Abstract
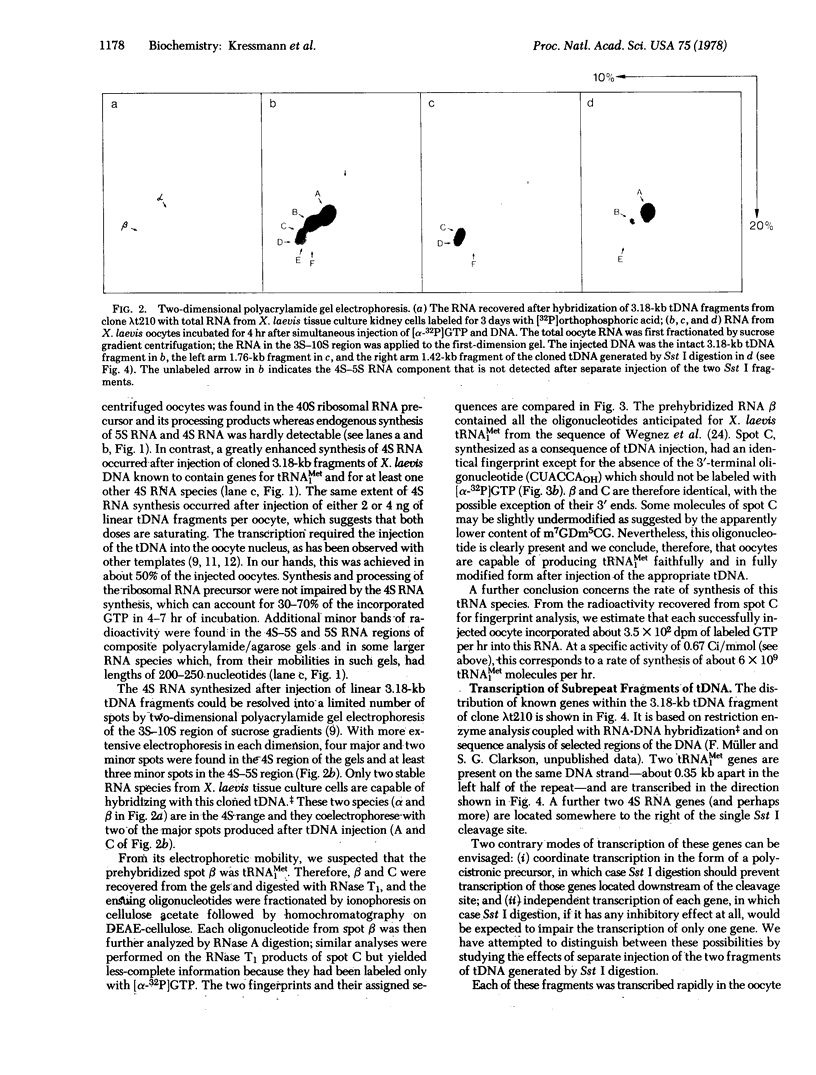
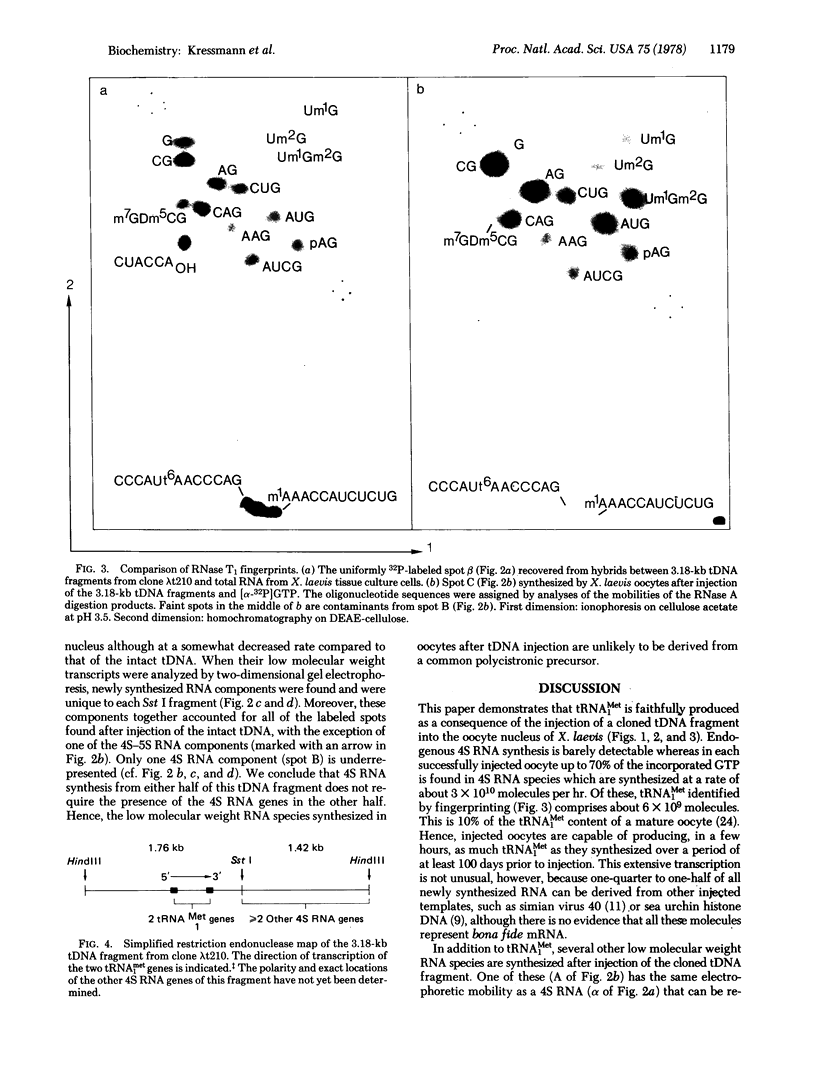
Cloned 3.18 kilobase fragments of Xenopus laevis DNA containing genes for tRNAMet1 and for at least one other 4S RNA species are transcribed rapidly after their injection into the nucleus of X. laevis oocytes. The newly synthesized RNA can be resolved by gel electrophoresis into a few predominant 4S RNA species and a series of slower migrating components. One of the 4S RNA species appears to be identical, by fingerprint analysis, to the tRNAMet1 isolated by hybridization of somatic cell RNA to this cloned tRNA gene fragment (tDNA). Thus, the tRNAMet1 produced after injection can be both fully processed and modified. Its rate of synthesis is estimated to be about 6 x 10(9) molecules/hr in each oocyte injected with 2 ng of tDNA. When the tDNA fragment is cleaved into two halves with restriction endonuclease Sst I, each injected half gives rise to a subset of the RNAs produced after injection of the intact fragment. This experiment thus suggests the presence of at least two transcriptional units on this cloned tDNA. This simple way of biologically testing defined restriction fragments may be of value for analyzing the functional organization of other cloned eukaryotic DNA units.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Smith L. D. Synthesis of heterogeneous nuclear RNA in full-grown oocytes of Xenopus laevis (Daudin). Cell. 1977 Jul;11(3):663–671. doi: 10.1016/0092-8674(77)90083-6. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Wallace H., Sirlin J. L., Fischberg M. Localization of the ribosomal DNA complements in the nucleolar organizer region of Xenopus laevis. Natl Cancer Inst Monogr. 1966 Dec;23:431–447. [PubMed] [Google Scholar]
- Birnstiel M., Telford J., Weinberg E., Stafford D. Isolation and some properties of the genes coding for histone proteins. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2900–2904. doi: 10.1073/pnas.71.7.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. High-fidelity transcription of 5S DNA injected into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 May;74(5):2064–2068. doi: 10.1073/pnas.74.5.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. Purification and some characteristics of 5S DNA from Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3175–3179. doi: 10.1073/pnas.68.12.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson S. G., Kurer V. Isolation and some properties of DNA coding for tRNA1met from Xenopus laevis. Cell. 1976 Jun;8(2):183–195. doi: 10.1016/0092-8674(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Colman A. Transcription of DNAs of known sequence after injection into eggs and oocytes of Xenopus laevis. Eur J Biochem. 1975 Sep 1;57(1):85–96. doi: 10.1111/j.1432-1033.1975.tb02279.x. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca M. J., Smith L. D., Strobel M. C. Quantitative and qualitative analysis of RNA synthesis in stage 6 and stage 4 oocytes of Xenopus laevis. Dev Biol. 1973 Sep;34(1):106–118. doi: 10.1016/0012-1606(73)90342-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Wegnez M., Mazabraud A., Denis H., Pétrissant G., Boisnard M. Biochemical research on oogenesis. Nucleotide sequence of initiator tRNA from oocytes and from somatic cells of Xenopus laevis. Eur J Biochem. 1975 Dec 1;60(1):295–302. doi: 10.1111/j.1432-1033.1975.tb21003.x. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Pestell R. Q. Determination of the nucleoside triphosphate contents of eggs and oocytes of Xenopus laevis. Biochem J. 1972 Apr;127(3):597–605. doi: 10.1042/bj1270597. [DOI] [PMC free article] [PubMed] [Google Scholar]