Abstract
There is scant published data about pulmonary hypertension (PH) from the developing countries. True prevalence of the disease, its biology, etiology and response to treatment are not well known, and they are likely to be somewhat different from that of the developed countries.
In this review, we will discuss the main challenges for managing PH in developing countries and propose real-life recommendations to deal with such difficulties.
Keywords: Developing world, pulmonary hypertension, Saudi association for pulmonary hypertension guidelines
There is scant published data about pulmonary hypertension (PH) from the developing countries.[1,2,3] True prevalence of the disease, its biology, etiology, and response to treatment are not well-known, and they are likely to be somewhat different from that of the developed countries. In this review, we will discuss the main challenges for managing PH in developing countries and propose real-life recommendations to deal with such difficulties.
Differences in Etiology of Pulmonary Hypertension between Developing and Developed Countries
Epidemiological data regarding PH and disease-specific registry data is available from many developed countries, but not from developing countries.[4] Many PH experts believe that the spectrum of diseases causing PH is likely to be different between the developed and the developing world's [Table 1].[5] PH due to congenital heart disease (CHD), rheumatic heart disease, schistosomiasis, hemolytic diseases, and HIV infection are more important causes for PH in the developing countries,[6] but they are much less common in the developed countries, where biological research or drug development takes place. Furthermore, clinical trials done to test new drugs may exclude the typical PH patients of the developing countries. By extrapolation, pulmonary arterial hypertension (PAH) specific drugs are used in these conditions, though their efficacy cannot be certain. For example, PAH specific therapy may not be effective in PH due to sickle cell disease or schistosomiasis.[7,8]
Table 1.
Causes of pulmonary vascular diseases with a large potential burden in the developing world
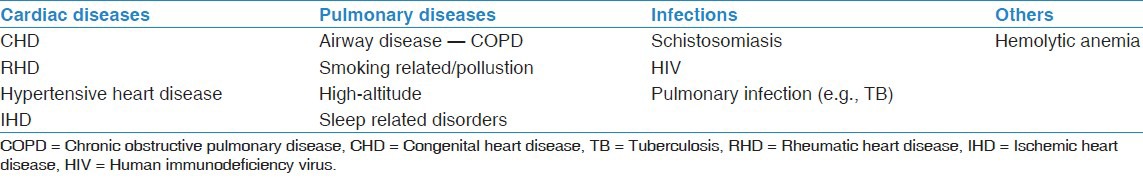
Congenital and rheumatic heart diseases
There are many developing countries with populations ranges between 15 and 100 million people in each country without a single pediatric cardiac center.[9] CHD in these countries is often missed in childhood.[10] Acute and chronic rheumatic heart disease, which is rarely seen in the developed countries is a common problem in many developing countries.[11] It has been estimated that 8% of those with CHD and as many as 70% of those with rheumatic heart disease will eventually develop PH.[12] Some patients with rheumatic valve disease develop progressive PH even after correction of the valvular disease. This is a specific group that behaves more like PAH. Early correction of valvular heart disease may prevent the development of PH postoperatively.
In many occasions, the cardiologists in the developing countries face a situation whether or not to send their patient with left to right shunt for corrective surgery in the presence of high pulmonary vascular resistance of >4-8 Wood unit.[13] In the developed countries, this problem is rarely seen since most of patients with left to right shunt lesions are operated at the right time. Though patients with PAH due to CHD are treated like idiopathic PAH (IPAH) with PH specific medication, their long-term management strategies are not well-studied and likely to be complicated in advanced cases. Their natural history differs from that of IPAH. Only a collaborative effort by the PH physicians in the developing world can find solutions to these problems.
Schistosomiasis
Schistosomiasis is highly prevalent in eastern South America, the Caribbean Islands, East Asia, and some parts of China and the Middle East, with sub-Saharan Africa being the most endemic area. It is an important cause of PH in these countries. In the absence of any systematic epidemiological study, it is speculated that schistosomiasis may be a major cause of PH worldwide.[5] Appropriate methods of treating PH due to schistosomiasis are still unknown and clinical research should be focused on this problem in the affected countries.
HIV and pulmonary hypertension
It has been estimated that 0.5% of HIV infected patients have symptomatic PAH that is much higher than the general population.[14] Though no epidemiological data is available from the developing countries, the prevalence may be the same (or even higher) in this population due to the high prevalence of common concomitant pulmonary infections, such as pulmonary tuberculosis.
Considering a huge burden of HIV in Africa, the magnitude of PH in this situation can be well imagined. The role of antiretroviral therapy in PAH is still being debated.[15,16] Systematic screening program for PAH in the infected patients and close collaboration with the research institutions of the developed world is highly desirable, but seems very difficult.
Pulmonary hypertension due to airways diseases
There are certain peculiar problems specific to developing countries that can cause PH secondary to chronic airway disease. These include domestic pollution caused by burning wood, charcoal, liquefied petroleum gas, or animal dung in poorly ventilated houses. This is seen in northern India, Africa, Middle East, and the highlands and has to be treated similarly to PH due to Group 3 disorders.
Challenges in Clinical Diagnosis
The median duration of symptoms in the NIH registry is more than 2 years. In developing countries with limited access to the primary health care, the duration of symptoms is likely to be more. In the early stages, patients and as well as physician tend to dismiss the symptoms. Paucity and subtlety of physical signs in early disease makes the diagnosis difficult. Breathlessness is often attributed to general asthenia, asthma, and even psychogenic. Syncope is often mistaken to be neurogenic. Cultural beliefs and practices, socio economic factors may delay medical consultation. Physicians with limited awareness and facilities to investigate may further delay the diagnosis. Although, the problem is universal, it is likely to be more in the developing countries.
The solution is to increase awareness among the doctors, so that clinical suspicion is increased and early diagnosis becomes a possibility. Availability of point of care to decide whether the patient has PH or not will improve early diagnosis. Such facilities are not widely available in developing countries for many reasons, such as financial.
Challenges in Diagnostic Evaluation
Echocardiography (ECG)
12-lead ECG and chest radiograph are not specific in early stages of the disease. 2D-ECG can be a screening test but has its own limitations as a confirmatory diagnostic tool. The findings may be subtle and an inexperienced operator can easily miss the diagnosis on echocardiography. Under, or over, diagnosis of PH is possible if the diagnosis is based only on echocardiography.[17] Many physicians do not realize that echocardiography only estimates and does not measure pulmonary artery pressures (PAP). Furthermore, mean PAP and cardiac output, the two important components of the definition of PH, cannot be accurately estimated by echocardiography. Hence, mistakes are common if the diagnosis is made solely based on echocardiography.
Furthermore, the inadequacy of tricuspid regurgitation (TR) jet, uncertainty in estimating right atrial pressure, and lack of uniform definition of PH based on ECG, make the echo based diagnosis less accurate. The sensitivity and specificity of Doppler ECG are even less satisfactory in estimating PAP in Group 3 PH disorders.[18] It is imperative to do right heart catheterization (RHC) to confirm the diagnosis of PH.
Right heart catheterization
Right heart catheterization is the key to diagnose PH. The facility is available in only a very few centers in developing countries. Cost is one of the important considerations in many developing countries where resources are limited.
Physician inertia as well as fear of complications is another reason for avoiding RHC. Many physicians have a mistaken notion that Doppler echocardiography is sufficient to diagnose and initiate treatment.
Acknowledging the scant resources, RHC may be avoidable if a patient found to have dilated right atrium and right ventricle with a D-shaped left ventricle and has unambiguous TR, which has velocity >3 m/s (level of evidence D). In the absence of such clear Doppler echocardiographic evidence, one should have a lower threshold to perform RHC. Furthermore, patients should not receive a sinister diagnosis like PAH and lifelong medication in the absence of unequivocal data. It is important to emphasize that RHC is in general safe in the hands of experienced physicians.[19]
Acute vasoreactivity testing
About 10% of patients with PAH have positive vasoreactivity testing. They respond well to long-term oral calcium channel blocker (CCB) therapy. In many centers in the developing countries the facilities are not available to do this test. Unfortunately, many physicians continue to advise CCBs to their patients empirically without doing this test. This practice can be harmful to the patient and should certainly be avoided. In such situations, where vasoreactive testing cannot be done, it is better to treat patients with PAH specific therapy than empiric CCBs. Physician education is the key to solve this problem.
Treatment Options
In many developing countries, PAH specific therapy is not available. It is too costly for patients to buy and use them on their own. For economic reasons insurance and governmental agencies may not sponsor or reimburse the costs of the medication. Unfortunately, there is no simple solution for this and physicians should learn to use the available medication appropriately. In many occasions, available medications are used at sub-optimal doses to save money. Some physicians mistakenly treat right heart failure due to PAH with beta-blockers and angiotensin converting enzyme inhibitors. That can be detrimental to the patients. To avoid such mistakes and to get better outcomes, patients should always be referred to PH physician and not to be treated by ordinary cardiologist or pulmonologist.
Pulmonary arterial hypertension specific therapy
Treatment options like continuous infusion of epoprostenol are a standard treatment option in developed countries. These are not available in developing countries due to its prohibitive costs. Even when the drug is made available, standard of care does not permit its administration in these countries. A continuous infusion of drug needs much more than physicians’ knowledge and availability of the drug. A clinical trial in India had to be abandoned half way through due to unacceptably high rate of infections. In modified New York Heart Association functional Class IV patient epoprostenol is the drug of choice. In its absence, upfront combination therapy with phosphodiesterase-5 inhibitor and endothelin receptor antagonist is a good option.[20] The availability of generic alternative to these drugs in the developing countries has solved the problem partially; however, the efficacy of the generic drugs will always be questionable.
Balloon atrial septostomy
Though it is considered as a bridge to transplantation,[21,22] it is not an unrealistic therapeutic option for sick patients in the developing world, who fail medical therapy or if the medical therapy is not available. It carries a high mortality when carried out in advanced patients, and its role is still uncertain in less sick patients. Obviously, high degree of expertize is needed to perform this procedure.
Lung transplantation
Pulmonary arterial hypertension is a progressive disease and some patients may eventually require bilateral lung transplantation despite advanced therapy. The facilities for lung transplantation and expertize are hardly available in many of the developing countries. Development of facilities for lung transplantation is so complex and beyond the physicians’ domain.
Chronic thromboembolic pulmonary hypertension (CTEPH)
Chronic thromboembolic pulmonary hypertension is less often reported from developing countries and is less often diagnosed. High index of suspicion is needed and complete workup in that direction is needed. Many physicians do not consider the diagnosis even in advanced cases. Despite its limitation in the diagnosis of CTEPH, many physicians still depend upon computed tomography (CT) pulmonary angiography as a screening test although many international guidelines have emphasized on ventilation perfusion (V/Q) lung scan as the ideal screening test. The diagnosis of CTEPH can certainly be missed by CT pulmonary angiography. Both V/Q lung scan and conventional pulmonary angiography are less often done in developing countries. Even when the diagnosis is made, there are less number of centers with expertize to offer surgical treatment. On the whole physician education to detect the CTEPH cases and development of surgical expertize in a few centers in the developing countries is required.
Pulmonary hypertension centers
In many developing countries, PH referral centers are not available. Individual physicians treat PH patients without seeking any specialist advice. This leads to inadequate and incomplete diagnostic workup and inappropriate treatment. It is to be expected that patient's outcomes will always be inferior to specialist centers practice in the developed world. National health services should endeavor to develop such nodal centers and resources should be pooled to develop expertize.
It may be a worthwhile idea that established PH centers in the developed countries pair with and adopts a PH center in the developing countries. Such a partnership in which exchange of ideas occurs regularly will help improving the patient outcomes in the developing countries.
Finally, PH is encountered by and treated by different physicians in the developing countries, such as cardiologists, pulmonologists, rheumatologists, and hematologists. It would be better if physicians in any hospital team up and establish a PH clinic in their hospital in order to give comprehensive and evidence-based care to the patient. Diagnostic algorithms and treatment pathways can be established based on the local practices and resources. Mistakes in diagnosis and treatment would be less in such organized system.
The Way Forward
There are many knowledgeable and competent physicians in the developing countries who are interested in PH management. They should work together in their respective regions and strive to educate the primary physicians in early diagnosis of PH.
Regional referral centers should be developed to optimize resources and to give best evidence-based medicine with better patient outcomes. Physicians should be encouraged to refer suitable patients to such centers.
These physician groups should partner with other stakeholders including patient groups, government, and pharmaceutical industry and research laboratories in the universities. Networking combined with educational exchange programs with established centers in the developed countries can be beneficial. Such centers in the developing countries can attract clinical trials that will benefit the hospitals, physicians, patients, and the industry as well. Development of regional registries can give insight to the magnitude of the problem and can give directions to the local governments in formulating public health problems in relation to PH. Combined efforts by all the stakeholders in the presence of adversity will lead to certain victory over this sinister disease.
Conclusion
The burden of PH in the developing countries is much more than that of the developed world and is different in etiology, natural history, and response to treatment. Very little knowledge in the presence of resource crunch and poor health care delivery systems makes it a challenging disease to manage. The situation is very challenging, but the solutions have to come from within.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Idrees MM, Al-Hajjaj M, Khan J, Al-Hazmi M, Alanezi M, Saleemi S, et al. Saudi guidelines on diagnosis and treatment of pulmonary arterial hypertension. Ann Thorac Med. 2008;3:1–57. Supplement. [Google Scholar]
- 2.Alhamad EH, Cal JG, Alfaleh HF, Alshamiri MQ, Alboukai AA, Alhomida SA. Pulmonary hypertension in Saudi Arabia: A single center experience. Ann Thorac Med. 2013;8:78–85. doi: 10.4103/1817-1737.109816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Idrees MM. Pulmonary hypertension: More to be done. Ann Thorac Med. 2009;4:107–8. doi: 10.4103/1817-1737.53343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: Epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–9. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008;118:1758–66. doi: 10.1161/CIRCULATIONAHA.107.727289. [DOI] [PubMed] [Google Scholar]
- 6.Rich S, Herskowitz A. Targeting pulmonary vascular disease to improve global health: Pulmonary vascular disease: The global perspective. Chest. 2010;137:1S–5. doi: 10.1378/chest.09-2813. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: Results of the ASSET studies. Br J Haematol. 2010;149:426–35. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–64. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yacoub MH. Establishing pediatric cardiovascular services in the developing world: A wake-up call. Circulation. 2007;116:1876–8. doi: 10.1161/CIRCULATIONAHA.107.726265. [DOI] [PubMed] [Google Scholar]
- 10.Larrazabal LA, Jenkins KJ, Gauvreau K, Vida VL, Benavidez OJ, Gaitán GA, et al. Improvement in congenital heart surgery in a developing country: The Guatemalan experience. Circulation. 2007;116:1882–7. doi: 10.1161/CIRCULATIONAHA.107.695403. [DOI] [PubMed] [Google Scholar]
- 11.McLaren MJ, Markowitz M, Gerber MA. Rheumatic heart disease in developing countries: The consequence of inadequate prevention. Ann Intern Med. 1994;120:243–5. doi: 10.7326/0003-4819-120-3-199402010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Radulescu D, Pripon S, Duncea C, Constantea NA, Gulei I. Conventional radiology and right heart catheterization in estimating primary pulmonary hypertension and pulmonary hypertension secondary to left-sided valvular disease. Acta Med Indones. 2008;40:24–8. [PubMed] [Google Scholar]
- 13.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, et al. HIV-associated pulmonary arterial hypertension: Survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 15.Pellicelli AM, D’Ambrosio C, Vizza CD, Borgia MC, Tanzi P, Pino P, et al. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol. 2004;59:323–30. doi: 10.2143/AC.59.3.2005190. [DOI] [PubMed] [Google Scholar]
- 16.Reinsch N, Buhr C, Krings P, Kaelsch H, Kahlert P, Konorza T, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: Results of the HIV-HEART Study. HIV Med. 2008;9:550–6. doi: 10.1111/j.1468-1293.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 17.Rich JD. Counterpoint: Can Doppler echocardiography estimates of pulmonary artery systolic pressures be relied upon to accurately make the diagnosis of pulmonary hypertension? No. Chest. 2013;143:1536–9. doi: 10.1378/chest.13-0297. [DOI] [PubMed] [Google Scholar]
- 18.Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, et al. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J. 2007;30:914–21. doi: 10.1183/09031936.00033007. [DOI] [PubMed] [Google Scholar]
- 19.Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546–52. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 20.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Austen WG, Morrow AG, Berry WB. Experimental studies of the surgical treatment of primary pulmonary hypertension. J Thorac Cardiovasc Surg. 1964;48:448–55. [PubMed] [Google Scholar]
- 22.Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med. 2001;22:547–60. doi: 10.1016/s0272-5231(05)70291-4. [DOI] [PubMed] [Google Scholar]


