SUMMARY
The present protocol details the synthesis of zinc bis(alkanesulphinate)s that can be used as general reagents for the formation of radical species. The zinc sulphinates described herein have been generated from the corresponding sulphonyl chlorides by treatment with zinc dust. The products may be used crude, or a simple purification procedure may be performed to minimize incorporation of water and zinc chloride. Elemental analysis has been conducted in order to confirm the purity of the zinc sulphinate reagents; reactions with caffeine have also been carried out to verify the reactivity of each batch that has been synthesized. Although the synthesis of the zinc sulphinate salts generally proceeds within 3 h, workup can take up to 24 h and purification can take up to 3 h. Following the steps in this protocol would enable the user to generate a small toolkit of zinc sulphinate reagents over the course of one week.
INTRODUCTION
Nitrogen-containing heteroaromatic compounds are omnipresent in both nature and society. They can be found throughout the human body and in other organisms, as well as in vitamins, drugs, dyes, pesticides, polymers and literally all other aspects of life. Despite the tremendous amount of work describing the functionalization of heteroarenes, the time and cost constraints of ever-growing societal needs incessantly call for more efficient synthetic methods in this area. With the goal of affixing carbon atoms to heteroarenes in a practical manner (i.e., a direct transformation of C–H bonds into C–C bonds), we have developed a radical-based functionalization strategy1,2 that involves the use of zinc bis(alkanesulphinate) reagents (Figure 1).3,4 Many of these zinc sulphinate reagents can be readily prepared in large quantities and are stable under ambient conditions, allowing the formation and commercialization (Sigma–Aldrich catalogue numbers are shown in Figure 1) of a small toolkit of reagents that include zinc trifluoromethanesulphinate (TFMS; 1), zinc difluoromethanesulphinate (DFMS; 2), zinc trifluoroethanesulphinate (TFES; 3), and zinc isopropylsulphinate (IPS; 4). While the synthesis of these reagents have been described in previous reports,3,4 further purification and analysis have since been conducted. A walkthrough of the experimental procedure and possible troubleshooting guidelines for the synthesis of zinc bis(alkanesulphinate)s from their respective alkanesulphonyl chlorides are detailed herein (Figure 2). The reactivity of each reagent batch was verified by a test reaction on caffeine as described in a previous publication.4
Figure 1.
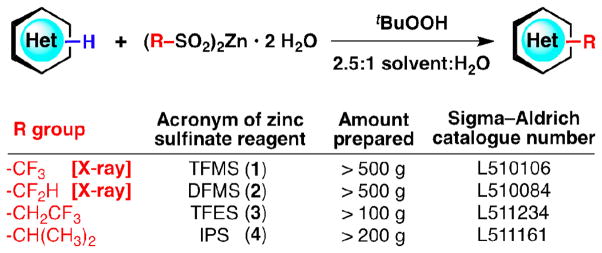
Heterocycle functionalization using recently developed and commercialized zinc bis(alkanesulphinate) reagents.
Figure 2.
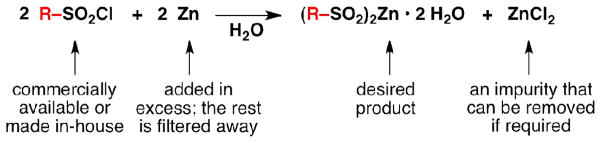
The focus of this article: the synthesis of zinc bis(alkanesulphinate) reagents.
It is of note that the prepared zinc sulphinate salts3,4 are likely to be dihydrates in their solid state5–8 (see Supplementary Information for details); while this does not affect the reagents’ reactivity, it affects the calculation of stoichiometry. It is also of note that the previously prepared reagents can contain up to one equivalent of zinc chloride (ZnCl2) per equivalent of zinc sulphinate (see Supplementary Information for details). This crude material may be used for the previously described heterocycle functionalizations without detriment, and it is more convenient to use these crude salts (in fact, all of the reported reactions3,4 were conducted with zinc sulphinate reagents containing ZnCl2). However, if a ZnCl2-free zinc sulphinate salt is required for other transformations,9 the purification procedure described below can be followed.
MATERIALS
REAGENTS
Zinc powder (6–9 micron; Alfa Aesar catalogue number 10835)
Trifluoromethanesulphonyl chloride (Sigma–Aldrich catalogue number 164798)
Difluoromethanesulphonyl chloride (Enamine Ltd. catalogue number EN300-31728)
2,2,2-Trifluoroethanesulphonyl chloride (Sigma–Aldrich catalogue number 324787)
2-Propanesulphonyl chloride (Sigma–Aldrich catalogue number 242705)
Sea sand (Fisher Scientific catalogue number S25-10)
Distilled water
Methanol (certified ACS; Avantor Performance Materials, catalogue number 3016-22)
Acetone (certified ACS; Avantor Performance Materials, catalogue number 2440-22)
Ethyl acetate (EtOAc; certified ACS; Avantor Performance Materials, catalogue number 4992-19)
Toluene (PhMe; certified ACS; Avantor Performance Materials, catalogue number 8608-22)
Dichloromethane (CH2Cl2; certified ACS; Fisher Scientific, Inc., catalogue number D37-20)
Deuterated solvents for NMR such as CDCl3, acetone-d6 and DMSO-d6 (Cambridge Isotope Laboratories, Inc.)
EQUIPMENT
Weighing balance
Weighing paper
Weighing boats
Round bottom flasks (Quark Glass; 100 mL (14/20 joint) 250 mL (24/40 joint), 500 mL (24/40 joint))
Sintered glass funnel (Quark Glass; 60 mL (14/20 joint), 150 mL (24/40 joint))
Porcelain Büchner funnel (Chemglass; 100 mL)
Erlenmeyer filtering flask (Quark Glass; 250 mL)
Graduated cylinder (Kimble Chase; 250 mL)
Yellow Teflon caps
Disposable syringes (1 mL, 5 mL, 10 mL, 20 mL)
Disposable needles (18, 20 and 22 gauge; 2, 4 and 6 inches long)
Metal spatula
Eppendorf pipette
Pasteur pipettes
Teflon-coated magnetic stir bar
Magnetic hotplate stirrer
Dewar flask
Ultrasonicator
Rotary evaporator
Vacuum pump
Vacuum manifold with vacuum line
Rubber vacuum tubes
0.25 mm E. Merck silica plates (60F-254)
QUANTOFIX® chloride test strips (MACHEREY-NAGEL GmbH & Co. KG.)
NMR tubes (5 mm width)
Access to NMR, IR, mass spectrometry and elemental analysis instruments
PROCEDURE
Note: This procedure is based on using 100 mmol of alkanesulphonyl chloride used. When using other amounts of alkanesulphonyl chloride, all reagents, solvents, and flask sizes must be scaled accordingly. (CAUTION: If these reactions need to be performed on a larger scale, the rate of cooling of the reaction mixture must be considered since the reaction warms up during the sulphonyl chloride addition.)
-
1|
On a weighing boat, weigh zinc powder (45.77 g, 700 mmol, 14.0 equiv.).
-
2|
In a 250 mL round bottom flask (with a 24/40 ground glass joint), place an egg-shaped Teflon-coated magnetic stir bar, followed by the zinc dust weighed in step 1.
-
3|
In a 250 mL graduated cylinder, measure water (110 mL) and then pour it into the round bottom flask prepared in step 2.
-
4|
Cool the round bottom flask prepared in step 3 in an ice-water bath. Stir the zinc-water mixture by turning on the magnetic stir plate; make sure that a constant stirring rate is achieved. Wait 5 min to allow the zinc-water mixture to cool. Keep the round bottom flask uncapped during this time.
-
5|
To the cooled zinc-water mixture prepared in step 4, add alkanesulphonyl chloride (100 mmol, 2.0 equiv.) dropwise by a syringe over 20 min (all the alkanesulphonyl chlorides described herein are liquid at ambient temperature and pressure). Keep the round bottom flask uncapped during this time. (CAUTION: This reaction is mildly exothermic and therefore the temperature of the reaction mixture needs to be monitored carefully if this reaction were to be performed on a larger scale.)
-
6|
Cap the reaction flask prepared in step 5 with a yellow Teflon cap. Keep the reaction flask in the ice bath but do not add any more ice to the bath; slowly allow the reaction mixture to warm to room temperature over 3 h.
-
7|
Secure a 500 mL round bottom flask (with a 24/40 ground glass joint) using a clamp. On top of it, place a 150 mL sintered glass funnel with a vacuum adaptor (with a 24/40 ground glass joint). Add sand (approximately 1 cm high) to the sintered glass funnel. Connect the vacuum outlet of the sintered glass funnel to a vacuum source (in-house vacuum is sufficient) using a rubber vacuum tube. Turn on the vacuum source.
-
8|
Remove the yellow cap from the reaction flask prepared in step 6. Pour the reaction mixture onto the sintered glass funnel prepared in step 7. Make sure to pour all the contents of the reaction flask into the sintered glass funnel, spraying a small amount of water (approximately 10 mL) with a wash bottle if necessary. All the excess zinc powder should be removed from the crude reaction mixture in this manner and the crude product solution should be clear. (TROUBLESHOOTING)
-
9|
Rinse the reaction flask with methanol from a wash bottle (approximately 60 mL) and pour the rinsate into the sintered glass funnel. This ensures that product is not lost by adhering onto the walls of the reaction flask.
-
10|
Break the vacuum by removing the rubber vacuum tube from the vacuum source and then turn off the vacuum source. Remove the sintered glass funnel from the 500 mL round bottom flask.
-
11|
Place the 500 mL round bottom flask described in step 10 onto a rotary evaporator. Remove methanol and water from the crude reaction mixture under vacuum.
-
12|
A further evaporation step is necessary to remove more water; this is achieved by adding toluene (25 mL) to the crude product and removing off the toluene (along with water as an azeotropic mixture) with a rotary evaporator. Repeat step 12 especially if water droplets are visible in the flask. (TROUBLESHOOTING)
-
13|
For TFMS (1), see steps 14 through 20. For DFMS (2), TFES (3), and IPS (4), the crude product is dried further by removing all the remaining solvent under high vacuum overnight (8 h or more). Purification of all these salts is described in step 21. (TROUBLESHOOTING)
PAUSE POINT These crude zinc sulphinates can be left overnight under vacuum at room temperature.
-
14|
For TFMS (1), additional washing steps are necessary. Add acetone (40 mL) to the round bottom flask containing crude product from step 12. Separate into two roughly equal portions (to allow for easier washing and drying later on) and transfer this solution into two 250 mL round bottom flasks (with 24/40 ground glass joints) using a Pasteur pipet.
-
15|
Place each 250 mL round bottom flask onto a rotary evaporator. Remove acetone from the crude product.
-
16|
Add toluene (100 mL) to each 250 mL round bottom flask prepared in step 15 and sonicate in a water bath for 4 h (water bath temperature rises from 20 to 45°C during this time).
-
17|
Collect the solid from each flask separately onto a filter paper by vacuum filtration through a 35 mL porcelain Büchner funnel, allowing all of the toluene to be drained into a 250 mL Erlenmeyer filtering flask.
-
18|
Place each portion of solid collected in step 17 into a 250 mL round bottom flask (with 24/40 ground glass joints) and add acetone (40 mL) to dissolve the solid completely.
-
19|
Repeat steps 15 through 18 three more times on each of the two portions of product.
-
20|
Combine the two portions from step 19 into a 250 mL round bottom flask (with a 24/40 ground glass joint) and remove all the acetone using a rotary evaporator. (TROUBLESHOOTING)
ANTICIPATED RESULTS
After step 20 (for TFMS (1)) and step 13 (for DFMS (2), TFES (3), and IPS (4)), the following amounts of crude products should be obtained if 100 mmol of alkanesulphonyl chloride is used. Typically however, TFES (3) is made on 10 mmol scale.
TFMS (1): 21.3 g (a mixture of 12.7 g of TFMS (1) + 4.6 g ZnCl2 + 4.0 g H2O as determined by elemental analysis; this represents a 1:1:6.5 molar ratio of 1:ZnCl2:H2O).
DFMS (2): 25.9 g (a mixture of 15.6 g of DFMS (2) + 6.3 g ZnCl2 + 4.0 g H2O as determined by elemental analysis; this represents a 1:1:4.7 molar ratio of 2:ZnCl2:H2O).
TFES (3): 26.5 g (a mixture of 18.2 g of TFES (3) + 4.4 g ZnCl2 + 3.9 g H2O as determined by elemental analysis; this represents a 1:0.7:4.7 molar ratio of 3:ZnCl2:H2O).
IPS (4): 24.0 g (a mixture of 15.9 g of IPS (4) + 6.8 g ZnCl2 + 1.4 g H2O as determined by elemental analysis; this represents a 1:1:1.5 molar ratio of 4:ZnCl2:H2O).
CRITICAL STEP
The above procedure (steps 1 through 20) for the synthesis of zinc bis(alkanesulphinate) salts does not remove ZnCl2 that is generated in the reaction (see Figure 2). While this crude material may be used for the previously described heterocycle functionalizations without detriment, if a pure zinc sulphinate salt is required for other applications,5 the procedure below (steps 21–26) should be followed.
-
21|
ZnCl2 dissolves well in 1:1 EtOAc:CH2Cl2, but generally zinc sulphinate reagents do not. Thus, ZnCl2 can be removed by a simple washing process (with the exception of IPS (4), where not all of the chloride can be removed by this method, likely because there is chloride incorporated into the structure of the reagent). To this end, secure a 100 mL round bottom flask (with a 14/20 ground glass joint) using a clamp. On top of it, place a 60 mL sintered glass funnel with a vacuum adaptor (with a 14/20 ground glass joint). Connect the vacuum outlet of the sintered glass funnel to a vacuum source (in-house vacuum is sufficient) using a rubber vacuum tube. Do not turn on the vacuum source yet.
-
22|
Prepare a solution of 1:1 EtOAc:CH2Cl2 (50 mL) in a 100 mL graduated cylinder.
-
23|
Place 1 gram of crude zinc sulphinate into the sintered glass funnel prepared in step 21.
-
24|
Pour 1:1 EtOAc:CH2Cl2 (10–15 mL; as prepared in step 22) onto the zinc sulphinate. With a spatula, swirl the suspension and crush large chunks of the zinc sulphinate (if there are any) against the inner sides of the sintered funnel to make sure that it is all exposed to the organic solvent. After 30 s, turn on the vacuum source to drain all the organic solvent away. Break the vacuum by removing the rubber vacuum tube from the vacuum source and then turn off the vacuum source. Re-connect the vacuum outlet of the funnel to the vacuum source.
-
25|
Repeat step 24 two to three times.
-
26|
Take the purified zinc sulphinate salt from the sintered funnel and place it into a 100 mL round bottom flask. Evaporate any remaining solvent under high vacuum (2 h or more). (TROUBLESHOOTING)
ANTICIPATED RESULTS
Steps 21–26 allow for the removal of ZnCl2 from the zinc sulphinate salts (with the exception of IPS (4)) as evidenced by QUANTOFIX® chloride test strips and elemental analysis (see Supplementary Information). The following amounts of pure zinc bis(alkanesulphinate) dihydrates should be obtained if 0.5 to 2 g of crude zinc bis(alkanesulphinate) is used.
TFMS (1): 2 g (a mixture of 1.19 g of 1 + 0.43 g ZnCl2 + 0.38 g H2O) gives 0.753 g of pure 1 after washing. Elemental analysis suggests that the washed reagent is of the formula (CF3SO2)2Zn • 2 H2O, with a molar mass of 367.56 g/mol (see Supplementary Information for details).
DFMS (2): 2 g (a mixture of 1.21 g of 2 + 0.49 g ZnCl2 + 0.31 g H2O) gives 1.21 g of pure 2 after washing. Elemental analysis suggests that the washed reagent is of the formula (CF2HSO2)2Zn • 2 H2O, with a molar mass of 331.58 g/mol (see Supplementary Information for details). TFES (3): 1 g (a mixture of 0.69 g of 3 + 0.17 g ZnCl2 + 0.15 g H2O) gives 0.622 g of pure 3 after washing. Elemental analysis suggests that the washed reagent is of the formula (CF3CH2SO2)2Zn • 2 H2O, with a molar mass of 395.62 g/mol (see Supplementary Information for details).
IPS (4): 0.5 g (a mixture of 0.33 g of 4 + 0.09 g ZnCl2 + 0.08 g H2O) gives 0.312 g of 4 with a reduced chloride content. The exact structure of this reagent is currently unknown; chloride could not be fully removed from 4 using this washing procedure. If chloride removal is necessary, it can be removed by addition of silver carbonate to an aqueous solution of the salt, followed by filtration to remove the silver chloride and zinc carbonate that are produced. Concentration and drying gives a product without trace of chloride.
TIMING
Steps 1–5: 30 min
Step 6: 3 h
Steps 7–12: 4 h
Step 13: 8 h
Steps 14–20: 20 h
Steps 21–25: 15 min
Step 26: 2 h
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 8 | The obtained solution is cloudy. | Inorganic salts (including Zn) may be present. | Repeat Steps 7 and 8 using more sand; alternatively, use Celite® instead of sand. |
| 12 | Water droplets are visible in the flask. | Too much water is left in the product. | Fix/obtain new vacuum pump; add toluene and repeat evaporation in vacuo. |
| 13/20 | Excessively high yields of the desired crude salt. | Too much water is left in the product. | Fix/obtain new vacuum pump; add toluene and repeat evaporation in vacuo. |
| 13/20 /26 | Low yields of the desired crude salt. | Starting materials are not pure. | Check the purity of the starting materials, repurify them if necessary. |
| 26 | Elemental analysis shows %Cl of more than a few % for TFMS (1), DFMS (2) and TFES (3). | ZnCl2 was not fully removed from the product. | Repeat wash with 1:1 EtOAc:CH2Cl2. For TFES (3), the impure product can be sonicated in the presence of 1:1 EtOAc:CH2Cl2 to help remove more chloride. |
Supplementary Material
Acknowledgments
We thank Troy Ryba and Chris Thomas from Sigma-Aldrich Inc. for a donation of chemicals. Financial support for this work was provided by the National Institutes of Health (research grant GM-073949 and a postdoctoral fellowship for R.D.B.), Pfizer Inc., US-UK Fulbright Commission (postdoctoral fellowship for F.O.), and the Uehara Memorial Foundation (postdoctoral fellowship for Y.F.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permission information is available online at http://www.nature.com/reprints/index.html.
ASSOCIATED PUBLICATIONS
This protocol is related to the following publications:
Fujiwara, Y. et al. A new reagent for direct difluoromethylation. J. Am. Chem. Soc. 134, 1494–1497 (2012).
Fujiwara, Y. et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012).
References
- 1.Ji Y, et al. Innate C–H trifluoromethylation of heterocycles. Proc Natl Acad Sci. 2011;108:14411–14415. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brückl T, Baxter RD, Ishihara Y, Baran PS. Innate and guided C–H functionalization logic. Acc Chem Res. 2012;45:826–839. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara Y, et al. A new reagent for direct difluoromethylation. J Am Chem Soc. 2012;134:1494–1497. doi: 10.1021/ja211422g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara Y, et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature. 2012;492:95–99. doi: 10.1038/nature11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidner JP, Block SS. Infrared spectra of zinc sulfinates. Appl Spectroscopy. 1969;23:337–341. [Google Scholar]
- 6.Deacon GB, Cookson PG. The nature of sulphinate coordination in zinc and cadmium arenesulphinates. Inorg Nucl Chem Lett. 1969;5:607–608. [Google Scholar]
- 7.Lindner E, Vitzthum G, Langner D, Lorenz IP. Metal-ligand bonding in sulfinato complexes of transition metals. Angew Chem Int Ed. 1970;9:160–161. [Google Scholar]
- 8.Lindner E, Frembs DWR, Krug D. Gezielte Synthese von Sulfinato-O- und -S-Komplexen einiger Übergangsmetalle, XI. Bindungsisomerie bei Sulfinato-Komplexen von Zink(II) Chem Ber. 1975;108:291–300. [Google Scholar]
- 9.Li Z, Cui Z, Liu ZQ. Copper- and iron-catalyzed decarboxylative tri- and difluoromethylation of α,β-unsaturated carboxylic acids with CF3SO2Na and (CF2HSO2)2Zn via a radical process. Org Lett. 2013;15:406–409. doi: 10.1021/ol3034059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


