Abstract
Objectives
We assessed in the Multi-Ethnic Study of Atherosclerosis (MESA) whether impaired fasting glucose, insulin resistance, and waist-to-hip ratio had effects on cardiac remodeling, independent of obesity.
Background
Recent studies suggest that central obesity and insulin resistance may be primary mediators of obesity-related cardiac remodeling independent of body mass index (BMI).
Methods
We investigated 4,364 individuals without diabetes in MESA. Impaired fasting glucose (IFG: 100-125 mg/dl) or insulin resistance (by homeostatic model assessment of insulin resistance, HOMA-IR) and waist-to-hip ratio (WHR) were used for cardiometabolic phenotyping. Multivariate linear regression analysis was used to determine the effects of the cardiometabolic markers on LV remodeling, assessed primarily through the LV mass-to-volume ratio obtained by cine cardiac magnetic resonance imaging.
Results
Individuals with IFG were more likely to be older, hypertensive, with increased prevalence of cardiometabolic risk factors regardless of BMI. In each quartile of BMI, individuals with above-median HOMA-IR, above-median WHR, or IFG had a higher LV mass-to-volume ratio (p<0.05 for all). HOMA-IR (p<0.0001), WHR (p<0.0001), and the presence of IFG (p=0.04), but not BMI (p=0.24), were independently associated with LV mass-to-volume ratio after adjustment for age, gender, hypertension, race, and dyslipidemia.
Conclusions
Insulin resistance and waist-to-hip ratio are associated with concentric LV remodeling independent of BMI. These results support the emerging hypothesis that the cardiometabolic phenotype, defined by insulin resistance and central obesity, may play a critical role in LV remodeling independently of BMI.
Keywords: obesity, metabolic syndrome, LV remodeling, MESA
INTRODUCTION
Affecting nearly 1 in 3 adults, obesity represents a growing, critical risk to cardiovascular health [1]. With associated abnormalities in left and right ventricular structure and function [2], obesity imposes a higher lifetime risk of heart failure independent of co-morbid illness (hypertension, coronary artery disease, and type 2 diabetes) [3-5]. Recently, abnormal neurohormonal activation from visceral adipocyte stores has been postulated to drive insulin resistance and a pro-inflammatory metabolic syndrome linking obesity to heart failure [6]. In effect, the insulin resistance that accompanies central obesity (“adiposopathy”) has been proposed to resolve an “obesity paradox,” the observation that not all obese individuals are subject to similarly elevated cardiovascular risk [6]. In several large studies, markers of central obesity and insulin resistance, but not body mass index (BMI), predict cardiac mortality and heart failure, even with a normal BMI [7-12]. The hypothesized role of insulin resistance independent of BMI has recently given rise to the concept of a “metabolically healthy” obese and “metabolically unwell” non-obese individual. Indeed, obese individuals without other components of the metabolic syndrome have a lower lifetime heart failure risk, compared to non-obese individuals with metabolic syndrome [9]. However, whether presence of central obesity and insulin resistance is associated with adverse cardiac remodeling independent of BMI in patients without diabetes or established cardiovascular disease is unknown.
To provide evidence to support the effect of insulin resistance on cardiac remodeling across BMI, we investigated markers of insulin resistance, central obesity, and ventricular structure and function in participants of the Multi-Ethnic Study of Atherosclerosis (MESA). Using a cross-sectional design, we tested whether insulin resistance and waist-to-hip ratio are associated with concentric LV remodeling (as defined by LV mass-to-volume ratio) independent of BMI. Given the important links between inflammation and cardiovascular disease across BMI, we further tested the hypothesis that systemic inflammation is associated with concentric LV remodeling, independent of BMI, insulin resistance, or waist-to-hip ratio.
METHODS
Participant population
The overall design of the MESA study has been described previously [13]. In brief, the MESA study consists of 6,814 men and women of different ethnicities (white, African American, Chinese American, and Hispanic) enrolled from six different national sites, all of whom were free of clinical cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke, or peripheral arterial disease) at the time of enrollment. Baseline demographics and medical history (including cardiac risk factors), as well as height, weight, waist and hip dimensions, were collected at the index examination (July 2000-August 2002 cycle). Body mass index was calculated as weight divided by the square of height (in kg/m2). Resting systolic blood pressure was measured three times in the seated position using a Dinamap model Pro 100 sphygmomanometer (Critikon, Wipro GE Healthcare, Waukesha, WI). Fasting blood glucose (Vitros analyzer, Johnson and Johnson Clinical Diagnostics, Rochester, NY) and fasting insulin (radioimmunoassay, Linco Human Insulin Specific RIA kit, Linco Research) were assessed during the baseline visit. In addition, selected biomarkers of inflammation, including interleukin-6, C-reactive protein, tumor necrosis factor-α receptor 1, and plasminogen activator inhibitor-1 (PAI-1), and cardiac remodeling were collected, as previously described [14]. Hours of physical activity per week were extracted from self-reported questionnaires conducted at the initial study visit. Protocols were approved by the Institutional Review Board at each participating institution. All participants provided written informed consent.
From the initial sample enrolled in the first MESA examination (N=6,814), individuals with data missing for fasting blood glucose, body mass index, waist or hip circumference (N=26, 0.4%) were excluded. In addition, individuals with diabetes mellitus (as defined by a fasting blood sugar ≥ 126 mg/dl at screening, history of treated or untreated diabetes, use of anti-diabetic medications) were excluded (N=925), leaving 5,863 individuals with complete data on adiposity measures, fasting blood glucose, and without established diabetes.
Cardiac magnetic resonance (CMR) assessment of LV structure and function
CMR imaging was performed at 1.5 Tesla at the index examination as previously described [15, 16]. Assessment of ventricular function was performed using electrocardiographically-gated fast gradient echo cine images (repetition time 6 msec, minimal echo time, flip angle 20°, 8 mm slice thickness with 2 mm gap, matrix 256×160, field of view adjusted to body size, receiver bandwidth 32 kHz). LV volumes and mass were determined by short-axis volumetric coverage, normalized to body surface area. Papillary muscles were included in the LV volumes and excluded from LV mass. LV mass to LV end-diastolic volume was calculated as an index of concentric remodeling.
To analyze regional myocardial systolic and diastolic function, CMR tagging and regional systolic wall shortening analyses were performed in a subgroup of the population [17]. Global systolic wall shortening was calculated for the mid-LV short-axis slice as the average of segmental fractional shortening: (LV end-diastolic – LV end-systolic)/LV end-diastolic thickness (N=4,215). CMR tagging analysis was performed with two sets of cine images with spatially modulated magnetization in orthogonal directions, using 7 mm tag line spacing, and analyzed by harmonic phase techniques (HARP software, Diagnosoft, Morrisville, NC) over 19-27 phases/cardiac cycle (average repetition time 6 msec, echo time 3 msec, flip angle 12°, matrix 256×96-140, average temporal resolution 40 msec). Peak systolic strain (a marker of subclinical ventricular systolic dysfunction) was calculated as the average of segmental peak systolic strain in the mid-LV slice (N=880). MASS software (version 4.2, Medis, The Netherlands) at a single reading center by readers blinded to clinical data was used to quantify data.
Statistical analysis
We examined impaired fasting glucose (IFG; 100-125 mg/dl) and insulin resistance (by homeostasis model assessment of insulin resistance, HOMA-IR; fasting insulin in μU/mL × fasting glucose in mg/dL/405) [18]. We assessed central obesity by waist-to-hip ratio (WHR). Baseline clinical, demographic, and measures of ventricular structure/function were stratified by obesity (BMI ≥ 30 kg/m2) and by IFG. Data normality was assessed by visual inspection of distributions and normal quantile-quantile plots. The Student's t-test (normal continuous data) or Kruskal Wallis test (non-normal continuous data) were used for comparisons. Chi-square or Fisher exact testing was used for categorical covariates. Biomarker concentrations were compared by non-parametric (Wilcoxon rank-sum) testing.
The association of insulin resistance and central obesity with cardiac structure was assessed by Spearman correlation between CMR LV structure and function, and HOMA-IR, WHR, or fasting glucose, in the whole population, and in obese and non-obese individuals separately. To examine the association of HOMA-IR or WHR with concentric LV remodeling, we compared the LV mass-to-volume ratio across quartiles of BMI, stratified around median HOMA-IR, median WHR, or by presence of IFG. Two-way analysis of variance and post-hoc Student's t-tests (with Bonferroni correction for multiple hypothesis testing) were used to compare across strata.
To investigate how insulin resistance or central obesity modifies the association between BMI and LV mass-to-volume ratio, we built a multivariate linear regression model with LV mass-to-volume ratio as the dependent variable, and BMI (continuous variable), presence of IFG, HOMA-IR (dichotomized by median), and WHR (dichotomized by median) included as explanatory variables, as well as interaction terms of each marker (IFG, HOMA-IR, or WHR) with BMI. We also adjusted for age, gender, race, prior and current smoking status, history of hypertension (by Joint National Committee VI guidelines), high-density lipoproteins, and triglycerides in the model (hereafter referred to collectively as “clinical covariates”). Continuous variables (age, triglycerides, HDL and BMI) were centered and standardized in this model. A separate model (without the interactions) was constructed to check the stability of the main effects.
We examined associations between LV mass-to-volume ratio, a marker of concentric LV remodeling, and inflammatory biomarkers. For all regression analyses, biomarkers of systemic inflammation were log-transformed to establish normality. Spearman correlation coefficients were used to measure association of inflammatory biomarkers with BMI, fasting glucose, HOMA-IR and WHR in the overall population, and in the obese and non-obese groups separately.
PAI-1 is known to be elevated in obesity and the metabolic syndrome, and its relationship with the cardiometabolic phenotype markers and the LV structure parameters was closely examined. To assess whether HOMA-IR or WHR would affect the relationship between PAI-1 and obesity, we compared with two-way analysis of variance mean PAI-1 levels across quartiles of BMI, with PAI-1 stratified by median HOMA-IR, or WHR. Student's t-tests (with Bonferroni correction for multiple hypothesis testing for 4 comparisons, one for each quartile) were used for post-hoc comparisons. The effect of PAI-1 on concentric LV remodeling was assessed with a linear regression model for LV mass-to-volume ratio, with PAI-1 (log transformed), HOMA-IR, WHR, IFG, BMI, as predictors, and simultaneous adjustment by clinical covariates. To examine links between PAI-1 and cardiometabolic markers, we also built a model for PAI-1 which included BMI, HOMA-IR (dichotomized by median), WHR (dichotomized by median), IFG, and the interaction of each parameter with BMI as predictors. Continuous variables were centered and standardized. The number of measurements for each analysis varied, based on the availability of CMR data. A p < 0.05 was considered significant. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) or R (version 2.15.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
Clinical and biochemical characteristics by obesity and impaired fasting glucose
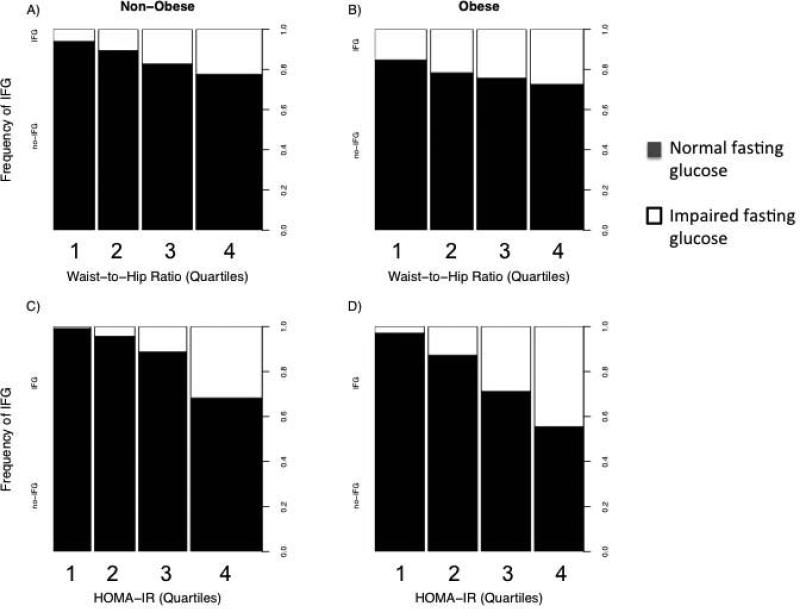
Baseline clinical, demographic, and biochemical characteristics of the study population are shown in Table 1. Of the overall population, 1,734 (30%) were obese by BMI criteria. In the obese subgroup, 386 (22%) individuals had IFG, as compared to 515 (12%) subjects with IFG in the non-obese stratum (p <0.0001). The presence of IFG in both the non-obese and obese groups was associated with a more adverse clinical, metabolic, and inflammatory profile. Compared to individuals without IFG, individuals with IFG (in both obese and non-obese groups) were older, more often male, more often hypertensive, with a greater waist circumference, WHR, HDL and triglyceride concentrations, fasting glucose, and HOMA-IR. In addition, markers of systemic adiposity-related inflammation (e.g., C-reactive protein, plasminogen activator inhibitor-1, interleukin-6, soluble TNFα receptor-1, urine albumin) were higher in the obese and in individuals with IFG. In addition, there was a statistically significant increase in prevalence of IFG across quartiles of HOMA-IR and WHR in both the obese and non-obese cohorts (p < 0.05 for all panels; Figure 1). Finally, a greater proportion of obese individuals did not engage in any weekly exercise (27% vs. 20% in non-obese, p < 0.0001), (Table 1). Subjects with IFG exercised less per week as compared to subjects with normal fasting glucose, regardless of obesity status (p = 0.001). Obese individuals with IFG had a lower amount of weekly intentional exercise than those with NFG (p = 0.006).
Table 1.
Baseline demographic, clinical, and biochemical characteristics, stratified by normal (NFG) or impaired (IFG) fasting glucose and by body mass index.
| BMI < 30 kg/m2 (N = 4129) |
BMI ≥ 30 kg/m2 (N=1734) |
Obese vs. non-obese (p-value) |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | NFG (N=3614) | IFG (N=515) | p-value | NFG (N=1348) | IFG (N=386) | p-value | NFG | IFG |
| Age, years | 61.9±10.5 | 65.8±9.7 | <.0001 | 59.9±9.7 | 62.2±9.6 | <.0001 | <.0001 | <.0001 |
| Male, n (%) | 1697 (47) | 321 (62) | <.0001 | 521 (39) | 185 (48) | .001 | <.0001 | <.0001 |
| Body mass index, kg/m2 | 25.1±2.9 | 26.3±2.6 | <.0001 | 34.2±4.0 | 35.1±4.4 | .0007 | <.0001 | <.0001 |
| Systolic blood pressure, mmHg | 123.5±20.8 | 131.5±21.9 | <.0001 | 128.7±20.3 | 133.5±19.6 | <.0001 | <.0001 | .15 |
| Hypertension, % | 1279 (35) | 267 (52) | <.0001 | 655 (49) | 232 (60) | <.0001 | <.0001 | 0.01 |
| Current or former smoker, % | 1762 (49) | 250 (49) | 0.96 | 686 (51) | 195 (51) | 1.0 | 0.17 | 0.52 |
| Race, n (%) | <.0001 | .004 | <.0001 | <.0001 | ||||
| Caucasian | 1638 (45) | 167 (32) | 517 (38) | 115 (30) | ||||
| Chinese-American | 538(15) | 124 (24) | 19 (1) | 11 (3) | ||||
| African-American | 764 (21) | 115 (22) | 496 (37) | 150 (39) | ||||
| Hispanic | 674 (19) | 109 (21) | 316 (23) | 110 (29) | ||||
| Metabolic syndrome | 110.9±11.2 | <.0001 | <.0001 | |||||
| Waist circumference, cm | 90.3±10.0 | 95.2±8.6 | <.0001 | 0.96 (0.90-1.00) | 114.4±11.8 | <.0001 | <.0001 | <.0001 |
| Waist-to-hip ratio | 0.91 (0.85-0.96) | 0.95 (0.91-0.99) | <.0001 | 48.8±13.2 | 0.98 (0.93-1.01) | <.0001 | <.0001 | .002 |
| HDL, mg/dl | 53.9±15.6 | 48.2±13.5 | <.0001 | 118.0 (83.0-168.0) | 45.7±11.1 | <.0001 | <.0001 | .32 |
| Triglycerides, mg/dl | 102.0 (72.0-145.0) | 126.0 (87.0-173.0) | <.0001 | 94.5±14.1 | 131.0 (88.0-185.0) | .004 | <.0001 | <.0001 |
| Weight, kg | 70.3±12.3 | 74.0±11.9 | <.0001 | 87.6±6.7 | 96.9±14.3 | .003 | <.0001 | .02 |
| Fasting glucose, mg/dl | 85.7±7.1 | 107.1±6.9 | <.0001 | 10.6 (7.8-14.5) | 108.1±7.0 | <.0001 | <.0001 | <.0001 |
| Fasting insulin, μU/mL | 6.65 (5.15-9.02) | 9.64 (7.02-12.89) | <.0001 | 2.28 (1.66-3.19) | 13.9 (10.8-18.9) | <.0001 | <.0001 | <.0001 |
| HOMA-IR | 1.41 (1.07-1.95) | 2.50 (1.89-3.38) | <.0001 | 3.65 (2.87-5.09) | <.0001 | |||
| Intentional weekly exercise | 990 (210-2235) | 840 (210-2062.5) | .40 | 667 (0-1777.5) | 510 (0-1410) | .006 | ||
| (METS-minute/week) | <.0001 | <.0001 | ||||||
| Biomarkers, median (IQR) | ||||||||
| Estimated GFR, ml/min/1.73 m2 | 78.9 (69.4-90.4) | 79.3 (69.1-91.2) | .36 | 79.4 (69.3-91.3) | 80.4 (67.6-93.1) | .19 | .87 | .49 |
| Urine albumin present, n (%) | 192 (5) | 55 (11) | <.0001 | 99 (7) | 50 (13) | <.0001 | .006 | .29 |
| NT-proBNP, pg/ml | 58.6 (27.8-115.7) | 52.2 (22.8-108.5) | .02 | 51.4 (20.4-107.0) | 42.7 (20.9-89.6) | .13 | <.0001 | .11 |
| C-reactive protein, mg/l | 1.34 (0.62-3.06) | 1.65 (0.81-3.53) | .0001 | 3.22 (1.56-6.78) | 4.05 (1.92-7.40) | .01 | <.0001 | <.0001 |
| PAI-I, ng/ml | 14.0 (8.0-25.0) | 25.0 (17.0-41.5) | <.0001 | 24.5 (13.0-48.0) | 39.5 (24.0-71.5) | .0001 | <.0001 | .001 |
| MMP-3, ng/ml | 12.2 (8.3-19.1) | 14.6 (10.4-21.4) | .07 | 10.8 (7.6-16.0) | 9.4 (7.5-15.2) | .36 | .03 | .003 |
| MMP-9, ng/ml | 207.1 (151.4-292.3) | 184.1 (134.9-254.5) | .23 | 228.3 (157.6-333.7) | 239.2 (176.7-315.0) | .79 | .04 | .04 |
| IL-6, pg/ml | 0.97 (0.66-1.54) | 1.21 (0.81-1.85) | <.0001 | 1.53 (1.05-2.28) | 1.74 (1.21-2.74) | <.0001 | <.0001 | <.0001 |
| TNFa receptor 1, pg/ml | 1243 (1063-1469) | 1287 (1109-1565) | .03 | 1345 (1176-1554) | 1416 (1222-1652) | .04 | <.0001 | .0008 |
Normal variables reported as mean±standard deviation. Non-normal variables and biomarkers reported as median (interquartile range). Abbreviations: BMI = body mass index; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; GFR = glomerular filtration rate; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PAI-I = platelet activator inhibitor-1; MMP = matrix metalloproteinase; IL-6 = interleukin-6; TNF = tumor necrosis factor; METS = metabolic equivalents. Variables expressed as mean and standard deviation were compared using the Student's t-test, and variables expressed as median (IQR) were compared using a Wilcoxon (Kruskal-Wallis) test. Categorical variables were compared using Chi-Square (for 2x2 comparisons) or Fisher exact tests (otherwise).
Figure 1. Prevalence of impaired fasting glucose stratified by BMI and waist-to-hip ratio.
Distribution of impaired and normal fasting glucose across obese (BMI ≥30 kg/m2) and non-obese (BMI < 30 kg/m2) strata and quartiles of WHR or HOMA-IR.
Association between insulin resistance, central obesity, and LV remodeling in obese and non-obese individuals
CMR indices of LV remodeling and systolic function, stratified by obesity and IFG, are shown in Table 2. Regardless of obesity status, individuals with IFG had a higher LV mass-to-volume ratio, a higher ventricular mass index and lower end-diastolic volume index. In the overall cohort, higher fasting glucose, HOMA-IR, BMI, and WHR were all positively associated with concentric LV remodeling (all p < 0.0001; Supplementary Table 1). Markers of cardiometabolic health (IFG, WHR) also had an effect of regional LV function: in both the obese and non-obese groups, individuals with IFG had a small reduction in peak systolic strain (Table 2). A less negative peak systolic strain (denoting subclinical systolic dysfunction) was associated with a higher fasting glucose (p < 0.01), HOMA-IR, BMI, and WHR (p < 0.0001 for rest). When limited to patients with IFG, male gender (β=1.93, p < 0.0001) and higher BMI (β=0.14, p=0.01) were associated with lower peak systolic strain, after adjustment for age, gender, race, history of hypertension, triglycerides, BMI, HDL cholesterol, and log-transformed HOMA-IR.
Table 2.
Ventricular remodeling and regional function by CMR, stratified by BMI and normal (NFG) or impaired (IFG) fasting glucose.
| Variable | BMI < 30 kg/m2 |
p (NFG vs. IFG) | BMI ≥ 30 kg/m2 |
p (NFG vs. IFG) |
Obese vs. non-obese (p-value)
|
|||
|---|---|---|---|---|---|---|---|---|
| NFG | IFG | NFG | IFG | NFG | IFG | |||
| Ventricular remodeling (N=4364) | ||||||||
| LV ejection fraction, % | 69.1±7.1 | 69.4±8.1 | .50 | 69.0±7.0 | 68.6±7.8 | .55 | .56 | .24 |
| LV end-diastolic volume index, ml/m2 | 69.0±13.8 | 65.9±13.2 | <.0001 | 68.0±13.0 | 65.4±12.1 | .005 | .06 | .59 |
| LV end-systolic volume index, ml/m2 | 21.6±8.0 | 20.5±8.2 | .01 | 21.3±7.5 | 20.7±7.6 | .24 | .43 | .76 |
| LV mass index, grams/m2 | 76.5±16.1 | 78.3±14.6 | .02 | 78.0±15.2 | 82.5±18.7 | .0009 | .009 | .004 |
| LV mass to volume ratio | 1.13±0.22 | 1.22±0.25 | <.0001 | 1.17±0.24 | 1.28±0.27 | <.0001 | <.0001 | .002 |
| Regional function (mid-LV) | ||||||||
| Systolic wall shortening, % (N=4215) | −78.1±26.9 | −77.4±27.2 | .62 | −77.7±26.5 | −75.8±29.0 | .36 | .66 | .50 |
| Peak systolic strain, % (N=880) | −17.6±2.4 | −17.0±2.7 | .03 | −17.0±2.8 | −16.1±3.1 | .04 | .01 | .05 |
Strain data was calculated as a segmental average in the mid-ventricular slice. The number of observations available in the whole population are as indicated. All values are mean±standard deviation. Variables were compared with t-testing unless otherwise specified. Abbreviations: LV = left ventricular.
Incremental effects of insulin resistance and central obesity on concentric LV remodeling across BMI
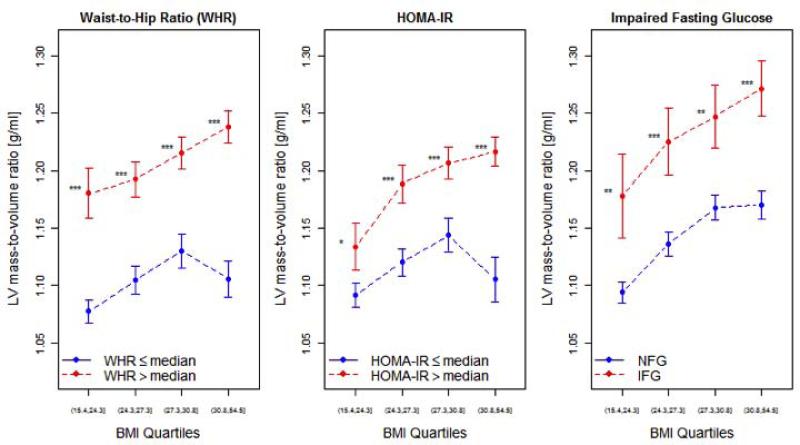
When stratified by quartiles of BMI, LV mass-to-volume ratio was significantly higher in individuals with IFG, above-median HOMA-IR (HOMA-IR > 1.73) and above-median WHR (WHR > 0.92; Figure 2). Consistently, individuals with above-median WHR, HOMA-IR, or IFG had greater LV mass-to-volume ratio at every quartile of BMI. When HOMA-IR was stratified around 2.5 (as has been reported [19]), HOMA-IR continued to have a highly significant association with LV mass-to-volume ratio across BMI quartiles (p < 0.0001).
Figure 2. LV remodeling as a function of cardiometabolic risk and obesity.
LV mass-to-volume ratio across quartiles of BMI, stratified by above- and below-median HOMA-IR or WHR and the presence of impaired fasting glucose. Error bars represent 95% confidence intervals of the mean. Comparisons across HOMA-IR, WHR, or IFG groups made by Student's t-tests (with Bonferroni adjustment for multiple hypothesis testing). * P < 0.05; ** P < 0.01; *** P < 0.001, ns = not significant.
Table 3 with the regression coefficients of a multivariate linear regression model for LV mass-to-volume ratio demonstrates the association of LV mass-to-volume ratio with the cardiometabolic predictors (HOMA-IR, WHR, or IFG). In this model, BMI was not significantly associated with LV mass-to-volume ratio. In addition, BMI did not modify the association of HOMA-IR with LV mass-to-volume (p = 0.77), suggesting that above-median HOMA-IR is associated with concentric LV remodeling regardless of BMI. On the other hand, BMI did modify the association of WHR (p = 0.008) with LV mass-to-volume ratio, suggesting that a higher WHR may have a greater impact on concentric LV remodeling in more obese individuals. In a model without interaction terms adjusted for clinical covariates, HOMA-IR (β = 0.0205, p < 0.0001), WHR (β = 0.0247, p < 0.0001), and IFG (β = 0.0243, p = 0.02), but not BMI (p = 0.10), were still each independently associated with LV mass-to-volume ratio.
Table 3.
Table of regression coefficients from a multivariate linear regression model for LV mass-to-volume ratio.
| Covariate | Model Parameters |
|
|---|---|---|
| β | P value | |
| Age | 0.0424 | <0.0001 |
| Male gender | 0.105 | <0.0001 |
| Hypertension | 0.0623 | <0.0001 |
| Smoking | ||
| Past | 0.00822 | 0.25 |
| Current | 0.0705 | <0.0001 |
| Triglyceride level | 0.00455 | 0.21 |
| High-density lipoprotein level | −0.00505 | 0.21 |
| Race* | ||
| Chinese | −0.00730 | 0.50 |
| African-American | 0.0724 | <0.0001 |
| Hispanic | 0.000888 | 0.92 |
| BMI | 0.00581 | 0.24 |
| HOMA-IR | 0.0198 | <0.0001 |
| BMI × HOMA-IR (interaction) | 0.00119 | 0.77 |
| whr | 0.0258 | <0.0001 |
| BMI × WHR (interaction) | 0.00956 | 0.008 |
| Presence of IFG | 0.0207 | 0.04 |
| BMI × IFG (interaction) | 0.0161 | 0.13 |
Model was constructed with body mass index, age, male gender, history of hypertension, triglycerides, HDL, race, BMI, HOMA-IR, WHR, IFG, and interactions between insulin resistance and visceral adiposity with BMI as predictors. Continuous variables (age, triglycerides, HDL and BMI) were centered and standardized for inclusion as predictors.
Caucasian race was the reference category. Abbreviations: BMI = body mass index; WHR = waist-to-hip ratio; HOMA-IR = homeostatic model assessment of insulin resistance; IFG = impaired fasting glucose. The R2 for this multivariable model is 0.22.
Association of biomarkers of inflammation with concentric remodeling
Spearman correlations between inflammatory biomarkers and LV-mass-to-volume ratio, HOMA-IR, WHR and fasting glucose are shown in Supplementary Table 2 for the overall population and stratified by obesity. Although all inflammatory markers were associated with LV mass-to-volume ratio (in both obese and non-obese individuals), PAI-1 demonstrated a consistent, significant association with concentric LV remodeling, insulin resistance and central obesity, and was therefore selected for further analyses.
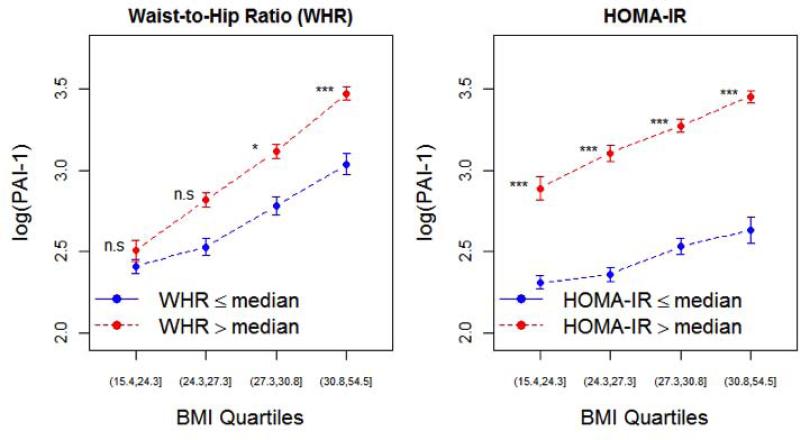
PAI-1 levels were higher in individuals with above-median HOMA-IR across quartiles of BMI (Figure 3). In a multivariate linear regression model for predicting PAI-1 (log-transformed), containing similar covariates as in the model in Table 3 (without interaction terms), BMI (p < 0.0001), HOMA-IR (p < 0.0001) and WHR (p = 0.04), but not IFG (p = 0.64) were associated with PAI-1. When PAI-1 was added as predictor to the model for LV mass-to-volume ratio (including covariates in Table 3, without interactions), it was found that PAI-1 had a significant association with concentric LV remodeling (β = 0.0226, p = 0.04), independent of BMI, WHR, HOMA-IR, and other clinical covariates. Of note, WHR (p=0.001) and IFG (p=0.01) maintained a significant association with concentric LV remodeling in this model. It should be noted that removal or addition of PAI-1 as predictor in the linear regression model for LV-mass-to-volume ratio did not cause the other predictors to loose or gain statistical significance, yet the addition of PAI-1 as predictor resulted in a significantly (p=0.038) better agreement with the data than the model without PAI-1.
Figure 3. PAI-1 as a function of cardiometabolic risk and obesity.
PAI-1 levels (log-transformed) across quartiles of BMI, stratified by median WHR or HOMA-IR. Error bars represent 95% confidence intervals of the mean. Comparisons across HOMA-IR and WHR groups made by Student's t-tests (with Bonferroni adjustment for multiple hypothesis testing). * P < 0.05; ** P < 0.01; *** P < 0.001, ns = not significant.
DISCUSSION
In this cross-sectional, multi-ethnic, community-based study, the presence of insulin resistance (by HOMA-IR) and central obesity (by WHR) was associated with concentric LV remodeling across the spectrum of BMI. Impaired fasting glucose identified a subgroup within both obese and non-obese individuals with a higher cardiometabolic risk (by metabolic syndrome criteria) and greater concentric LV remodeling. Impaired fasting glucose, a higher HOMA-IR, and higher WHR were significantly associated with concentric LV remodeling, after adjustment for BMI and clinical covariates. After adjustment for clinical covariates (as well as HOMA-IR, WHR, and IFG), BMI was not associated with LV mass-to-volume ratio, suggesting that insulin resistance may explain some of the effect of BMI on concentric LV remodeling in individuals without established diabetes or cardiovascular disease. These results highlight the importance of insulin resistance and central obesity in the pathogenesis of concentric LV remodeling across BMI.
Current World Health Organization guidelines define “obesity” as a BMI ≥ 30 kg/m2 [20]. However, a growing literature supports inflammation and insulin resistance as a potential etiology for cardiovascular damage in both “obese” and “non-obese” individuals [6]. Pro-inflammatory visceral adipocytes produce a variety of neurohormonal signals, including interleukin-6, and PAI-1 [6], which mediate hepatic insulin resistance [21], cardiac remodeling, and heart failure [22, 23]. In fact, cardiovascular function and clinical outcomes are associated with the extent and neurohormonal activity of visceral fat [7, 8, 11, 24], such that obese individuals without pro-inflammatory visceral adipose tissue remain metabolically neutral [25]. Ultimately, cardiovascular risk may depend more on insulin resistance than on BMI [26], with insulin resistance driving incident heart failure in non-obese individuals to a greater degree than in metabolically healthy obese [9]. Our results support the emerging hypothesis that “adiposopathy” (marked by insulin resistance and visceral adipose distribution) impacts cardiac remodeling across BMI beyond markers of clinical risk. These results are in agreement with a recent smaller study utilizing echocardiography, demonstrating a relationship between ventricular function, C-reactive protein, and HOMA-IR in patients with normal weight obesity (normal BMI, increased fat content) [27].
Our results provide additional insight into relationships between inflammation, insulin resistance, and remodeling. PAI-1 exhibited a significant association with HOMA-IR and WHR in the overall cohort and in both obese and non-obese strata, again confirming a connection between adiposopathy, inflammation, and systemic insulin resistance [28, 29]. When adjusted for clinical covariates, HOMA-IR, BMI, and WHR were all significantly associated with PAI-1 levels. Furthermore, PAI-1 was associated with concentric LV remodeling beyond that predicted by BMI, insulin resistance or central obesity. PAI-1 has been implicated in cardiac remodeling [22], and recent observations in normal weight mice with increased central obesity indicate that tissue-level insulin resistance, vascular dysfunction, adipocyte expression of PAI-1 and TNFα coexist and are reversible with anti-TNFα therapy [30], suggesting a link between inflammation, central obesity, and cardiovascular dysfunction independent of obesity. Ultimately, cardiometabolic health may be more completely described by integrating BMI with insulin resistance, fat distribution, and inflammation, rather than by BMI alone.
Prior observations from MESA included reported associations between inflammation and LV remodeling [14] or obesity, inflammation, and heart failure incidence [15, 31-33]. In a study of 5,098 individuals with CMR data in MESA, Turkbey et al. reported an association between BMI and LV mass-to-volume ratio, after adjustment for WHR and other clinical risk markers [32]. Of note, individuals with diabetes were included in their population, and models were not adjusted for measures of insulin resistance (e.g., fasting glucose, IFG, or HOMA-IR). These results extend these prior investigations to an even more pre-clinical population (without diabetes) to directly address the impact of insulin resistance and central obesity on concentric remodeling, suggesting that both BMI and IFG may contribute to subclinical dysfunction (e.g., strain) and concentric remodeling. Though our analysis was limited to non-diabetic participants of MESA, to allow for a focus on an earlier cardiometabolic phenotype, it should be pointed out that the effects of higher than median WHR are still significant when the analysis includes MESA participants with diabetes. Our results identify individuals with increased cardiometabolic risk as at risk for incident heart failure and an important population to target for prevention. Indeed, elevations in LV mass and concentric remodeling have been associated with adverse outcome in MESA [34]. Demonstrating that insulin resistance impacts LV remodeling before clinical heart failure across BMI suggests that adoption of more intensive strategies (e.g., bariatric surgery [26]) or novel drug therapies (e.g., incretin modulation) in this high-risk group may improve outcome. Ultimately, BMI may not the sole arbiter of cardiometabolic risk.
The results of this study must be viewed in light of its cross-sectional design. While measuring inflammation, insulin resistance, and concentric LV remodeling at the same time point renders difficult any suggestions of temporal sequence, the suggestion that insulin resistance may influence hypertrophy and remodeling is biologically plausible [35]. Although correlations of LV structure and functional parameters with inflammatory markers and insulin resistance were modest, fully-adjusted regression models suggest a significant association of metabolic dysfunction and LV remodeling. In addition, our adjustments for age, gender, race, and other important markers of cardiometabolic risk improves the robustness of any effects of these other confounding variables on the association between insulin resistance and concentric LV remodeling. Furthermore, although formal glucose tolerance testing is considered more prognostic than the parameters of cardiometabolic risk used in this study (e.g., HOMA-IR, WHR, and IFG) [36], this is not routinely performed in practice in all individuals, and associations we observed here were in agreement with a prior report using IFG [35]. Finally, we recognize that WHR is not an ideal marker of visceral adiposity, and more advanced measures (e.g., computed tomography) may be more specific. However, given that WHR is associated with inflammation and remodeling independent of BMI, it is likely that WHR more directly captures cardiometabolic disease relative to BMI.
In conclusion, we demonstrate an association among insulin resistance, central obesity, and concentric LV remodeling across BMI in MESA, independent of traditional and metabolic risk factors. These results provide evidence to support the emerging hypothesis that insulin resistance and central obesity mediates concentric LV remodeling in otherwise healthy individuals beyond that predicted by BMI. Efforts to target individuals at higher cardiometabolic risk, integrating assessments of insulin resistance and waist-to-hip ratio in addition to BMI, may prevent further LV remodeling and incident heart failure.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the participants in MESA for their ongoing participation and support and other MESA investigators for their valuable contributions.
FUNDING SOURCES
MESA was supported by contracts NO1-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. Dr. Shah is supported by an American Heart Association Post-Doctoral Fellowship Award (11POST000002) and a training grant from the Heart Failure National Institutes of Health Clinical Research Network (U01-HL084877). Dr. Jerosch-Herold receives support through R01-HL-65580. All other authors have no financial disclosures relevant to the content of this manuscript.
ABBREVIATIONS
- LV
left ventricular
- CMR
cardiac magnetic resonance
- BMI
body mass index
- HOMA-IR
homeostatic model assessment of insulin resistance
- WHR
waist-to-hip ratio
- IFG
impaired fasting glucose
- NFG
normal fasting glucose
- MESA
Multi-Ethnic Study of Atherosclerosis
- PAI-1
plasminogen activator inhibitor-1
- HDL
high-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
There are no disclosures relevant to the content of this manuscript.
REFERENCES
- 1.Flegal K, Carroll M, Ogden C, Curtin L. Prevalence and Trends in Obesity among US Adults, 1999-2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Garza C, Pellikka P, Somers V, et al. Structural and Functional Changes in Left and Right Ventricles after Major Weight Loss Following Bariatric Surgery for Morbid Obesity. Am J Cardiol. 2010;105(4):550–556. doi: 10.1016/j.amjcard.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Spies C, Farzaneh-Far R, Na B, et al. Relation of Obesity to Heart Failure Hospitalization and Cardiovascular Events in Persons with Stable Coronary Heart Disease (from the Heart and Soul Study). Am J Cardiol. 2009;104(7):883–889. doi: 10.1016/j.amjcard.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Wong C, Marwick T. Obesity Cardiomyopathy: Pathogenesis and Pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4(8):436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 5.Wong C, Marwick T. Alterations in Myocardial Characteristics Associated with Obesity: Detection, Mechanisms, and Implications. Trends Cardiovasc Med. 2007;17(1):1–5. doi: 10.1016/j.tcm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Bays H. Adiposopathy Is “Sick Fat” a Cardiovascular Disease? JACC. 2011;57(25):2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho T, Goel K, Correa de Sa D, et al. Central Obesity and Survival in Subjects with Coronary Artery Disease: A Systematic Review of the Literature and Collaborative Analysis with Individual Subject Data. JACC. 2011;57(19):1877–86. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 8.Kip K, Marroquin O, Kelley D, et al. Clinical Importance of Obesity Versus the Metabolic Syndrome in Cardiovascular Risk in Women. Circulation. 2004;109(6):706–13. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 9.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased Heart Failure Risk in Normal-Weight People with Metabolic Syndrome Compared with Metabolically Healthy Obese Individuals. JACC. 2011;58(13):1343–50. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of Body Mass Index and the Metabolic Syndrome on the Risk of Cardiovascular Disease and Death in Middle-Aged Men. Circulation. 2010;121(2):230–6. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 11.Ammar K, Redfield M, Mahoney D, Johnson M, Jacobsen S, Rodeheffer R. Central Obesity: Association with Left Ventricular Dysfunction and Mortality in the Community. American Heart Journal. 2008;156(5):975–81. doi: 10.1016/j.ahj.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libhaber C, Norton G, Majane O, et al. Contribution of Central and General Adiposity to Abnormal Left Ventricular Diastolic Function in a Community Sample with a High Prevalence of Obesity. Am J Cardiol. 2009;104(11):1527–33. doi: 10.1016/j.amjcard.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Bild D, Bluemke D, Burke G, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Arnett D, McClelland R, Bank A, et al. Biomarkers of Inflammation and Hemostasis Associated with Left Ventricular Mass: MESA. International Journal of Molecular Epidemiology and Genetics. 2011;2(4):391–400. [PMC free article] [PubMed] [Google Scholar]
- 15.Heckbert S, Post W, Pearson G, et al. Traditional Cardiovascular Risk Factors in Relation to Left Ventricular Mass, Volume, and Systolic Function by Cardiac Magnetic Resonance Imaging: MESA. JACC. 2006;48(11):2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan R, Bluemke D, Gomes A, et al. Regional Left Ventricular Myocardial Dysfunction as a Predictor of Incident Cardiovascular Events: MESA. JACC. 2011;57(17):1735–44. doi: 10.1016/j.jacc.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen B, Edvardsen T, Lai S, et al. Left Ventricular Concentric Remodeling Is Associated with Decreased Global and Regional Systolic Function: MESA. Circulation. 2005;112(7):984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 18.Tison G, Blaha M, Budoff M, et al. The Relationship of Insulin Resistance and Extracoronary Calcification in MESA. Atherosclerosis. 2011;218(2):507–10. doi: 10.1016/j.atherosclerosis.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calori G, Lattuada G, Piemonti L, et al. Prevalence, Metabolic Features, and Prognosis of Metabolically Healthy Obese Italian Individuals: the Cremona Study. Diabetes Care. 2011;34(1):210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Obesity: Preventing and Managing the Global Epidemic. Publication WHO/NUT/NCD/98.1. [PubMed] [Google Scholar]
- 21.Nov O, Kohl A, Lewis E, et al. Interleukin-1beta May Mediate Insulin Resistance in Liver-Derived Cells in Response to Adipocyte Inflammation. Endocrinology. 2010;151(9):4247–56. doi: 10.1210/en.2010-0340. [DOI] [PubMed] [Google Scholar]
- 22.Zaman A, French C, Schneider D, Sobel B. A Profibrotic Effect of Plasminogen Activator Inhibitor Type-1 (PAI-1) in the Heart. Experimental Biology and Medicine. 2009;234(3):246–54. doi: 10.3181/0811-RM-321. [DOI] [PubMed] [Google Scholar]
- 23.Kalogeropoulos A, Georgiopoulou V, Psaty B, et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults: The Health ABC Study. JACC. 2010;55(19):2129–37. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marfella R, Grella R, Rizzo M, et al. Role of Subcutaneous Abdominal Fat on Cardiac Function and Proinflammatory Cytokines in Premenopausal Obese Women. Annals of Plastic Surgery. 2009;63(5):490–5. doi: 10.1097/SAP.0b013e3181955cdb. [DOI] [PubMed] [Google Scholar]
- 25.Farb M, Bigornia S, Mott M, et al. Reduced Adipose Tissue Inflammation Represents an Intermediate Cardiometabolic Phenotype in Obesity. JACC. 2011;58(3):232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric Surgery and Long-Term Cardiovascular Events. JAMA. 307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 27.Kosmala W, Jedrzejuk D, Derzhko R, et al. Left Ventricular Function Impairment in Patients with Normal-Weight Obesity: Contribution of Abdominal Fat Deposition, Profibrotic State, Reduced Insulin Sensitivity, and Proinflammatory Activation. Circulation: Cardiovascular Imaging. 2012;5(3):349–56. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 28.Shoelson S, Herrero L, Naaz A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology. 2007;132(6):2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Shoelson S, Lee J, Goldfine A. Inflammation and Insulin Resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Hernandez A, Otero Y, de las Heras N, et al. Brown Fat Lipoatrophy and Increased Visceral Adiposity through a Concerted Adipocytokines Overexpression Induces Vascular Insulin Resistance and Dysfunction. Endocrinology. 2012;153(3):1242–55. doi: 10.1210/en.2011-1765. [DOI] [PubMed] [Google Scholar]
- 31.Bahrami H, Bluemke D, Kronmal R, et al. Novel Metabolic Risk Factors for Incident Heart Failure and Their Relationship with Obesity: MESA. JACC. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Turkbey E, McClelland R, Kronmal R, et al. The Impact of Obesity on the Left Ventricle: MESA. JACC: Cardiovascular Imaging. 2010;3(3):266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertoni A, Goff D, D'Agostino R, et al. Diabetic Cardiomyopathy and Subclinical Cardiovascular Disease: MESA. Diabetes Care. 2006;29(3):588–94. doi: 10.2337/diacare.29.03.06.dc05-1501. [DOI] [PubMed] [Google Scholar]
- 34.Bluemke D, Kronmal R, Lima J, et al. The Relationship of Left Ventricular Mass and Geometry to Incident Cardiovascular Events: MESA. JACC. 2008;52(25):2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velagaleti R, Gona P, Chuang M, et al. Relations of Insulin Resistance and Glycemic Abnormalities to Cardiovascular Magnetic Resonance Measures of Cardiac Structure and Function: The Framingham Heart Study. Circulation: Cardiovascular Imaging. 2010;3(3):257–63. doi: 10.1161/CIRCIMAGING.109.911438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Decode Study Group European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe. Glucose Tolerance and Mortality: Comparison of WHO and American Diabetes Association Diagnostic Criteria. Lancet. 1999;354(9179):617–621. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.