very low molecular weight glycosaminoglycan |
Glycosaminoglycan polysulfate | 3-amino-1-propanesulfonic acid (tramiprosate) |
| Administration | iv, sc, po | po | po |
| Pharmacology | Alzheimer’s disease, protection against neuronal cell death, neuronal repair, prevention of amyloid peptide- induced toxicity in the brain via a number of sites of activity, growth factor-like neurotrophism |
Multi-infarct dementia, ischemic vascular dementia, old-age dementia, the behav- ioral effects may be due to normalizing influence on dopamine neurotransmission in the nucleus accumbens |
Alzheimer’s disease, modify the course of disease through anti-amyloid activity: binding to soluble amyloid beta protein, preventing and stopping the formation and the deposition of amyloid fibrils in the brain, and reducing the amyloid-induced toxicity on neuronal and brain inflammatory cells |
| Adverse effects | Little anticoagulant effects, well tolerated in healthy volunteers |
Well tolerated in dementia patients; vital signs and laboratory measures did not show clinically significant changes |
Well tolerated in patients; most frequent adverse events were nausea and vomiting |
| Clinical trial | Single dose, Phase I study planned | Marketed in Europe and Asia | Phase III clinical trials |
| Website | http://www.hunter-fleming.com/ | http://www.cornelliconsulting.it | http://www.neurochem.com |
REFERENCES
References 108-110 are related articles recently published in Current Pharmaceutical Design.
- [1].McLean J. The discovery of heparin. Circulation. 1959;19:75. doi: 10.1161/01.cir.19.1.75. [DOI] [PubMed] [Google Scholar]
- [2].Best CH. Preparation of heparin and its use in the first clinical cases. Circulation. 1959;19:79. doi: 10.1161/01.cir.19.1.79. [DOI] [PubMed] [Google Scholar]
- [3].Fareed J, Hoppensteadt DA. Heparins in the new millennium: will unfractionated heparin survive? Epilogue. Semin Thromb Hemost. 2000;26(Suppl 1):87. [PubMed] [Google Scholar]
- [4].Rosenberg RD, Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci USA. 1979;76:1218. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Choay J. Chemically synthesized heparin-derived oligosaccharides. Ann N Y Acad Sci. 1989;556:61. doi: 10.1111/j.1749-6632.1989.tb22490.x. [DOI] [PubMed] [Google Scholar]
- [6].Fareed J, Hoppensteadt D, Jeske W, Clarizio R, Walenga JM. Low molecular weight heparins: are they different? Can J Cardiol. 1998;14(Suppl E):28E. [PubMed] [Google Scholar]
- [7].Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(Suppl 1):64S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- [8].Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- [9].Fareed J. Heparin, its fractions, fragments and derivatives: some newer perspectives. Semin Thromb Hemost. 1985;11:1. doi: 10.1055/s-2007-1004350. [DOI] [PubMed] [Google Scholar]
- [10].Walenga JM, Jeske WP, Bara L, Samama MM, Fareed J. Biochemical and pharmacologic rationale for the development of a synthetic heparin pentasaccharide. Thromb Res. 1997;86:1. doi: 10.1016/s0049-3848(97)00042-x. [DOI] [PubMed] [Google Scholar]
- [11].Ma Q, Dudas B, Daud A, Iqbal O, Hoppensteadt D, Jeske W, et al. Molecular and biochemical profiling of a heparin-derived oligosaccharide C3. Thromb Res. 2002;105:303. doi: 10.1016/s0049-3848(01)00413-3. [DOI] [PubMed] [Google Scholar]
- [12].Cornelli U. In: Casu B, Non-anticoagulant action of glycosaminoglycans. Plenum Press; New York: 1996. The therapeutic approach to Alzheimer’s disease; pp. 249–79. [Google Scholar]
- [13].McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- [14].Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253. [PubMed] [Google Scholar]
- [15].Tyrrell DJ, Horne AP, Holme KR, Preuss JM, Page CP. Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv Pharmacol. 1999;46:151. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- [16].Esmon CT. New mechanisms for vascular control of inflammation mediated by natural anticoagulant proteins. J Exp Med. 2002;196:561. doi: 10.1084/jem.20021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ekre HP. Inhibition of human and Guinea pig complement by heparin fractions differing in affinity for antithrombin III or in average molecular weight. Int J Immunopharmacol. 1985;7:271. doi: 10.1016/0192-0561(85)90036-0. [DOI] [PubMed] [Google Scholar]
- [18].Ekre HP, Fjellner B, Hagermark O. Inhibition of complement dependent experimental inflammation in human skin by different heparin fractions. Int J Immunopharmacol. 1986;8:277. doi: 10.1016/0192-0561(86)90109-8. [DOI] [PubMed] [Google Scholar]
- [19].Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins: implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmed T, Ungo J, Zhou M, Campo C. Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J Appl Physiol. 2000;88:1721. doi: 10.1152/jappl.2000.88.5.1721. [DOI] [PubMed] [Google Scholar]
- [21].Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 2002;8:1. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yanaka K, Nose T. Heparin ameliorates brain injury by inhibiting leukocyte accumulation. Stroke. 1996;27:2146. [PubMed] [Google Scholar]
- [23].Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein basic fibroblast growth factor is stored within basement membrane. Am J Pathol. 1988;130:393. [PMC free article] [PubMed] [Google Scholar]
- [24].Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- [25].Haynes LW. Fibroblast (heparin-binding) growing factors in neuronal development and repair. Mol Neurobiol. 1988;2:263. doi: 10.1007/BF02935635. [DOI] [PubMed] [Google Scholar]
- [26].Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998;95:5672. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Slack JM, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- [28].Mufson EJ, Kroin JS, Sendera TJ, Sobreviela T. Distribution and retrograde transport of trophic factors in the central nervous system: functional implications for the treatment of neurodegenerative diseases. Prog Neurobiol. 1999;57:451. doi: 10.1016/s0301-0082(98)00059-8. [DOI] [PubMed] [Google Scholar]
- [29].Miao HQ, Ornitz DM, Aingorn E, Ben-Sasson SA, Vlodavsky I. Modulation of fibroblast growth factor-2 receptor binding, dimerization, signaling, and angiogenic activity by a synthetic heparinmimicking polyanionic compound. J Clin Invest. 1997;99:1565. doi: 10.1172/JCI119319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vlodavsky I, Ishai-Michaeli R, Mohsen M, Bar-Shavit R, Catane R, Ekre HP, et al. Modulation of neovascularization and metastasis by species of heparin. Adv Exp Med Biol. 1992;313:317. doi: 10.1007/978-1-4899-2444-5_31. [DOI] [PubMed] [Google Scholar]
- [32].Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans: biological implications in neovascularization. Int J Clin Lab Res. 1996;26:15. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- [33].Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83:357. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- [34].D'Amore PA. Heparin-endothelial cell interactions. Haemostasis. 1990;20(Suppl 1):159. doi: 10.1159/000216175. [DOI] [PubMed] [Google Scholar]
- [35].Taniguchi T, Toi M, Tominaga T. Rapid induction of hepatocyte growth factor by heparin. Lancet. 1994;344:470. doi: 10.1016/s0140-6736(94)91797-3. [DOI] [PubMed] [Google Scholar]
- [36].Yamazaki H, Oi H, Matsushita M, Inoue T, Tang JT, Nose T, et al. Heparin induces rapid and remarkable elevation of hepatocyte growth factor/scatter factor during trans-arterial embolization of renal cell carcinoma. Anticancer Res. 1997;17:1435. [PubMed] [Google Scholar]
- [37].Conrad HE. Heparin-binding proteins. Academic Press; San Diego: 1998. [Google Scholar]
- [38].Barzu T, Lormeau JC, Petitou M, Michelson S, Choay J. Heparinderived oligosaccharides: affinity for acidic fibroblast growth factor and effect on its growth-promoting activity for human endothelial cells. J Cell Physiol. 1989;140:538. doi: 10.1002/jcp.1041400320. [DOI] [PubMed] [Google Scholar]
- [39].Ornitz DM, Herr AB, Nilsson M, Westman J, Svahn CM, Waksman G. FGF binding and FGF receptor activation by synthetic heparan-derived di- and trisaccharides. Science. 1995;268:432. doi: 10.1126/science.7536345. [DOI] [PubMed] [Google Scholar]
- [40].Lindahl B, Westling C, Gimenez-Gallego G, Lindahl U, Salmivirta M. Common binding sites for beta-amyloid fibrils and fibroblast growth factor-2 in heparan sulfate from human cerebral cortex. J Biol Chem. 1999;274:30631. doi: 10.1074/jbc.274.43.30631. [DOI] [PubMed] [Google Scholar]
- [41].Rabins PV. Developing treatment guidelines for Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(Suppl 11):17. [PubMed] [Google Scholar]
- [42].Meek PD, McKeithan K, Schumock GT. Economic considerations in Alzheimer’s disease. Pharmacotherapy. 1998;18:68. [PubMed] [Google Scholar]
- [43].Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- [44].Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- [45].Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- [46].Iqbal K, Grundke-Iqbal I. Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer’s disease. Mol Neurobiol. 1991;5:399. doi: 10.1007/BF02935561. [DOI] [PubMed] [Google Scholar]
- [47].Mayeux R, Sano M. Treatment of Alzheimer’s disease. N Engl J Med. 1999;341:1670. doi: 10.1056/NEJM199911253412207. [DOI] [PubMed] [Google Scholar]
- [48].Fisher A, Brandeis R, Haring R, Eshhar N, Heldman E, Karton Y, et al. Novel M1 muscarinic agonists in treatment and delaying the progression of Alzheimer’s disease: an unifying hypothesis. J Physiol Paris. 1998;92:337. doi: 10.1016/S0928-4257(99)80001-1. [DOI] [PubMed] [Google Scholar]
- [49].Yamada K, Nitta A, Hasegawa T, Fuji K, Hiramatsu M, Kameyama T, et al. Orally active NGF synthesis stimulators: potential therapeutic agents in Alzheimer’s disease. Behav Brain Res. 1997;83:117. doi: 10.1016/s0166-4328(97)86054-8. [DOI] [PubMed] [Google Scholar]
- [50].Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med. 1997;103:19S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- [51].Broe GA, Grayson DA, Creasey HM, Waite LM, Casey BJ, Bennett HP, et al. Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol. 2000;57:1586. doi: 10.1001/archneur.57.11.1586. [DOI] [PubMed] [Google Scholar]
- [52].Prasad KN, Cole WC, Hovland AR, Prasad KC, Nahreini P, Kumar B, et al. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr Opin Neurol. 1999;12:761. doi: 10.1097/00019052-199912000-00017. [DOI] [PubMed] [Google Scholar]
- [53].Howlett DR, Jennings KH, Lee DC, Clark MS, Brown F, Wetzel R, et al. Aggregation state and neurotoxic properties of Alzheimer beta-amyloid peptide. Neurodegeneration. 1995;4:23. doi: 10.1006/neur.1995.0003. [DOI] [PubMed] [Google Scholar]
- [54].Kisilevsky R, Lemieux LJ, Fraser PE, Kong X, Hultin PG, Szarek WA. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer’s disease. Nat Med. 1995;1:143. doi: 10.1038/nm0295-143. [DOI] [PubMed] [Google Scholar]
- [55].Snow AD, Kinsella MG, Parks E, Sekiguchi RT, Miller JD, Kimata K, et al. Differential binding of vascular cell-derived proteoglycans (perlecan, biglycan, decorin, and versican) to the beta-amyloid protein of Alzheimer’s disease. Arch Biochem Biophys. 1995;320:84. doi: 10.1006/abbi.1995.1345. [DOI] [PubMed] [Google Scholar]
- [56].Snow AD, Lara S, Nochlin D, Wight TN. Cationic dyes reveal proteoglycans structurally integrated within the characteristic lesions of Alzheimer’s disease. Acta Neuropathol. 1989;78:113. doi: 10.1007/BF00688198. [DOI] [PubMed] [Google Scholar]
- [57].Buee L, Ding W, Anderson JP, Narindrasorasak S, Kisilevsky R, Boyle NJ, et al. Binding of vascular heparan sulfate proteoglycan to Alzheimer’s amyloid precursor protein is mediated in part by the n-terminal region of A4 peptide. Brain Res. 1993;627:199. doi: 10.1016/0006-8993(93)90321-d. [DOI] [PubMed] [Google Scholar]
- [58].Buee L, Ding W, Delacourte A, Fillit H. Binding of secreted human neuroblastoma proteoglycans to the Alzheimer’s amyloid A4 peptide. Brain Res. 1993;601:154. doi: 10.1016/0006-8993(93)91706-x. [DOI] [PubMed] [Google Scholar]
- [59].Gupta-Bansal R, Frederickson RC, Brunden KR. Proteoglycanmediated inhibition of a beta proteolysis: a potential cause of senile plaque accumulation. J Biol Chem. 1995;10:18666. doi: 10.1074/jbc.270.31.18666. [DOI] [PubMed] [Google Scholar]
- [60].Snow AD, Sekiguchi R, Nochlin D, Fraser P, Kimata K, Mizutani A, et al. An important role of heparan sulfate proteoglycan (perlecan) in a model system for the deposition and persistence of fibrillar a beta-amyloid in rat brain. Neuron. 1994;12:219. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- [61].Kisilevsky R, Szarek WA, Ancsin J, Vohra R, Li Z, Marone S. Novel glycosaminoglycan precursors as antiamyloid agents: Part IV. J Mol Neurosci. 2004;24:167. doi: 10.1385/JMN:24:1:167. [DOI] [PubMed] [Google Scholar]
- [62].Patey SJ, Yates EA, Turnbull JE. Novel heparan sulphate analogues: inhibition of beta-secretase cleavage of amyloid precursor protein. Biochem Soc Trans. 2005;33:1116. doi: 10.1042/BST20051116. [DOI] [PubMed] [Google Scholar]
- [63].Scholefield Z, Yates EA, Wayne G, Amour A, McDowell W, Turnbull JE. Heparan sulfate regulates amyloid precursor protein processing by BACE1, the Alzheimer’s beta-secretase. J Cell Biol. 2003;163:97. doi: 10.1083/jcb.200303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Buee L, Hof PR, Delacourte A. Brain microvascular changes in Alzheimer’s disease and other dementias. Ann N Y Acad Sci. 1997;826:7. doi: 10.1111/j.1749-6632.1997.tb48457.x. [DOI] [PubMed] [Google Scholar]
- [65].Breteler MM. Risk factors for vascular disease and dementia. J Neural Transm. 1998;105:773. doi: 10.1159/000022428. [DOI] [PubMed] [Google Scholar]
- [66].Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann N Y Acad Sci. 2000;903:457. doi: 10.1111/j.1749-6632.2000.tb06399.x. [DOI] [PubMed] [Google Scholar]
- [67].de la Torre JC. Vascular basis of Alzheimer’s pathogenesis. Ann N Y Acad Sci. 2002;977:196. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- [68].Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barrett AJ. The many forms and functions of cellular proteinases. Fed Proc. 1980;39:9. [PubMed] [Google Scholar]
- [70].Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, et al. The Serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- [71].Potempa J, Korzus E, Travis J. The Serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957. [PubMed] [Google Scholar]
- [72].Janciauskiene S, Sun YX, Wright HT. Interactions of A beta with endogenous anti-inflammatory agents: a basis for chronic neuroinflammation in Alzheimer’s disease. Neurobiol Dis. 2002;10:187. doi: 10.1006/nbdi.2002.0519. [DOI] [PubMed] [Google Scholar]
- [73].Highsmith RF, Rosenberg RD. The inhibition of human plasmin by human antithrombin-heparin cofactor. J Biol Chem. 1974;249:4335. [PubMed] [Google Scholar]
- [74].Kalaria RN, Golde T, Kroon SN, Perry G. Serine protease inhibitor antithrombin III and its messenger RNA in the pathogenesis of Alzheimer’s disease. Am J Pathol. 1993;143:886. [PMC free article] [PubMed] [Google Scholar]
- [75].Hollister RD, Kisiel W, Hyman BT. Immunohistochemical localization of tissue factor pathway inhibitor-1 (TFPI-1), a Kunitz proteinase inhibitor in Alzheimer’s disease. Brain Res. 1996;728:13. [PubMed] [Google Scholar]
- [76].Lomas DA, Carrell RW. Serpinopathies and the conformational dementias. Nat Rev Genet. 2002;3:759. doi: 10.1038/nrg907. [DOI] [PubMed] [Google Scholar]
- [77].Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--A model for conformational diseases. N Engl J Med. 2002;346:45. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- [78].Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- [79].Santini V. A general practice trial of Ateroid 200 in 8,776 patients with chronic senile cerebral insufficiency. Mod Probl Pharmacopsychiatry. 1989;23:95. doi: 10.1159/000416683. [DOI] [PubMed] [Google Scholar]
- [80].Lorens SA, Guschwan M, Hata N, van de Kar LD, Walenga JM, Fareed J. Behavioral, endocrine, and neurochemical effects of sulfomucopolysaccharide treatment in the aged Fischer 344 male rat. Sem Thromb Hemost. 1991;17:S164. [PubMed] [Google Scholar]
- [81].Conti L, Placidi GF, Cassano GB. Ateroid in the treatment of dementia: results of a clinical trial Ateroid in the clinical treatment of multi-infarct dementia a general practice trial of Ateroid 200 in 8,776 patients with chronic senile cerebral insufficiency. Mod Probl Pharmacopsychiatry. 1989;23:76. doi: 10.1159/000416681. [DOI] [PubMed] [Google Scholar]
- [82].Passeri M, Cucinotta D. Ateroid in the clinical treatment of multiinfarct dementia. Mod Probl Pharmacopsychiatry. 1989;23:85. doi: 10.1159/000416682. [DOI] [PubMed] [Google Scholar]
- [83].Walzer M, Lorens S, Hejna M, Fareed J, Hanin I, Cornelli U, et al. Low molecular weight glycosaminoglycan blockade of betaamyloid induced neuropathology. Eur J Pharmacol. 2002;445:211. doi: 10.1016/s0014-2999(02)01759-4. [DOI] [PubMed] [Google Scholar]
- [84].Dudas B, Cornelli U, Lee JM, Hejna MJ, Walzer M, Lorens SA, et al. Oral and subcutaneous administration of the glycosaminoglycan C3 attenuates Abeta(25-35)-induced abnormal tau protein immunoreactivity in rat brain. Neurobiol Aging. 2002;23:97. doi: 10.1016/s0197-4580(01)00255-x. [DOI] [PubMed] [Google Scholar]
- [85].Snow AD, Wight TN. Proteoglycans in the pathogenesis of Alzheimer’s disease and other amyloidoses. Neurobiol Aging. 1989;10:481. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- [86].Leveugle B, Ding W, Laurence F, Dehouck MP, Scanameo A, Cecchelli R, et al. Heparin oligosaccharides that pass the blood–brain barrier inhibit beta-amyloid precursor protein secretion and heparin binding to beta-amyloid peptide. J Neurochem. 1998;70:736. doi: 10.1046/j.1471-4159.1998.70020736.x. [DOI] [PubMed] [Google Scholar]
- [87].Ma Q, Dudas B, Hejna M, Cornelli U, Lee JM, Lorens S, et al. The blood-brain barrier accessibility of heparin-derived oligosaccharides C3. Thromb Res. 2002;105:447. doi: 10.1016/s0049-3848(02)00050-6. [DOI] [PubMed] [Google Scholar]
- [88].Ma Q, Schultz C, Neville B, Jeske W, Hoppensteadt D, Cornelli U, et al. Pharmacodynamics and pharmacokinetics of C3, a heparinderived oligosaccharide mixture, in non-human primates. Thromb Res. 2003;112:249. doi: 10.1016/j.thromres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- [89].Bergamaschini L, Donarini C, Rossi E, De Luigi A, Vergani C, De Simoni MG. Heparin attenuates cytotoxic and inflammatory activity of Alzheimer amyloid-beta in vitro. Neurobiol Aging. 2002;23:531. doi: 10.1016/s0197-4580(02)00003-9. [DOI] [PubMed] [Google Scholar]
- [90].Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Res Bull. 1999;49:139. doi: 10.1016/s0361-9230(99)00027-1. [DOI] [PubMed] [Google Scholar]
- [91].Smith MA, Kalaria RN, Perry G. Alpha 1-trypsin immunoreactivity in Alzheimer disease. Biochem Biophys Res Commun. 1993;193:579. doi: 10.1006/bbrc.1993.1663. [DOI] [PubMed] [Google Scholar]
- [92].Ciallella JR, Figueiredo H, Smith-Swintosky V, McGillis JP. Thrombin induces surface and intracellular secretion of amyloid precursor protein from human endothelial cells. Thromb Haemost. 1999;81:630. [PubMed] [Google Scholar]
- [93].Haas C, Aldudo J, Cazorla P, Bullido MJ, de Miguel C, Vazquez J, et al. Proteolysis of Alzheimer’s disease beta-amyloid precursor protein by factor Xa. Biochim Biophys Acta. 1997;1343:85. doi: 10.1016/s0167-4838(97)00094-0. [DOI] [PubMed] [Google Scholar]
- [94].Saporito-Irwin SM, Van Nostrand WE. Coagulation factor XIa cleaves the RHDS sequence and abolishes the cell adhesive properties of the amyloid beta-protein. J Biol Chem. 1995;270:26265. doi: 10.1074/jbc.270.44.26265. [DOI] [PubMed] [Google Scholar]
- [95].Yasuhara O, Walker DG, McGeer PL. Hageman factor and its binding sites are present in senile plaques of Alzheimer’s disease. Brain Res. 1994;654:234. doi: 10.1016/0006-8993(94)90484-7. [DOI] [PubMed] [Google Scholar]
- [96].Shimizu-Okabe C, Yousef GM, Diamandis EP, Yoshida S, Shiosaka S, Fahnestock M. Expression of the kallikrein gene family in normal and Alzheimer’s disease brain. Neuroreport. 2001;12:2747. doi: 10.1097/00001756-200108280-00031. [DOI] [PubMed] [Google Scholar]
- [97].Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG. Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer’s disease brains. EMBO Rep. 2000;1:530. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-densitylipoprotein receptor-related protein. Ann Neurol. 1995;37:211. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- [99].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- [101].Gollin PA, Kalaria RN, Eikelenboom P, Rozemuller A, Perry G. Alpha 1-antitrypsin and alpha 1-antichymotrypsin are in the lesions of Alzheimer’s disease. Neuroreport. 1992;3:201. doi: 10.1097/00001756-199202000-00020. [DOI] [PubMed] [Google Scholar]
- [102].Akiyama H, Ikeda K, Kondo H, Kato M, McGeer PL. Microglia express the type 2 plasminogen activator inhibitor in the brain of control subjects and patients with Alzheimer’s disease. Neurosci Lett. 1993;164:233. doi: 10.1016/0304-3940(93)90899-v. [DOI] [PubMed] [Google Scholar]
- [103].Turgeon VL, Houenou LJ. The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res Brain Res Rev. 1997;25:85. doi: 10.1016/s0165-0173(97)00015-5. [DOI] [PubMed] [Google Scholar]
- [104].Cacabelos R. Pharmacogenomics for the treatment of dementia. Ann Med. 2002;34:357. doi: 10.1080/078538902320772115. [DOI] [PubMed] [Google Scholar]
- [105].Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28(4):537–47. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [106].Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, et al. A Phase II study targeting amyloid-beta with 3APS in mildto-moderate Alzheimer disease. Neurology. 2006;67:1757. doi: 10.1212/01.wnl.0000244346.08950.64. [DOI] [PubMed] [Google Scholar]
- [107].Aisen PS, Gauthier S, Briand R, Saumier D, Laurin J, Garceau D. Tramiprosate (Alzhemed™): a potential treatment for Alzheimer’s disease; Presented at 9th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy; Geneva, Switzerland. Apr 19-22, 2006. [Google Scholar]
- [108].D'Andrea MR, Nagele RG. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons. Curr Pharm Des. 2006;12(6):677–84. doi: 10.2174/138161206775474224. [DOI] [PubMed] [Google Scholar]
- [109].Morelle W, Michalski JC. Glycomics and mass spectrometry. Curr Pharm Des. 2005;11(20):2615–45. doi: 10.2174/1381612054546897. [DOI] [PubMed] [Google Scholar]
- [110].Rusnati M, Oreste P, Zoppetti G, Presta M. Biotechnological engineering of heparin/heparan sulphate: a novel area of multi-target drug discovery. Curr Pharm Des. 2005;11(19):2489–99. doi: 10.2174/1381612054367553. [DOI] [PubMed] [Google Scholar]