Abstract
Schistosomiasis is caused by infection with the parasite Schistosoma, which is a flat-worm or fluke. The dominant species are Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Schistosomiasis is the third most common parasitic disease in the world after malaria and amoebiasis. It is endemic in more than 70 countries affecting about 200 million people worldwide, of whom 80% are in sub-Saharan Africa. There are pockets of infection in north-eastern Brazil, near the Yangtze River in China, and some pockets in south East Asia. In the East Mediterranean regions, the Schistosoma have been reported in Iraq and Egypt as well as in Sudan. The latter has the highest infection rate nowadays, particularly in the Al Jazeera area, due to the poor Schistosoma control program. In the Arabian peninsula, schistosomiasis has been reported in southwest part of Saudi Arabia, mainly in the Asir province and Jizan province, which lay in the southwest corner of Saudi Arabia and directly north of the border with Yemen. The efforts to control schistosomiasis have been very successful in Saudi Arabia due to the irrigation system control. However, the infection is prone in Yemen, where the schistosomiasis control is much less strict. Thus as a result, the problem still exists due to transmigration of the populations from both countries.
As a cause of pulmonary arterial hypertension (PAH), schistosomiasis is still under diagnosed and undertreated. This article with give a highlight about the pathophysiology of the disease and both diagnostic and therapeutic strategies.
Keywords: Schistosomiasis, pulmonary arterial hypertension, praziquantel, Saudi association for pulmonary hypertension
Schistosomiasis is caused by infection with the parasite Schistosoma, which is a flat-worm or fluke. The dominant species are Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Schistosomiasis is the third most common parasitic disease in the world after malaria and amoebiasis. It is endemic in more than 70 countries affecting about 200 million people worldwide, of whom 80% are in sub-Saharan Africa.[1,2,3,4] There are pockets of infection in North-Eastern Brazil, near the Yangtze River in China, and some pockets in South East Asia. In the East Mediterranean regions, the Schistosoma have been reported in Iraq and Egypt as well as in Sudan.[5] The latter has the highest infection rate nowadays, particularly in the Al Jazeera area, due to the poor Schistosoma control program. In the Arabian peninsula, schistosomiasis has been reported in the southwest part of Saudi Arabia, mainly in the Asir province and Jizan province, which lay in the southwest corner of Saudi Arabia and directly north of the border with Yemen.[6,7,8] The efforts to control schistosomiasis have been very successful in Saudi Arabia due to the irrigation system control. However, the infection is prone in Yemen, where the schistosomiasis control is much less strict. Thus as a result, the problem still exists due to transmigration of the populations from both countries.
The Schistosoma worm usually resides in humans and animals in targeted organs, which are the liver and the gastrointestinal tract for S. mansoni or S. japonicum, and the urinary tract for S. haematobium. The worms may reside there for 30 years or more, where both male and female worms excrete eggs. The parasites are immunologically inert and cannot be identified by the human body, but the eggs are highly antigenic and the cause of disease. The eggs will produce pathological changes, mainly complex granuloma, which, after it has been resolved, will end-up with fibrous tissues, and cause diverse forms of fibrosis in various organs. In the liver, for example, the eggs will be trapped in the periportal venules, ultimately causing periportal fibrosis, which will eventually causes obstructions and lead to portal hypertension. The same pathology applies to the bladder and urinary tract by S. haematobium. Some of the eggs will be excreted, either by urine in case of S. haematobium, or, in the case of S. mansoni or S. japonicum, by the bowel. When the egg finds itself in fresh water, it will hatch to a small form, called miracidium. This form will find a host in the form of fresh water snails of a specific species, depending on the species of the Schistosoma, and it will undergo an extensive transformation that may lead to the formation of the second form called cercaria. The cercaria will be released back into the fresh water from the snails, where it will live for about 24 h. During that period, it will be able to penetrate into the skin of humans or animals in contact with the fresh water. If it succeeds, it will enter into the venous system, travel to the lung, and there undergo another transformation, which will later migrate to the final destination of either liver or urinary tract, where it will live as a flat-worm for 30 or more years, excreting eggs.[9]
The eggs will produce a severe localized immunological reaction characterized by granuloma formation with various different types of cells, and this will invoke both type 1 and 2 immunological reactions and excretion of various cytokines and other immunological mediators. Recent studies have shown that many of these cytokines will be responsible for remodeling and fibrosis.[10,11,12]
Some of the eggs may escape to other tissue, particularly to the lung, through collateral circulation, probably because of the reopening of some collateral vessels due to portal hypertension. They will be trapped in the small arterioles, and can induce granuloma. The immunological reaction triggered by these granuloma can modify the blood vessels adjacent to it, and this will likely cause some remodeling of the vessels.[11,12,13,14]
Lung tissues collected at autopsy from patients who died of schistosomiasis-related pulmonary arterial hypertension (schist-PAH) revealed a large number of granulomas in addition to a severe remodeling process including severe media hypertrophy, and even formation of plexiform lesions.[15] The importance of the immunological reaction has been manifested in the finding that patients treated with praziquantel reduced the incidence of pulmonary hypertension (PH), likely because praziquantel kills the Schistosoma worms and thereby reduces the number of eggs deposited in the tissue. This reduces the antigenicity load and thus the incidence of granuloma.[16]
Recent findings have shown that interleukin-4 (IL-4) and IL-13 (IL-13 is a key mediator of to several type II reactions) contributes to the development of the remodeling and fibrosis in the pulmonary vessels which may lead to PH.[13] Furthermore, the transforming growth factor-beta signaling, which is well-known to play an important part in the pathophysiology of the PAH[17,18] has also been shown to influence the pathophysiology of PAH secondary to schistosomiasis. However, in the mouse models, the rodent overexpression of IL-6 is inadequate to cause experimental PH, while a blockade of IL-6 suppresses hypoxia-induced PH.[19] It is therefore concluded that IL-6 could also be one of the cytokines responsible for balancing the remodeling process of the pulmonary vascular diseases secondary to Schistosoma eggs-induced granuloma.[20]
Clinical Presentation
Patients with schistosomiasis could be asymptomatic, whereby only a very severe remodeling of the vessels through a high antigenicity load of eggs from the Schistosoma can produce PAH.[11] These patients generally present with a classical form of PAH, including a significant increase in the pulmonary vascular resistance and consequently right heart failure.
The diagnosis follows the same line as patients with suspected PAH. This may involve transthoracic echocardiography, which can provide an initial data about the severity of the right ventricular functions, and probably give some indication of the PH through the measuring of the tricuspid jet regurgitation. However, the gold standard for the diagnosis is still through right heart catheterization (RHC), to provide direct measurement of the mean pulmonary arterial pressure, and also assess the right ventricular function and pulmonary artery wedge pressure (PAWP). In addition, acute vasodilator challenge may be performed, either through inhaled nitric oxide or intravenous prostacyclin, to determine if the patient is a candidate for calcium channel blocker therapy as per the current guidelines. However, in recent studies, none of the 54 patients with schist-PAH that underwent RHC had significant vasodilatory response.[21] In another study, only 3 of 84 patients with schist-PAH had acute vasodilatory response.[22] It is worth to mention that about 13% of patients will also have left ventricular function with the increase of the PAWP.[23,24]
To assess causality, it is important to rule out other causes of PAH, and to ensure evidence of previous schist-PAH,[9] as well as the presence of the prehepatic portal hypertension in the majority of patients.[21,23,24,25] We do not yet have biomarkers or any other clinical criteria to associate PAH with schistosomiasis in these cases. Methods for diagnosing Schistosoma infection using clinical, subclinical, or biochemical morbidity markers are not specific, given the generalized presentation of schistosomiasis. Eggs in the stool could be helpful in the diagnosis using common technique like the Kato-Katz method,[25] but many patients become egg-free, indicating egg-free stools can also be found in patients known to have Schist-PAH1*. Recent findings from Brazil have noticed that 7-10% of patients with Schist-PAH also have associated portal hypertension.[23,24]
Using serological tests for schistosomiasis can be helpful2*, but due to limitations in test sensitivities, it is recommend to use two or more assays in parallel.[26] However, patients who have lived in the endemic area are unlikely to have positive serological tests due to prior infections. Indirect methods include evidence of peripheral blood eosinophilia, hepatomegaly and splenomegaly, which develop in some patients as biochemical markers of liver fibrosis. Measured in serum3*, these specific tests have the potential to provide highly sensitive and cost-effective methods for assessment of Schistosoma induced fibrosis. A biopsy of the liver may be necessary in some patients with co-infection (e.g., with hepatitis virus).[2,4,9]
Treatment of Schistosomiasis Associated with Pulmonary Arterial Hypertension
There are no clinical trials to confirm the application of any of the current therapies for PAH. Our current evidence is mainly observational from various centers all over the world [Table 1].
Table 1.
Class of recommendation and level of evidence for management of schist-PAH
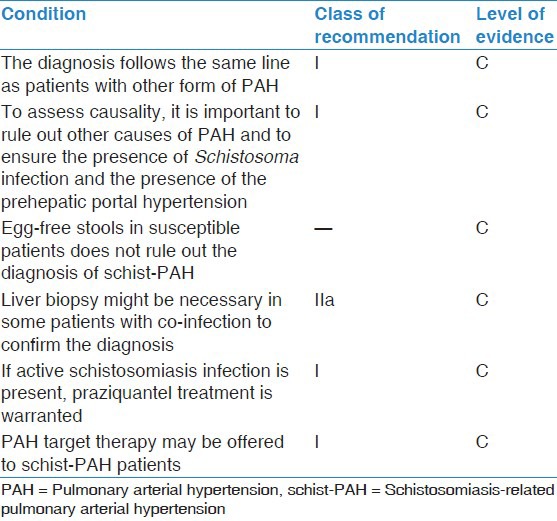
It is important to determine if active schistosomiasis infection is present, as treatment with an antihelmintic drug, such as praziquantel, is warranted. This will help to reduce the antigenic load, thus reducing the granuloma, and probably prevent further deterioration.[16] Other measurements for the consequences of infection, such as corticosteroids, may be useful, but it is uncertain whether they have any effect on the PAH.
Few case series, mainly from Brazil, have documented the successful treatment of schist-PAH with target therapies of PAH with drugs such as phosphodiesterase-5 inhibitors, or endothelial receptor antagonists,[27,28,29,30] or a combination of both therapies. Some patients have also used inhalations therapies with iloprost4*. In these small series, there was a significant improvement of the 6-min walk distance accompanied with the improvement of WHO functional class and hemodynamic parameters. In one study, cardiac magnetic resonance imaging showed a significant increase in right ventricular ejection fraction.[30]
The Prognosis of Schistosomiasis Associated with Pulmonary Arterial Hypertension
The prognosis of schist-PAH has not been systematically studied. Initial small observations noticed that the survival rate for patients with schist-PAH was not lower than patients with idiopathic PAH (IPAH) despite the lack of treatment (as treatment for schistosomiasis-associated PAH was not authorized at the time of the study).[21] However, the diagnosis of schist-PAH when compared to IPAH had a small (but not significant) increased hazard ratio for death of 1.16 (95% confidence interval range 0.29-4.68; P = 0.84).
Footnotes
Personal communication with Dr. Angela Bandeira.
There are serological tests available using different techniques like indirect immunofluorescent-antibody tests, indirect hemagglutination assays (IHAs), and enzyme-linked immunosorbent assays (ELISAs) etc. and using different antigens.[8,25,26]
Example: (pro-collagen peptides type III and IV, the P1 fragment of laminin, hyaluronic acid, fibrosin, tumour necrosis factor αR-II, and sICAM-1)
Personal communication with Dr. Angela Bandeira.
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–46. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 4.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 5.Badawy MS, El-Senbesy MA, Mahmoud HS. Evaluation of pulmonary hypertension in Bilharzial patients. Egypt J Chest Dis Tuberc. 2013;62:305–9. [Google Scholar]
- 6.Hotez PJ, Savioli L, Fenwick A. Neglected tropical diseases of the Middle East and North Africa: Review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2012;6:e1475. doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magzoub M, Kasim AA. Schistosomiasis in Saudi Arabia. Ann Trop Med Parasitol. 1980;74:511–3. doi: 10.1080/00034983.1980.11687377. [DOI] [PubMed] [Google Scholar]
- 8.Idrees MM. Pulmonary hypertension: More to be done. Ann Thorac Med. 2009;4:107–8. doi: 10.4103/1817-1737.53343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McWilliam HE, Driguez P, Piedrafita D, Maupin KA, Haab BB, McManus DP, et al. The developing schistosome worms elicit distinct immune responses in different tissue regions. Immunol Cell Biol. 2013;91:477–85. doi: 10.1038/icb.2013.33. [DOI] [PubMed] [Google Scholar]
- 11.Kolosionek E, King J, Rollinson D, Schermuly RT, Grimminger F, Graham BB, et al. Schistosomiasis causes remodeling of pulmonary vessels in the lung in a heterogeneous localized manner: Detailed study. Pulm Circ. 2013;3:356–62. doi: 10.4103/2045-8932.114764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008;118:1758–66. doi: 10.1161/CIRCULATIONAHA.107.727289. [DOI] [PubMed] [Google Scholar]
- 13.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, et al. Schistosomiasis-induced experimental pulmonary hypertension: Role of interleukin-13 signaling. Am J Pathol. 2010;177:1549–61. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, et al. Transforming growth factor-β signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation. 2013;128:1354–64. doi: 10.1161/CIRCULATIONAHA.113.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauad T, Pozzan G, Lanças T, Overbeek MJ, Souza R, Jardim C, et al. Immunopathological aspects of schistosomiasis-associated pulmonary arterial hypertension. J Infect. 2014;68:90–8. doi: 10.1016/j.jinf.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Crosby A, Jones FM, Kolosionek E, Southwood M, Purvis I, Soon E, et al. Praziquantel reverses pulmonary hypertension and vascular remodeling in murine schistosomiasis. Am J Respir Crit Care Med. 2011;184:467–73. doi: 10.1164/rccm.201101-0146OC. [DOI] [PubMed] [Google Scholar]
- 17.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–44. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:1340–8. doi: 10.1164/rccm.200311-1602OC. [DOI] [PubMed] [Google Scholar]
- 19.Crosby A, Jones FM, Southwood M, Stewart S, Schermuly R, Butrous G, et al. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med. 2010;181:279–88. doi: 10.1164/rccm.200903-0355OC. [DOI] [PubMed] [Google Scholar]
- 20.Graham BB, Chabon J, Kumar R, Kolosionek E, Gebreab L, Debella E, et al. Protective role of IL-6 in vascular remodeling in Schistosoma pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:951–9. doi: 10.1165/rcmb.2012-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.dos Santos Fernandes CJ, Jardim CV, Hovnanian A, Hoette S, Dias BA, Souza S, et al. Survival in schistosomiasis-associated pulmonary arterial hypertension. J Am Coll Cardiol. 2010;56:715–20. doi: 10.1016/j.jacc.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 22.Japyassú FA, Mendes AA, Bandeira ÂP, Oliveira FR, Sobral Filho D. Hemodynamic profile of severity at pulmonary vasoreactivity test in schistosomiasis patients. Arq Bras Cardiol. 2012;99:789–96. doi: 10.1590/s0066-782x2012005000071. [DOI] [PubMed] [Google Scholar]
- 23.Lapa M, Dias B, Jardim C, Fernandes CJ, Dourado PM, Figueiredo M, et al. Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation. 2009;119:1518–23. doi: 10.1161/CIRCULATIONAHA.108.803221. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira RC, Domingues AL, Bandeira AP, Markman Filho B, Albuqerque Filho ES, Correiade de Araújo AC, et al. Prevalence of pulmonary hypertension in patients with schistosomal liver fibrosis. Ann Trop Med Parasitol. 2009;103:129–43. doi: 10.1179/136485909X398168. [DOI] [PubMed] [Google Scholar]
- 25.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 26.Kinkel HF, Dittrich S, Bäumer B, Weitzel T. Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin Vaccine Immunol. 2012;19:948–53. doi: 10.1128/CVI.05680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bierman WF, Wetsteyn JC, van Gool T. Presentation and diagnosis of imported schistosomiasis: Relevance of eosinophilia, microscopy for ova, and serology. J Travel Med. 2005;12:9–13. doi: 10.2310/7060.2005.00003. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes CJ, Dias BA, Jardim CV, Hovnanian A, Hoette S, Morinaga LK, et al. The role of target therapies in schistosomiasis-associated pulmonary arterial hypertension. Chest. 2012;141:923–8. doi: 10.1378/chest.11-0483. [DOI] [PubMed] [Google Scholar]
- 29.Bandeira A, Mendes A, Santos-Filho P, Sa DT, Loureiro R. Clinical efficacy of oral sildenafil in severe pulmonary hypertension in patients with chronic pulmonary schistosomiasis. Circulation. 2004;110(Suppl III):296. [Google Scholar]
- 30.Loureiro R, Mendes A, Bandeira A, Cartaxo H, Sa DT. Oral sildenafil improves functional status and cardiopulmonary hemodynamics in patients with severe pulmonary hypertension secondary to chronic pulmonary schistosomiasis: a cardiac magnetic resonance study. Circulation. 2004;110(Suppl III):572. [Google Scholar]


