Abstract
Objective
The phenotypic modulation of vascular smooth muscle cells (VSMCs) to a synthetic phenotype is vital during pathological vascular remodeling and the development of various vascular diseases. An increase in type I collagen (collagen I) has been implicated in synthetic VSMCs, and cyclic nucleotide signaling is critical in collagen I regulation. Herein, we investigate the role and underlying mechanism of cyclic nucleotide phosphodiesterase 1 (PDE1) in regulating collagen I in synthetic VSMCs.
Methods and Results
The PDE1 inhibitor IC86340 significantly reduced collagen I in human saphenous vein explants undergoing spontaneous remodeling via ex vivo culture. In synthetic VSMCs, high basal levels of intracellular and extracellular collagen I protein were markedly decreased by IC86340. This attenuation was due to diminished protein but not mRNA. Inhibition of lysosome function abolished the effect of IC86340 on collagen I protein expression. PDE1C but not PDE1A is the major isoform responsible for mediating the effects of IC86340. Bicarbonate-sensitive soluble adenylyl cyclase/cAMP signaling was modulated by PDE1C, which is critical in collagen I degradation in VSMCs.
Conclusion
These data demonstrate that PDE1C regulates soluble adenylyl cyclase/cAMP signaling and lysosome-mediated collagen I protein degradation, and they suggest that PDE1C plays a critical role in regulating collagen homeostasis during pathological vascular remodeling.
Keywords: vascular smooth muscle cell, phosphodiesterase, collagen, lysosome, soluble adenylyl cyclase
During physiological states, vascular smooth muscle cells (VSMCs) residing in the media layer are quiescent and contractile. Their principal function is to maintain vascular tone. In response to biological and mechanical injury, VSMCs exhibit phenotypic plasticity and undergo modulation from a quiescent/contractile phenotype to an active synthetic one.1 Synthetic VSMCs contribute to vascular remodeling and dysfunction by downregulating contractile proteins and acquiring the capacity to proliferate, migrate, and produce extracellular matrix (ECM) proteins. Therefore, synthetic VSMCs play a key role in the pathogenesis of cardiovascular disorders such as atherosclerosis, postangioplasty restenosis, bypass vein graft failure, and cardiac allograft vasculopathy. Elucidating molecules that control the phenotypic changes may be critical to circumvent pathological vascular remodeling.
The major components of the ECM of the vessel wall are collagens.2 Several genetically distinct collagens are present in the vessel wall, including collagen types I, III, IV, V, and VIII.2 In normal vascular tissue, collagens play important roles in maintaining vascular structural integrity, regulating vascular mechanical properties (such as extensibility and stiffness), and regulating cellular function through receptor mediated cell-collagen interaction.2 Synthetic VSMCs, in the atherosclerotic and neointimal lesions, produce abundant ECM, particularly type I collagen (collagen I). The ECM, together with cellular components in the lesions, is responsible for vessel wall thickening and eventual occlusion of the vessel lumen. In addition, collagen I in vascular lesions may also regulate VSMC proliferation/migration, monocyte activation, platelet circulation, lipid accumulation, calcification, and plaque stability.2
cAMP and cGMP have a variety of biological effects in VSMCs, such as promoting VSMC relaxation and inhibiting VSMC proliferation, migration, and ECM synthesis. cAMP-mediated signaling has been shown to inhibit agonist-stimulated collagen I synthesis in smooth muscle cells.3,4 Cyclic nucleotide phosphodiesterases (PDEs), by catalyzing the hydrolysis of cAMP and cGMP, regulate the amplitude, duration, and compartmentalization of intracellular cyclic nucleotide signaling. To date, more than 60 different isoforms have been identified and grouped into 11 broad families by differences in structure, kinetic and regulatory properties, and sensitivity to chemical inhibitors.5 The Ca2+/calmodulin-stimulated PDE1 family enzymes are encoded by 3 distinct genes, PDE1A, PDE1B, and PDE1C. Both PDE1A and PDE1C have been previously shown to regulate synthetic VSMC growth.6,7 In the present study, we interrogated the role and underlying mechanism of PDE1 isozymes in regulating collagen I in synthetic VSMCs and defined a novel mechanism by which PDE1C/cAMP signaling regulates collagen I protein degradation through a lysosome-dependent mechanism.
Materials and Methods
IC86340 was provided by ICOS Corp, and the primary antibody against collagen I (LF-67) was kindly provided by Dr Fisher (National Institutes of Health, Bethesda, MD). Rat aortic VSMCs were prepared using enzymatic digestion of aortas as previously described.6 VSMCs (passages 7 to 12) were used for the experiments. Human saphenous veins (SVs), not required for surgery, were collected from patients after coronary artery bypass surgery. SVs were cultured for 7 days as previously described.8 The medium and drug were changed every other day.
An expanded Methods section is available in the online supplement (http://atvb.ahajournals.org).
Results
Effects of PDE1 Inhibition on Collagen I Protein Levels in Human SV Explants
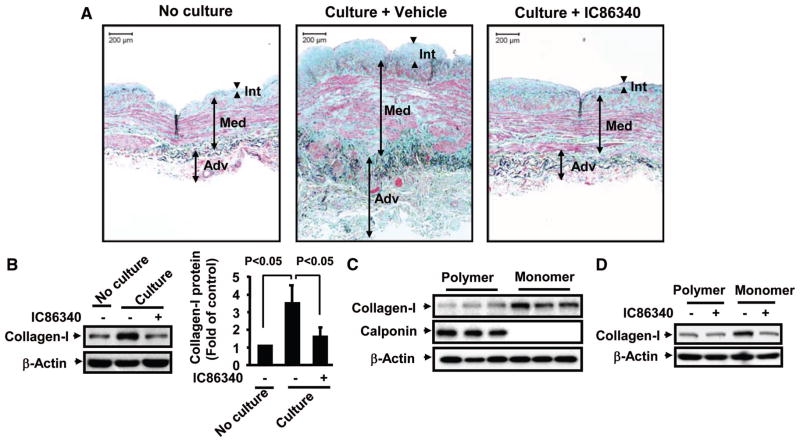
Human SV is the most commonly used vessel to bypass blocked coronary arteries; however, late vein graft failure occurs because of the development of stenosis or occlusion.9 When human SVs are cultured in vitro, they spontaneously undergo remodeling, which predominantly involves smooth muscle cell growth and ECM deposition.10 As shown in Figure 1A (left panel), the SV wall can be divided into 3 zones, the internal zone (intima), the medial zone (inner and outer media), and the external zone (inner and outer adventitia).11,12 With Verhoeff Masson Trichrome combination staining, smooth muscles are stained with red, collagen with light blue or blue-green, and elastin with dark blue. When SVs were cultured in vitro for 1 week, SV remodeling occurred, revealing a larger amount of collagen and elastic fiber deposition and a less organized smooth muscle (Figure 1A, middle panel). This led to an increased thickness of the SV vessel wall. We found that collagen deposition was significantly reduced in human SV by the PDE1 inhibitor IC86340, accompanied by a significant reduction of remodeling (Figure 1A, right panel). Consistently, collagen I protein levels measured by Western blotting were increased in cultured SV compared with the same SV before culture. PDE1 inhibitor IC86340 significantly attenuated the increased collagen I protein levels (Figure 1B). According to the IC50 values of IC86340 on inhibiting different PDE family members (Supplemental Table), the doses of IC86340 used in this study should preferentially inhibit PDE1 isozymes in vascular cells. These results suggest that PDE1 is involved in regulating collagen I during SV remodeling. To further demonstrate the effects of PDE1 inhibition on collagen I accumulation in neointimal formation in vivo, we performed immunostaining of collagen I in a mouse arterial injury model induced by flow-cessation via carotid ligation.4 As shown in Supplemental Figure I, collagen I staining intensities are increased in the neointimal regions of the injured vessel, which is significantly reduced by PDE1 inhibitor IC86340.
Figure 1.
Effects of PDE1 inhibition on collagen I protein levels in human SV explants and VSMCs cultured on polymerized or monomeric collagen matrix. A, Images of human SVs. Sections of human SVs, before or after culture for 7 days in the presence of vehicle or 30 μmol/L IC86340, were subjected to Verhoeff Masson Trichrome combination staining. Int indicates intima; Med: media; Adv: adventitia. B, Western blotting showing IC86340 decreased collagen I expression in cultured human SV. Collagen I protein levels were detected by Western blotting using collagen I–specific antibody (kindly provided by Dr Fisher, National Institutes of Health). Band intensities were quantified, and results are shown as mean±SD (n=6). C, Collagen I protein levels in VSMCs cultured on polymerized collagen matrix or on monomeric collagen matrix. D, The effect of IC86340 on collagen I expression. After being cultured on polymerized or monomeric collagen with vehicle or 15 μmol/L IC86340 for 48 hours, cells were harvested, and the expression of collagen I and smooth muscle calponin was examined by Western blotting.
Upregulation of Collagen I Protein in Synthetic VSMCs
VSMCs in vitro show distinct phenotypes in response to different types of extracellular matrices. When cultured on dishes coated with polymerized collagen, VSMCs elicit a “contractile”-like phenotype and mimic many characteristics of VSMCs in the normal medial layer in vivo.13 In contrast, VSMCs cultured on monomer collagen or noncoated plastic dishes have synthetic phenotype and retain many properties of VSMCs in developing vascular lesions.13 We thus compared collagen I protein levels in contractile-like and synthetic VSMCs (Figure 1C). As expected, we observed that contractile-like VSMCs (on polymerized collagen) expressed much higher levels of contractile marker proteins, such as smooth muscle calponin, compared with synthetic VSMCs (on monomeric collagen). In contrast, the collagen I protein levels were much higher in the synthetic VSMCs, consistent with the previous finding that synthetic rabbit VSMCs have significantly higher collagen I than contractile VSMCs.14 Interestingly, PDE1 inhibition blocked collagen I upregulation in synthetic VSMCs (Figure 1D), further supporting the role of PDE1 in the regulation of collagen I in synthetic VSMCs.
Mechanism of Collagen I Regulation by PDE1 in Synthetic VSMCs
As shown in Supplemental Figure II, synthetic VSMCs had a high basal level of collagen I. Stimulation with serum or transforming growth factor-β further increased collagen I protein (Supplemental Figure IIA) and mRNA (Supplemental Figure IIB). Interestingly, PDE1 inhibitor IC86340 inhibited not only agonist-stimulated collagen I protein but also the basal collagen I protein to a great extent (Supplemental Figure IIA). In contrast, inhibiting PDE1 reduced only the amount of agonist-stimulated collagen I mRNA, not basal mRNA levels (Supplemental Figure IIB). These observations suggest that PDE1 regulates both basal and agonist-stimulated collagen I in synthetic VSMCs, very likely via distinct mechanisms. For instance, PDE1 regulates the basal collagen I at the protein level and the agonist-stimulated collagen I at the mRNA level. Because the role of cyclic nucleotide-mediated signaling in agonist-stimulated collagen I expression is well described,15,16 in this study we specifically focused on the function and underlying mechanism of PDE1 in regulating phenotype-associated basal collagen I in synthetic VSMCs.
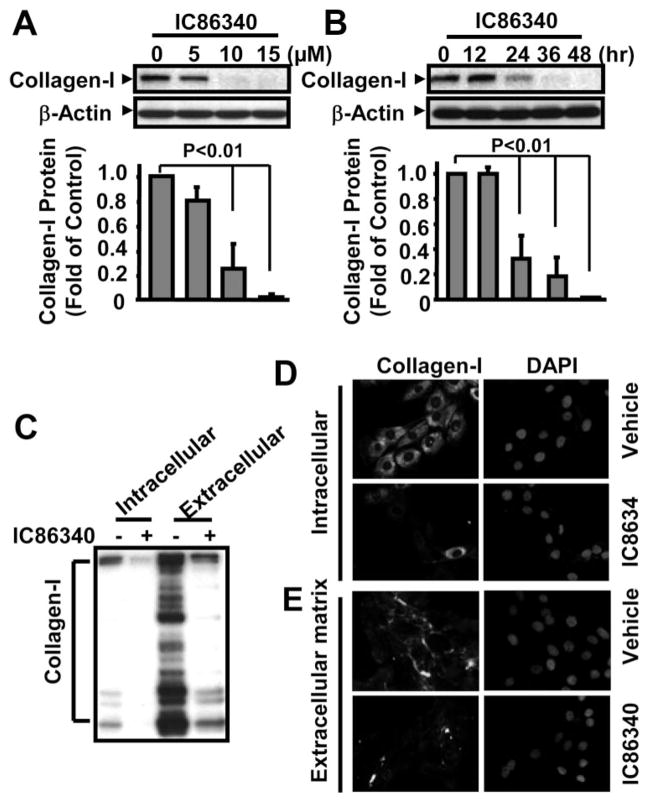
Using synthetic VSMCs in the absence of stimulation, we found that PDE1 inhibitor IC86340 caused a significant decrease in intracellular procollagen I protein levels in a dose- and time-dependent manner (Figure 2A and 2B). Because collagen I is a secretory protein and the major component of interstitial connective tissue, we also analyzed extracellular collagen I. As shown in Figure 2C, the extracellular secreted collagen I protein in the culture medium was much higher than intracellular collagen I. There were also large amounts of cleaved collagen I fragments in the extracellular fraction, consistent with reports stating that extracellular collagen I undergoes fragmentation via free radicals and proteinases.17 We consistently observed concomitantly reduced intracellular and extracellular collagen I (Figure 2C). Immunofluorescent staining also revealed that the collagen I staining intensities are decreased by IC86340 in both intracellular (Figure 2D) and extracellular space (Figure 2E). Taken together, these results provide support that PDE1 plays a critical role in regulating collagen I protein levels in synthetic VSMCs.
Figure 2.
PDE1 inhibitor IC86340 decreased collagen I protein levels. A and B, IC86340 dose-dependently (A) and time-dependently (B) decreased collagen I expression. Rat aortic VSMCs were plated for 24 hours and then treated with different doses of IC86340 for 24 hours or treated with vehicle or 15 μmol/L IC86340 for the indicated times. C, Western blot showing that IC86340 decreased both intracellular and extracellular secreted collagen I levels. VSMCs in the serum-free medium were treated with 15 μmol/L IC86340 for 24 hours. Collagen I protein levels in cell lysates or in culture medium were determined by Western blotting. Band intensities were quantified and values are mean±SD of 3 independent experiments. D and E, Immunostaining images showing that IC86340 decreased both intracellular (D) and ECM (E) collagen I. For staining ECM collagen I, the permeabilization step was omitted.
Role of Lysosome in PDE1-Mediated Regulation of Collagen I Protein Levels
To further confirm that the basal collagen I protein reduction by PDE1 inhibition is not due to decreased collagen I gene expression, we measured mRNA levels by reverse transcription–polymerase chain reaction. As expected, basal collagen I mRNA levels were not significantly altered by IC86340 (Supplemental Figure IIIA). We next determined whether IC86340 treatment causes proteasome-mediated collagen degradation and found that the proteasome inhibitor MG132 did not significantly influence IC86340-mediated collagen I protein reduction (Supplemental Figure IIIB). This suggests that a proteasome-dependent mechanism is likely not involved.
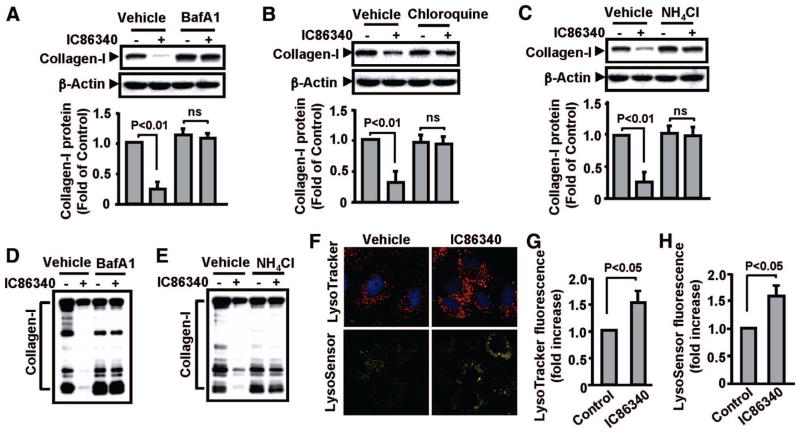
Because lysosome-dependent degradation of collagen I is an important mechanism in fibroblast-like cells,17 we determined the role of lysosomes in IC86340-mediated regulation of collagen I in VSMCs. Within lysosomes, digestive enzymes function in an acidic condition (around pH 5.0), which is maintained by vacuolar-type H(+)-ATPase (V-ATPase). Therefore, the V-ATPase inhibitor or lysosomal pH neutralizer is commonly used to inhibit lysosome function. As shown in Figure 3A, bafilomycin A1 (a specific inhibitor of the vacuolar type H(+)-ATPase) significantly blocked intracellular collagen I reduction by IC86340. Similarly, chloroquine and NH4Cl (neutralizing lysosomal pH and thus decreasing the lysosomal function) also prevented intracellular collagen I reduction (Figure 3B and 3C). The reduction of extracellular collagen I levels was also blocked by lysosome inhibitor bafilomycin A1 (Figure 3D) and NH4Cl (Figure 3E). A similar observation was also made in human VSMCs (Supplemental Figure IIIC). These results suggest that PDE1 inhibition stimulates lysosome-mediated degradation of collagen I, which leads to a decrease of intracellular and extracellular collagen I protein levels.
Figure 3.
Lysosome inhibitors blocked IC86340-mediated collagen I reduction. A to C, Effects of lysosome inhibitors on intracellular collagen I protein. Rat aortic VSMCs were pretreated with vehicle or lysosome inhibitor 50 nmol/L bafilomycin A1 (A), 40 μmol/L chloroquine diphosphate (B), or 10 mmol/L NH4Cl (C) for 30 minutes, followed by treatment with vehicle or 15 μmol/L IC86340 for 24 hours. Intracellular collagen I expression was analyzed by Western blotting. Band intensities were quantified, and values are mean±SD of 3 independent experiments. D and E, Effects of lysosome inhibitors on extracellular secreted collagen I. Cells were treated in a manner similar to the procedures described above except using serum-free medium. F to H, Effects of PDE1 inhibition on lysosome-like organelles. Cells were treated with vehicle or 15 μmol/L IC86340 for 24 hours, labeled with LysoTracker Red DND-99 or LysoSensor Yellow/Blue DND-160, and imaged with a confocal microscope as described in Supplemental Methods.
To determine the role of PDE1 on lysosome regulation, we first performed cytochemical analysis of lysosomes with LysoTracker Red DND-99, a fluorescent acidtropic probe for labeling and tracking acidic organelles in live cells. We found that the number and fluorescent intensity of lysosome-like organelles in the perinuclear regions of VSMCs are significantly increased on PDE1 inhibition (Figure 3F and 3G). In addition, we also analyzed a LysoSensor Yellow/Blue DND-160, a fluorescent pH indicator used to detect active lysosomes. We observed a similar increase of fluorescence intensity in the presence of IC86340 (Figure 3F and 3H). These observations suggest that PDE1 may regulate lysosome function in an as-yet-unknown manner.
Role of cGMP-Dependent Protein Kinase, cAMP-Dependent Protein Kinase, and Exchange Protein Activated by cAMP in PDE1-Mediated Regulation of Collagen I Protein
PDE1 isozymes are dual specificity enzymes, which hydrolyze both cAMP and cGMP in vitro. We then examined the effects of several common cyclic nucleotide effector molecules on PDE1 inhibition-mediated collagen I reduction, including cGMP-dependent protein kinase (PKG), cAMP-dependent protein kinase (PKA), and exchange protein activated by cAMP (Epac). As shown in Supplemental Figure IVA, when PKG protein levels were significantly downregulated with 2 different PKG-I short interfering RNA (siRNA) duplexes, the effects of IC86340 on collagen I protein were not altered. The fact that downregulation of PKG by siRNA blocked PKG-mediated VASP Ser239 phosphorylation (Supplemental Figure IVB) indicates that the VSMCs have functional PKG. We obtained very similar results when using the PKG inhibitor DT-3 or Rp-8-Br-PET-cGMPs to inhibit PKG activity (Supplemental Figure IVC). These results suggest that activation of PKG is not involved in PDE1-mediated regulation of collagen I protein degradation.
We next examined the role of PKA by expressing the endogenous PKA inhibitor protein called PKI. As shown in Supplemental Figure VA, adenoviral mediated PKI expression did not significantly alter IC86340-mediated reduction of collagen I compared with control or LacZ expression. However, expression of PKI inhibited PKA-dependent phosphorylation of Ser157 of VASP induced by cAMP analog Sp-8-CPT-cAMPs (Supplemental Figure VB), suggesting that PKI is functional in blocking PKA activity.
Because cAMP can also activate Epac, we examined the effects of Epac stimulation and inhibition on the ability of IC86340 to reduce collagen I protein. We found 2 Epac-specific activators, 8-CPT-2′-O-Me-cAMP and 8-pCPT-2′-O-Me-cAMP-AM, neither altered collagen I reduction in response to IC86340 (Supplemental Figure VIA). In addition, knocking down Epac1 using siRNA had no effect on IC86340-mediated collagen I reduction (Supplemental Figure VIB and VIC). These data suggest that Epac1 is also not likely involved.
Role of Cyclic Nucleotide Gated Channel in PDE1-Mediated Regulation of Collagen I Protein
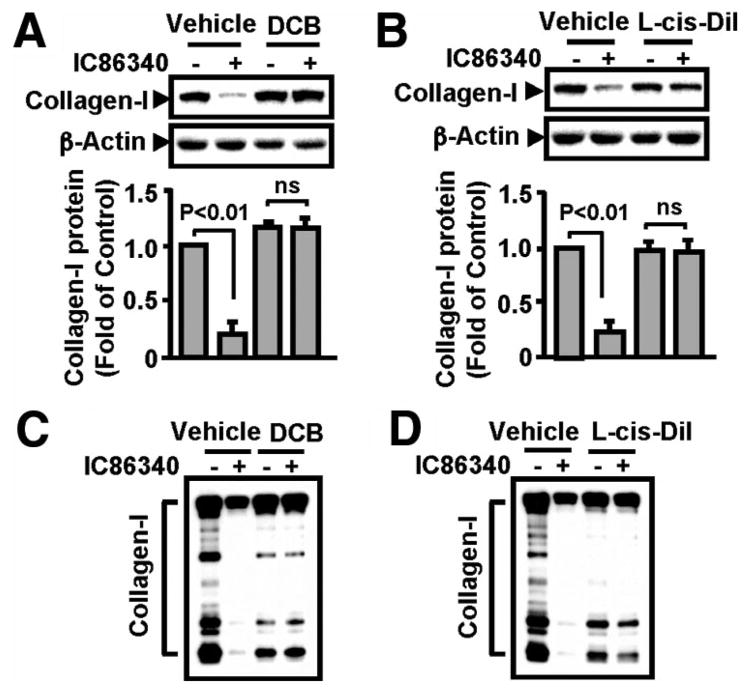
cAMP and cGMP can activate cyclic nucleotide gated (CNG) channels leading to intracellular Ca2+ elevation,18 and Ca2+ might be important for lysosome-mediated degradation.19,20 CNGs have been reported in vascular cells; however, their functions in vasculature are unclear.21,22 Therefore, we investigated the potential involvement of CNG using pharmacological CNG inhibitors. As shown in Figure 4A, 2′,4′-dichlorobenzamil, a nonselective CNG channel blocker, prevented the effect of IC86340 on collagen I reduction. In addition, another, more specific CNG channel blocker, L-cis-diltiazem, almost completely reversed the effect of IC86340 on collagen I (Figure 4B). The fact that 2 very different chemical inhibitors had similar results in IC86340-mediated collagen I reduction strongly supports the role of CNG channels in the regulation of collagen I degradation. Consistently, CNG channel blockers also abolished the effects of IC86340 on extracellular collagen I (Figure 4C and 4D).
Figure 4.
Inhibition of CNG channels blocked IC86340-mediated collagen I reduction. VSMCs were pretreated with vehicle or CNG channels inhibitors 50 μmol/L L-cis-diltiazem (A) or 5 μmol/L 2′,4′-dichlorobenzamil (B) for 30 minutes, followed by treatment of 15 μmol/L IC86340 for 24 hours. The level of collagen I protein was detected by Western blotting. Band intensities were quantified and values are mean±SD of 3 independent experiments. C and D, Effects of CNG channel inhibitors on extracellular collagen I. Cells were treated in a manner similar to the procedures described above except using serum-free Dulbecco’s modified Eagle’s medium.
Role of Bicarbonate-Sensitive Soluble Adenylyl Cyclase in PDE1-Mediated Regulation of Collagen I Protein
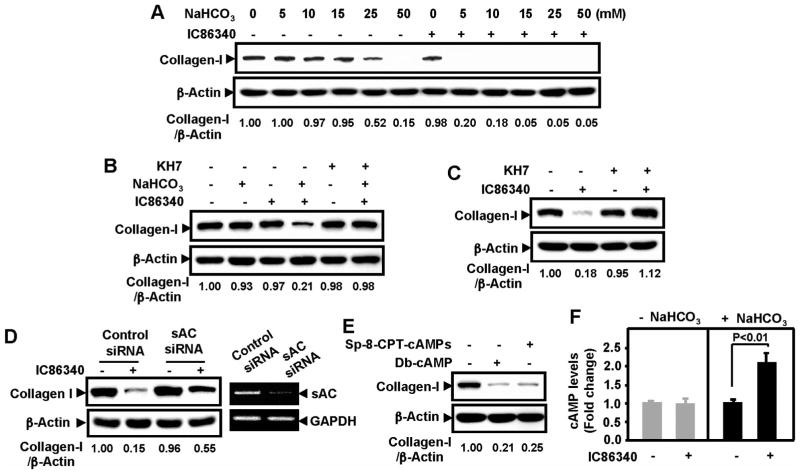
Bicarbonate-sensitive soluble adenylyl cyclase (sAC) has been shown to regulate V-ATPase in germ cells and renal epithelial cells.23,24 Given that V-ATPase is critical for lysosome function, we determined the role of PDE1 in the regulation of sAC signaling and collagen I reduction. We found that sAC stimulus with bicarbonate at low doses (5 and 10 μmol/L) did not affect collagen I protein, whereas high doses of bicarbonate (25 and 50 mmol/L) gradually reduced collagen I protein (Figure 5A). In the absence of bicarbonate, 5 μmol/L of IC86340 itself had no effect on collagen I protein. Interestingly, bicarbonate and IC86340 together synergistically reduced collagen I protein. The synergistic effect of bicarbonate and IC86340 was blocked by the sAC inhibitor KH7 (Figure 5B). In addition, in the normal bicarbonate-containing medium, the effect of IC86340 on collagen reduction was diminished by KH7 (Figure 5C). Similar effects of KH7 on IC86340-mediated regulation of collagen I protein were also observed in cultured human VSMCs (Supplemental Figure VIIA), as well as in human SV explants cultured ex vivo (Supplemental Figure VIIB). These results suggest that the effect of PDE1 inhibitor IC86340 on collagen I reduction likely require the activation of sAC. We detected sAC expression in VSMCs via reverse transcription–polymerase chain reaction. As shown in Supplemental Figure VIIIA, rat aortic VSMCs express sAC at a level that is comparable to PC12 cells, in which sAC has been shown to be functionally important.25,26 To specifically target sAC, we used sAC siRNA. We found that downregulation of sAC with sAC siRNA (Figure 5D, right) significantly attenuated the effects of IC86340 on collagen I protein, similar to KH7 (Figure 5D, left).
Figure 5.
Role of bicarbonate-sensitive sAC in PDE1-mediated regulation of collagen I protein. A, Synergistic effect of bicarbonate and PDE1 inhibitor IC86340 on collagen I protein reduction. Rat aortic VSMCs were preincubated in bicarbonate-free medium overnight and then stimulated with various doses of bicarbonate as indicated in the presence of vehicle or a low concentration of IC86340 (5 μmol/L) for 24 hours. B, Effects of the sAC inhibitor KH7 on bicarbonate- and IC86340-induced collagen I reduction. VSMCs precultured in bicarbonate-free medium were treated with or without 15 mmol/L bicarbonate, 5 μmol/L IC86340, or both in the presence or absence of 10 μmol/L KH7. C, Effects of KH7 on IC86340-induced collagen I reduction in VSMCs cultured in normal bicarbonate-containing medium. VSMCs cultured in normal bicarbonate-containing medium were treated with vehicle or 15 μmol/L IC86340 in the presence of vehicle or 10 μmol/L KH7. D, Effects of sAC siRNA on IC86340-induced collagen I reduction. VSMCs were transfected with sAC siRNA for 2 days and treated with vehicle or 15 μmol/L IC86340 in normal bicarbonate-containing medium. sAC mRNA levels were measured by reverse transcription–polymerase chain reaction. E, Effects of cAMP analogs on collagen I protein reduction. VSMCs were treated with vehicle, 250 μmol/L Sp-8-CPT-cAMPs, or 500 μmol/L Db-cAMP. Levels of collagen I were analyzed by Western blotting. Equal loading was confirmed by immunoblotting for β-actin. The blots were analyzed by densitometry. Fold changes normalized to the left lane are shown below the blots (n=2 to 3). F, Effect of PDE1 inhibition on intracellular cAMP. VSMCs precultured in bicarbonate-free medium were stimulated with or without 15 mmol/L bicarbonate for 10 minutes in the presence of vehicle or 15 μmol/L IC86340. Intracellular cAMP levels measured using a cAMP radioimmunoassay. Values are mean±SD (n=4).
To further confirm the involvement of cAMP, we used nonhydrolyzable cAMP analogs. We found that 2 different cAMP analogs, Db-cAMP and Sp-8-CPT-cAMPs, significantly reduced collagen I protein levels, similar to the PDE1 inhibitor IC86340 (Figure 5E). In contrast, a cGMP analog (8-pCPT-cGMP) and a nitric oxide donor (S-nitroso-N-acetylpenicallamine) had no effect on collagen I protein (Supplemental Figure VIIIB). These data together suggest that cAMP but not cGMP regulates collagen I protein in VSMCs, consistent with the role of sAC.
To examine the effect of PDE1 inhibition on intracellular cAMP, we analyzed cellular cAMP via radioimmunoassay. We found that without bicarbonate stimulation, IC86340 did not significantly change intracellular cAMP levels (Figure 5F), which is consistent with our previous observation that IC86340 does not significant alter basal cAMP in VSMCs.6 However, with bicarbonate stimulation, IC86340 significantly elevated cAMP levels (Figure 5F).
These results together suggest that activation of sAC/cAMP signaling is essential for collagen I degradation. PDE1 may represent a major PDE activity that terminates sAC/cAMP signaling. Inhibition of PDE1 potentiates sAC/cAMP signaling and synergistically promotes collagen I degradation.
PDE1C but Not PDE1A Regulates Collagen I Protein Degradation in VSMCs
Both PDE1A and PDE1C were detected in growing rat aortic and human SV VSMCs, whereas PDE1B was minimally detected (Supplemental Figure IXA and IXB). To determine the specific function of PDE1 isoform in the regulation of collagen I protein degradation, we used isoform-specific short hairpin RNA (shRNA) via the adenoviral expression system. As expected, PDE1A and PDE1C shRNA selectively knocked down PDE1A and PDE1C gene expression, respectively (Supplemental Figure IXC). However, PDE1C shRNA but not PDE1A shRNA significantly reduced the collagen I protein level (Figure 6A). Consistent with IC86340, basal collagen I mRNA was not affected by PDE1A and PDE1C shRNA (Supplemental Figure IXD). In addition, similar to IC86340, the effect of PDE1C shRNA on collagen I protein was blocked by the lysosome inhibitor bafilomycin A1 or NH4Cl (Figure 6B), in the absence of sAC activator bicarbonate (Figure 6C), or in the presence of sAC inhibitor KH7 (Figure 6D). Consistent with the role of PDE1C in synthetic VSMCs, we observed an upregulation of PDE1C expression in VSMCs cultured on monomeric collagen relative to polymerized collagen (Supplemental Figure IXE), and in cultured human SV explants as compared with noncultured explants (Supplemental Figure IXF). These results demonstrate that PDE1C but not PDE1A inhibition contributed to the effect of IC86340 on collagen I reduction in synthetic VSMCs.
Figure 6.

Downregulation of PDE1C but not PDE1A decreases collagen I protein. A, The effect of PDE1A shRNA and PDE1C shRNA on collagen I protein. VSMCs were transduced without or with Ad-LacZ shRNA, 100 multiplicity of infection Ad-PDE1A shRNA, or Ad-PDE1C shRNA for 72 hours. Collagen I protein was evaluated by Western blotting. Band intensities were quantified and values are mean±SD of at least 3 independent experiments. B, Effects of lysosome inhibitors on PDE1C shRNA-induced collagen I protein reduction. Virus-transduced VSMCs were treated with or without 50 nmol/L bafilomycin A1 or 10 mmol/L NH4Cl for 24 hours. C, Effects of bicarbonate on PDE1C shRNA-induced collagen I protein reduction. Virus-transduced VSMCs were precultured in bicarbonate free medium overnight, followed with or without 25 mmol/L bicarbonate treatment for 24 hours. D, Effects of the sAC inhibitor KH7 on PDE1C shRNA-induced collagen I protein reduction. Virus-transduced VSMCs were cultured in normal bicarbonate-containing medium for 48 hours and then treated with vehicle or 10 μmol/L KH7 for 24 hours. Collagen I protein was analyzed by Western blotting. The blots were analyzed by densitometry. Fold changes normalized to the left lane are shown below the blots (n=2 to 3).
Discussion
In this study, we demonstrated that synthetic VSMCs associate with a high basal level of collagen I. PDE1 plays a critical role in regulating the phenotype-associated basal collagen I protein level via modulating lysosome-mediated collagen I protein degradation. Besides agonist-stimulated collagen I gene expression, our finding suggests that regulation of lysosome-mediated collagen I protein degradation represents one of the important mechanisms for controlling collagen I homeostasis in synthetic VSMC. Both PDE1A and PDE1C are expressed in synthetic VSMCs and have been shown to regulate VSMC growth.6,7 However, here we demonstrate that PDE1C but not PDE1A is specifically involved in lysosome-mediated collagen I degradation. Together with our unpublished observation that PDE1A but not PDE1C specifically regulates agonist-stimulated collagen I mRNA expression in cardiac myofibroblasts (Miller C, Dikawa M, Cai Y, Yan C, unpublished data, 2010), these data suggest that PDE1A and PDE1C differentially regulate collagen I, probably through modulating distinctly compartmentalized cyclic nucleotide pools.
In addition, we also demonstrate that the effect of PDE1 inhibition on collagen I degradation appears to involve CNG channel activation. The expression of CNGs has been reported in endothelial cells and VSMCs in vitro and in vivo21,22; however the function of these channels in the vasculature is unknown. Our finding of CNG in collagen I degradation indicates for the first time that the CNG channel(s) may play important roles in regulating the nonsensory function. Although the mechanism by which CNG channel(s) involves lysosome-mediated collagen I degradation remains to be investigated, it is known that CNG channel activation leads to intracellular Ca2+ elevation,18 and Ca2+ might be critical in phagosome-lysosome fusion19 and lysosome exocytosis.20 Finally, we demonstrated that the activation of sAC/cAMP signaling is essential for collagen I degradation. PDE1C specifically regulates sAC/cAMP signaling, which identifies PDE1C as the first PDE isoform shown to modulate sAC/cAMP signaling. Taken together, our findings reveal a novel mechanism for the regulation of collagen I in synthetic VSMCs by PDE1C/cAMP through lysosome-dependent degradation of collagen I protein (Supplemental Figure XI) and imply that PDE1C is an important regulator of collagen I metabolism and a potential therapeutic target for vascular remodeling. It should be noted that despite these novel findings, how sAC-PDE1C-cAMP signaling regulates lysosome function through CNG channels remains to be investigated in the future.
Collagen I levels are controlled by both its biosynthesis and degradation. Biosynthesis of collagen I is regulated mainly at the transcription level by growth factors and cytokines.27 Accumulating evidence demonstrates that degradation of collagen I is an important process in both physiological (during developmental growth and wound healing) and pathological circumstances.17 For example, excessive collagen I degradation causes tissue destruction, whereas insufficient degradation leads to scar formation and fibrosis. Collagen I degradation can occur either before or after secretion from cells. The pathways for degradation of intracellular procollagen I molecules differ from those for extracellular fibrillar collagen I. The intracellular degradation before secretion can occur in 2 different sites: the Golgi apparatus and the lysosome.28 Also, secreted collagen I is degraded mainly by 2 consecutive routes: proteolytic and phagocytotic. Proteolytic degradation occurs mainly through matrix metalloproteinase–mediated cleavage.17 The resultant collagen I fragments are phagocytosed by cells29 and degraded in lysosomes within the cell.17 It is believed that during ECM remodeling, phagocytosis of collagen I fibrils by fibroblasts appears to be a continuous process.17 In addition to the intracellular lysosome, evidence has also revealed the existence of Ca2+-dependent exocytosis of lysosomes in many different tissues and cell types and that the extracellular lysosome enzymes contribute to degradation of connective tissues.30 Although matrix metalloproteinase–mediated cleavage appears to be important for collagen phagocytosis and degradation by lysosome,17 we found that the effect of PDE1 inhibition on collagen I degradation is unlikely through regulating matrix metalloproteinase (Supplemental Figure X). Taken together, the lysosome appears to be a central player for collagen I degradation, regardless of intracellular or extracellular mechanisms. This may provide an explanation for our findings that intracellular and extracellular collagen I is concomitantly regulated by the inhibitors of PDE1, CNG channel, and lysosome.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by grants from the National Institutes of Health (HL077789 and HL088400) and the American Heart Association (0740021N).
Footnotes
Disclosures
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–525. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and total protein synthesis in vascular smooth muscle cells. Hypertension. 1999;33:190–194. doi: 10.1161/01.hyp.33.1.190. [DOI] [PubMed] [Google Scholar]
- 4.Chen YM, Wu KD, Tsai TJ, Hsieh BS. Pentoxifylline inhibits PDGF-induced proliferation of and TGF-β-stimulated collagen synthesis by vascular smooth muscle cells. J Mol Cell Cardiol. 1999;31:773–783. doi: 10.1006/jmcc.1998.0910. [DOI] [PubMed] [Google Scholar]
- 5.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 6.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98:777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 8.Rey J, Probst H, Mazzolai L, Bosman FT, Pusztaszeri M, Stergiopulos N, Ris HB, Hayoz D, Saucy F, Corpataux JM. Comparative assessment of intimal hyperplasia development after 14 days in two different experimental settings: tissue culture versus ex vivo continuous perfusion of human saphenous vein. J Surg Res. 2004;121:42–49. doi: 10.1016/j.jss.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Cable DG, Caccitolo JA, Caplice N, O’Brien T, Simari RD, Daly RC, Dearani JA, Mullany CJ, Orszulak TA, Schaff HV. The role of gene therapy for intimal hyperplasia of bypass grafts. Circulation. 1999;100:II392–396. doi: 10.1161/01.cir.100.suppl_2.ii-392. [DOI] [PubMed] [Google Scholar]
- 10.Mekontso-Dessap A, Kirsch M, Guignambert C, Zadigue P, Adnot S, Loisance D, Eddahibi S. Vascular-wall remodeling of 3 human bypass vessels: organ culture and smooth muscle cell properties. J Thorac Cardiovasc Surg. 2006;131:651– 658. doi: 10.1016/j.jtcvs.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 11.Slomp J, Gittenberger-deGroot AC, van Munsteren JC, Huysmans HA, van Bockel JH, van Hinsbergh VW, Poelmann RE. Nature and origin of the neointima in whole vessel wall organ culture of the human saphenous vein. Virchows Arch. 1996;428:59–67. doi: 10.1007/BF00192928. [DOI] [PubMed] [Google Scholar]
- 12.Janowski K, Sopinski M, Topol M. Changes in the wall of the great saphenous vein at consecutive stages in patients suffering from chronic vein disease of the lower limbs. Folia Morphol (Warsz) 2007;66:185–189. [PubMed] [Google Scholar]
- 13.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann N Y Acad Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. discussion 51–32. [DOI] [PubMed] [Google Scholar]
- 14.Ang AH, Tachas G, Campbell JH, Bateman JF, Campbell GR. Collagen synthesis by cultured rabbit aortic smooth-muscle cells. Alteration with phenotype. Biochem J. 1990;265:461–469. doi: 10.1042/bj2650461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-β-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF. Atrial natriuretic peptide inhibits transforming growth factor β-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 17.Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- 18.Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009:111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- 19.Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerasimenko JV, Gerasimenko OV, Petersen OH. Membrane repair: Ca(2+)-elicited lysosomal exocytosis. Curr Biol. 2001;11:R971–R974. doi: 10.1016/s0960-9822(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng KT, Chan FL, Huang Y, Chan WY, Yao X. Expression of olfactory-type cyclic nucleotide-gated channel (CNGA2) in vascular tissues. Histochem Cell Biol. 2003;120:475–481. doi: 10.1007/s00418-003-0596-2. [DOI] [PubMed] [Google Scholar]
- 22.Yao X, Leung PS, Kwan HY, Wong TP, Fong MW. Rod-type cyclic nucleotide-gated cation channel is expressed in vascular endothelium and vascular smooth muscle cells. Cardiovasc Res. 1999;41:282–290. doi: 10.1016/s0008-6363(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 23.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol. 2008;294:C488–C494. doi: 10.1152/ajpcell.00537.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol. 298:F643–F654. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JJ, Mehdi A, Stohl LL, Levin LR, Buck J, Wagner JA, Stessin AM. “Soluble” adenylyl cyclase-generated cyclic adenosine mono-phosphate promotes fast migration in PC12 cells. J Neurosci Res. 2008;86:118–124. doi: 10.1002/jnr.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aigner T, Stove J. Collagens–major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569–1593. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987;252:C1–9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Sodek KL, Hwang Q, Brown TJ, Ringuette M, Sodek J. Phagocytosis of collagen by fibroblasts and invasive cancer cells is mediated by MT1-MMP. Biochem Soc Trans. 2007;35:704–706. doi: 10.1042/BST0350704. [DOI] [PubMed] [Google Scholar]
- 30.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.