Abstract
Cardiac fibroblasts become activated and differentiate to smooth muscle-like myofibroblasts in response to hypertension and myocardial infarction (MI), resulting in extracellular matrix (ECM) remodeling, scar formation and impaired cardiac function. cAMP and cGMP-dependent signaling have been implicated in cardiac fibroblast activation and ECM synthesis. Dysregulation of cyclic nucleotide phosphodiesterase (PDE) activity/expression is also associated with various diseases and several PDE inhibitors are currently available or in development for treating these pathological conditions. The objective of this study is to define and characterize the specific PDE isoform that is altered during cardiac fibroblast activation and functionally important for regulating myofibroblast activation and ECM synthesis. We have found that Ca2+/calmodulin-stimulated PDE1A isoform is specifically induced in activated cardiac myofibroblasts stimulated by Ang II and TGF-β in vitro as well as in vivo within fibrotic regions of mouse, rat, and human diseased hearts. Inhibition of PDE1A function via PDE1-selective inhibitor or PDE1A shRNA significantly reduced Ang II or TGF-β-induced myofibroblast activation, ECM synthesis, and pro-fibrotic gene expression in rat cardiac fibroblasts. Moreover, the PDE1 inhibitor attenuated isoproterenol-induced interstitial fibrosis in mice. Mechanistic studies revealed that PDE1A modulates unique pools of cAMP and cGMP, predominantly in perinuclear and nuclear regions of cardiac fibroblasts. Further, both cAMP-Epac-Rap1 and cGMP-PKG signaling was involved in PDE1A-mediated regulation of collagen synthesis. These results suggest that induction of PDE1A plays a critical role in cardiac fibroblast activation and cardiac fibrosis, and targeting PDE1A may lead to regression of the adverse cardiac remodeling associated with various cardiac diseases.
Keywords: Cyclic nucleotide, Phosphodiesterase, Myofibroblast, Cardiac fibrosis
Introduction
Cardiac fibroblasts are essential for the orderly synthesis and degradation of ECM proteins and paracrine signaling with myocytes [9]. Myocardial infarction (MI) or hypertension triggers the differentiation of cardiac fibroblasts to smooth muscle-like myofibroblasts, which have enhanced ECM synthesis, migration, and contractile properties [49]. Following an inflammatory response in hypertrophic or infarcted hearts, activated myofibroblasts orchestrate structural remodeling leading to fibrotic scar formation [4, 49, 63]. The phenotypic transformation to myofibroblasts is distinguished by the expression of α-smooth muscle actin (α-SMA), which contributes to stress fiber organization required for scar contraction and wound healing [61]. The excessive deposition of ECM by myofibroblasts promotes myocardial stiffness, impairs cardiac function, and contributes to the progression of heart failure [7].
Cyclic nucleotides, cAMP and cGMP are ubiquitous second messengers that mediate a number of physiological processes in the heart, from acute effects on contractility to chronic effects on growth and metabolism [5, 34]. A few reports demonstrated anti-fibrotic actions of both cAMP [45, 57, 69] and cGMP [35, 37, 60] dependent signaling in the heart. For instance, cGMP-dependent protein kinase (PKG) was shown to mediate the effects of ANP on reduced TGF-β-Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts [35]. Similarly, activation of the Rap1 guanine exchange factor activated by cAMP (Epac) was shown to modulate collagen synthesis in rat cardiac fibroblasts [64, 69]. Cyclic nucleotides exist in multiple discrete compartments to mediate different cellular functions, which are spatiotemporally regulated by unique cyclases, anchoring proteins, and cyclic nucleotide phosphodiesterase (PDE) isoforms [70]. To date, at least 60 different PDE isozymes derived from 22 genes are identified and grouped into 11 broad families (PDE1–PDE11). Given that PDEs are tightly coupled to specific cyclic nucleotide signaling components, selective activation or inhibition of distinct PDE isozymes may represent a critical mechanism to modulate cardiac pathophysiology. Therefore, it is of great interest and therapeutic importance to define the PDE isoforms that specifically regulate cardiac fibroblast activation.
Our primary screening results have revealed that among 21 known PDE genes, PDE1A is one of a few PDE isoforms that are highly induced in activated cardiac fibroblasts. PDE1A belongs to the Ca2+/calmodulin-stimulated PDE (PDE1) family that consists of three genes, PDE1A, PDE1B and PDE1C. In vitro, the activity of PDE1 family members can be stimulated up to tenfold by Ca2+/cal-modulin [56], thus PDE1 isozymes may integrate Ca2+ and cyclic nucleotide signaling in various cell types [68]. PDE1 family members are considered as dual substrate enzymes. In vitro, PDE1A and PDE1B isozymes hydrolyze cGMP with much higher affinity than cAMP [55]; and PDE1C isozymes hydrolyze both cAMP and cGMP with equally high affinity. Herein, we illustrate the regulation of PDE1A expression during cardiac fibroblast activation in vitro and in vivo. We further report a critical role of PDE1A in regulating myofibroblast formation and ECM synthesis in intact hearts and in vitro, through modulating PKG and Epac1-Rap1 signaling. These observations are supported by unique PDE1-regulated cGMP and cAMP signaling in intact cardiac fibroblasts. Collectively, these results may provide mechanistic insight in targeting PDE1 to circumvent adverse cardiac remodeling.
Materials and methods
A detailed description of materials and methods is available in the Online Supplement.
Adenovirus production
Adenovirus vectors encoding PDE1A and LacZ shRNA were constructed using BLOCK-iT Adenoviral shRNA Expression System (Invitrogen) according to the manufacturer’s instructions as described previously [11].
Animal models
All animal procedures were performed in accordance with the National Institutes of Health (NIH) and University of Rochester institutional guidelines. Isoproterenol was delivered at 30 mg/kg/day for 1 week via subcutaneous osmotic minipumps and IC86340 (3 mg/kg/day) was administered daily via intraperitoneal injection in 10–12 week old C57Bl/6 mice as previously described [38]. Myocardial infarction was induced via ligating the left anterior descending coronary artery (LAD) in 8–12 week old C57Bl/6 mice for 2–3 weeks as previously described [44, 54]. Ang II was delivered at 0.7 mg/kg/day for 2 weeks via subcutaneous osmotic minipump in 200–250 g Sprague–Dawley rats as previously described.
Primary rat cardiac fibroblasts
Primary rat cardiac fibroblasts were isolated from 3 to 4 day old Sprague–Dawley rats and used at passage 1 for all experiments. Cardiac fibroblasts were cultured in Dulbecco’s modified eagle medium containing 1 g/L glucose, 10% fetal bovine serum for 24 h before switching to serum-free medium.
Human myocardial infarction tissue
Human heart explants were obtained with prior Institutional Review Board approval and written informed consent from MI donors, and cardiac dysfunction was assessed via echocardiography as previously described [16]. Information regarding the age, gender and list of prescribed medications was not disclosed.
Histological and immunohistochemistry analysis
Excised mouse or rat hearts or human heart explants were washed, fixed in 10% buffered formalin, and paraffin embedded as previously described [39]. Hearts were transversely sectioned (5 μm), deparaffinized and stained for histopathology markers or antigens of interest as previously described [38].
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was isolated from cardiac fibroblasts using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Semi-quantitative or quantitative RT-PCR was performed with an iCycler thermocycler (Bio-Rad) by synthesizing cDNA with the Reverse Transcription PCR kit (Promega) followed by standard PCR using GoTaq (Pro-mega) or real-time PCR using SYBR green (Applied Biosystems) according to the manufacturer’s instructions as previously described [38]. Primer sequences are available in the Online Supplement.
Ca2+/CaM-dependent PDE assay
PDE assays were performed using the established radio-labeled nucleotide method [6]. PDE1 activity was measured using 1 μmol/L cGMP substrate in the presence of 4 μg/mL calmodulin and 0.8 mmol/L CaCl2 (Ca2+/CaM-dependent PDE assay), as previously described [27].
Western blotting
Cell lysates were prepared by adding ice cold modified RIPA buffer containing the following: 50 mmol/L Tris–HCl pH 7.4, 1% NP-40, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 1 mmol/L sodium orthovanadate, 1 mmol/L NaF, and 1 μ/mL each of aprotinin, leupeptin, and pepstatin as proteins were separated by SDS-PAGE as previously described [38].
Small interfering RNA transfection
Cardiac fibroblasts were transiently transfected with Epac1, Rap1, or non-targeting control siRNA duplexes using Oligofectamine reagent (Invitrogen) in Dulbecco’s modified eagle medium containing reduced serum according to the manufacturer’s instructions. A detailed description containing Epac1, Rap1 and control siRNA sequences is available in the Online Supplement.
Luciferase assays
Cardiac fibroblasts were transfected with indicated luciferase reporters: α-SMA-luc and Smad binding element (SBE-luc), along with indicated plasmid constructs and β-galactosidase as an internal control using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s instructions and as previously described [26].
[3H]-proline incorporation
Collagen synthesis was determined by incorporating 1 μCi/mL [3H]-proline upon Ang II or TGF-β stimulation for up to 48 h. Cells were washed twice with HBSS and incubated in 10% trichloroacetic acid for 30 min to precipitate the protein. Precipitates were washed twice with cold 95% ethanol, solubilized in 0.5 N NaOH for 30 min, and neutralized with 1 N HCl. Radioactivity was quantified using a liquid scintillation counter. Samples were normalized to the total precipitable protein per well using a Bradford assay.
Immunocytochemistry
Immunostaining was performed as previously described [39]. Briefly, cardiac fibroblasts were fixed in 4% paraformaldehyde and permeabilized in PBS/0.2% Triton-X-100. Cells were incubated in DAKO serum-free protein block followed by incubation with anti-phosphorylated-Smad2/3 (Santa Cruz) primary antibody.
Real-time cAMP and cGMP measurements
Cardiac fibroblasts were transiently transfected with a nuclear targeted Epac1-H30 FRET cAMP sensor (CFP-Epac1-H30-YFP-NLS) [59] using Lipofectamine 2000 and transduced with adenovirus expressing the cytosolic Epac1-camps (Ad-CFP-Epac1-camps-YFP) [48]. After 24–48 h, the culture media were replaced with HEPES buffered Ringer external solution containing 120 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mmol/L KH2PO4, 10 mmol/L HEPES–NaOH, pH 7.4 and 2 mmol/L D-glucose. CFP was excited at 440 nm and dual emission three channel corrected FRET timelapse images at 485 and 535 nm were visualized on a climate controlled (37°C, 5% CO2, and 40% humidity) inverted epifluorescent microscope (Olympus IX81) at 50–100 ms exposure at 5 s intervals. Data were represented as the baseline normalized 485/585 FRET ratios (ΔR/R0). For cGMP measurements, cardiac fibroblasts were transduced with adenovirus expressing the cGMP sensor δ-FlincG (Ad-δ-FlincG) for 48–72 h [41]. The sensor was excited at 485 nm and emission at 510 nm was captured using epifluorescence timelapse imaging at 50–100 ms exposure and 2–5 s intervals. Data were represented as the baseline (F0) normalized changes in the background-subtracted fluorescence intensity (ΔF/F0).
Rap1 activity assay
Cells were treated as indicated and lysed in buffer containing 50 mM Tris–HCl, pH 7.4, 500 mM NaCl, 1% NP40, 2.5 mM MgCl2, and 10% glycerol containing 10 μg/mL aprotinin and 10 μg/mL leupeptin. Lysates were incubated with 20–30 μg GST-RalGDS-RBD beads for 1 h followed by four washes in lysis buffer. Samples were loaded on a 15% SDS-PAGE gel, transferred to PVDF membranes and probed with anti-Rap1 (BD Transduction) antibodies to determine GTP-Rap1. Total cell lysates were used to determine total Rap1.
Statistical analysis
Experiments were performed and quantified in a randomized and unbiased manner using at least three independent preparations with treatments done in triplicate when possible. Data are presented as mean ± SD. GraphPad Prism 5.0 was used for statistical analysis. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to determine the statistical significance, as appropriate. Comparisons between two groups were performed using unpaired Student’s t-test. P < 0.05 was considered statistically significant.
Results
PDE1A is upregulated during cardiac fibroblast activation in vitro
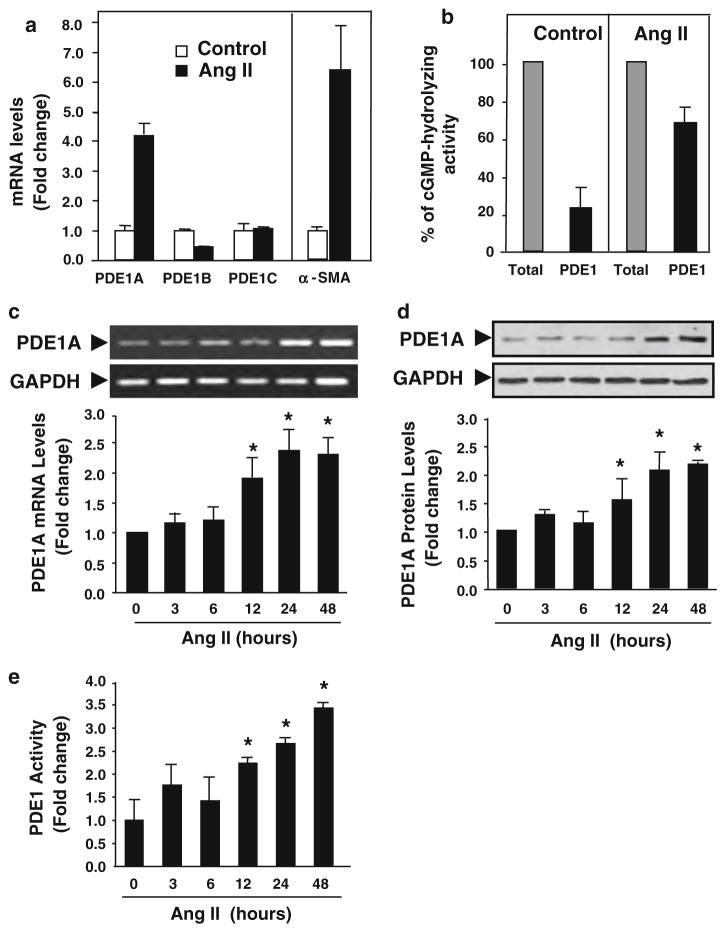
Transformation of quiescent cardiac fibroblasts to active myofibroblasts, distinguished by expression of α-SMA and ECM production, is mediated by fibrotic stimuli such Ang II and TGF-β [23, 61]. Given that cAMP and cGMP are implicated as negative mediators of myofibroblast transformation, we defined the PDE isoforms that are specifically upregulated during this phenotypic transformation. Using quantitative real-time RT-PCR, we assessed the expression levels of 21 known PDE genes in relatively quiescent cardiac fibroblasts (serum-free conditions) and active cardiac myofibroblasts (Ang II stimulation for 24 h) (Fig. S1). We found that among a few PDEs that were significantly regulated during cardiac fibroblast transformation, PDE1A, but not PDE1B and 1C, was a major PDE (and PDE1 specific) isoform highly induced by Ang II in cardiac fibroblasts (Fig. S1 and Fig. 1a). Induction of α-SMA expression represented the activated myofibroblast phenotype (Fig. 1a) Consistently, PDE1 contributed to around 20 and 70% of the total cGMP-hydrolyzing PDE activity under control and Ang II stimulated conditions, respectively, suggesting that PDE1 represents the major PDE in Ang II-activated fibroblasts (Fig. 1b). Our findings also coincide with previous reports that PDE1 represents a predominant PDE activity in adult rat cardiac fibroblasts [8, 19, 25]. RT-PCR analysis further demonstrated Ang II time-dependently increased PDE1A mRNA expression up to 48 h (Fig. 1c). Consistent with mRNA changes, PDE1A protein levels and PDE1 activity were also upregulated by Ang II (Fig. 1d, e). Similarly, PDE1A mRNA levels were also time-dependently increased by TGF-β stimulation (Fig. S2a).
Fig. 1.
PDE1A expression and activity are increased by Ang II stimulation in rat cardiac fibroblasts. a Real-time quantitative RT-PCR results comparing the mRNA expression levels of PDE1 isoforms in control (serum-free) and Ang II stimulation conditions for 24 h in rat cardiac fibroblasts, normalized to GAPDH mRNA levels. α-SMA levels represent the conversion to cardiac myofibroblasts. Values represent mean ± SD. Results are typical from two independent experiments performed in triplicate. b cGMP PDE1 assay performed in the presence of 1 μM cGMP substrate demonstrating the effects of Ang II (100 nmol/L) stimulation (24 h) on PDE1 inhibitable (via 10 μmol/L IC86340) cGMP hydrolyzing activity in rat cardiac fibroblasts. Results are presented as the percentage of total cGMP hydrolyzing activity. Values represent mean ± SD from at least three independent experiments. c Semi-quantitative RT-PCR results showing the effects of Ang II stimulation for the indicated times on PDE1A mRNA expression relative to GAPDH mRNA levels. Values represent mean ± SD from four experiments. d Western blot showing the effects of Ang II stimulation for the indicated times on PDE1A protein levels in cardiac fibroblasts. Values represent mean ± SD from three experiments. e Ca2+-dependent PDE assay showing the effects of Ang II stimulation for various times on PDE1 cGMP hydrolyzing activity in rat cardiac fibroblasts. Values are mean ± SD of triplicates from a typical experiment and are representative of five independent experiments. *P < 0.05 versus 0 h
PDE1A is upregulated in activated cardiac fibroblasts in fibrotic rodent and human hearts
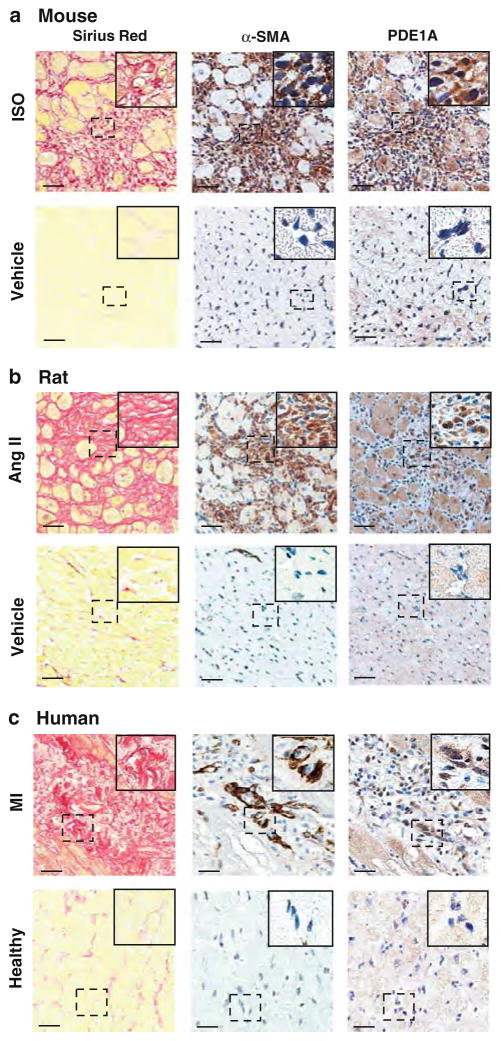
Next, we specifically examined PDE1A expression in fibrotic scar regions enriched with activated cardiac fibroblasts from diseased rodent and human hearts (Fig. 2). We first evaluated mouse models of pathological cardiac remodeling including chronic isoproterenol (ISO) infusion for 1 week and post-MI remodeling induced by left anterior descending coronary artery (LAD) ligation for 2 weeks [15, 54]. Adjacent sections were immunostained for PDE1A and α-SMA, and collagen accumulation was detected by picrosirius red. We found that PDE1A protein was weakly detected in quiescent cardiac fibroblasts (α-SMA negative) in normal control hearts (Fig. 2a, lower panels). In addition to previous findings in cardiomyocytes [38], PDE1A staining intensity was highly increased in α-SMA positive myofibroblasts in fibrotic regions of the endomyocardium in mouse hearts subjected to ISO infusion (Fig. 2a, upper panels). Similar observations were revealed at the border zone of the infarct in MI hearts (Fig. S2b). We also found that PDE1A protein expression is elevated in α-SMA positive myofibroblasts in remodeled rat hearts subjected to Ang II infusion for 2 weeks compared to vehicle controls (Fig. 2b). Cardiac myofibroblasts may persist in human infarct scars for many years to exacerbate fibrosis after completing tissue repair [66]. Thus, we examined the fibrotic scar regions from human diseased hearts after MI. We found that PDE1A was also highly induced in human cardiac myofibroblasts compared to quiescent cardiac fibroblasts in control hearts (Fig. 2c). PDE1A appears to be expressed in both cytosolic and nuclear compartments of myofibroblasts in the various models examined (Fig. 2, right panels, insets). Together these data indicate that PDE1A induction in activated cardiac fibroblasts may be a conserved regulatory mechanism in various paradigms of pathological cardiac remodeling.
Fig. 2.
PDE1A protein levels are increased in activated cardiac myofibroblasts in fibrotic regions of mouse, rat, and human diseased hearts. a Mouse hearts subjected to 1-week ISO infusion (30 mg/kg/day) or vehicle control and stained for collagen deposition and immunostained for α-SMA and PDE1A (n = 6–8 per group). b Rat hearts subjected to 2 weeks Ang II infusion (0.7 mg/kg/day) or vehicle control and stained for collagen deposition and immunostained for α-SMA and PDE1A (n = 3 per group). c Post-MI remodeled human hearts or normal control hearts stained for collagen deposition, and immunostained for α-SMA and PDE1A. (n = 4 per group). Left panels Representative high magnification images of picro-sirius red staining depicting increased interstitial collagen deposition in mouse, rat, and human hearts. Middle panels Adjacent sections immunostained for α-SMA confirm the induction of cardiac myofibroblasts. Right panels PDE1A immunostaining depicts increased PDE1A expression in interstitial myofibroblasts in remodeled hearts. Insets, higher magnification of boxed areas. PDE1A expression is differentially localized in nuclear and cytosolic compartments in cardiac fibroblasts within the left ventricle. Scale bar = 20 μm
Role of PDE1A in cardiac fibroblast activation and extracellular matrix synthesis
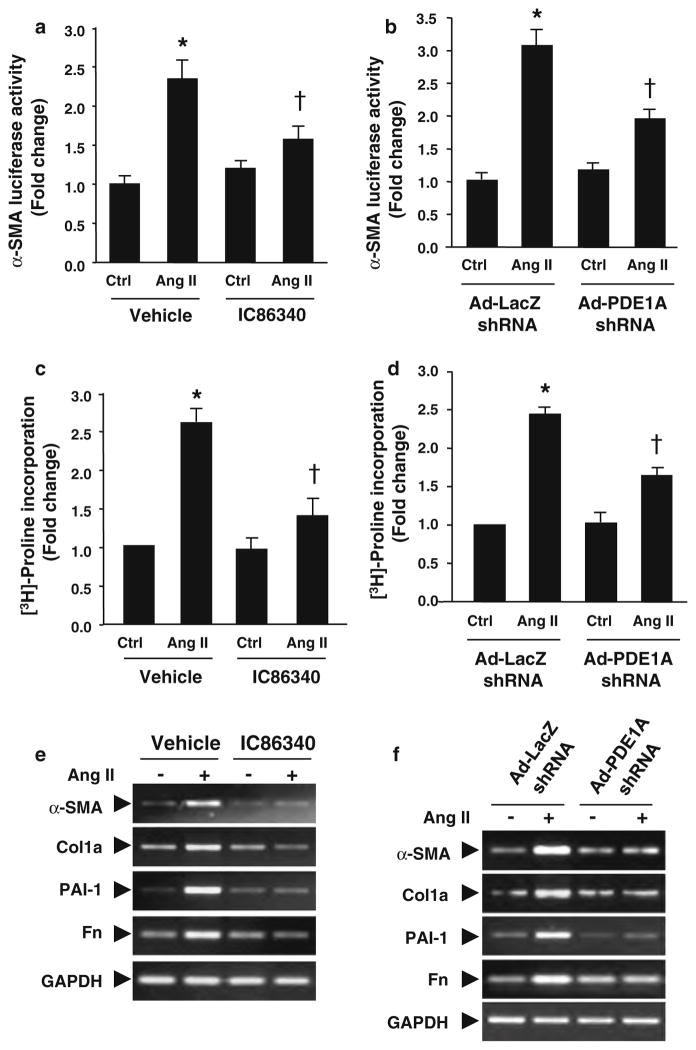
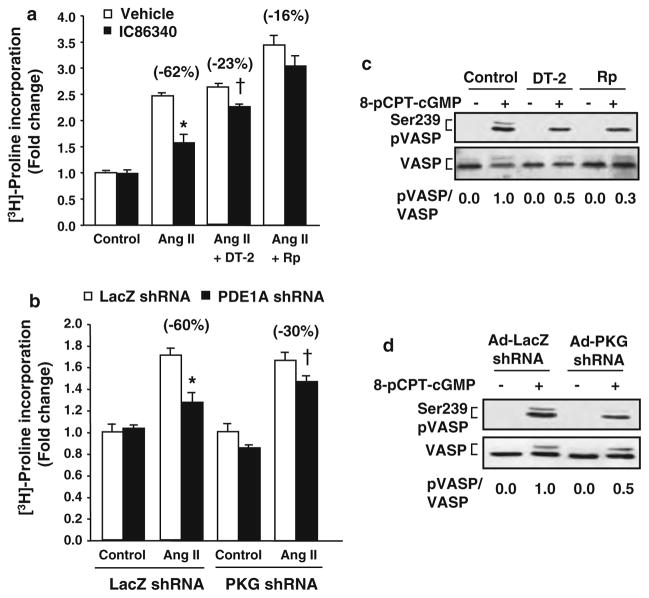
To determine the specific contribution of PDE1A in regulating myofibroblast function, we measured the effects of PDE1 inhibitors IC86340 and PDE1A knockdown. Based on the published selectivity of the PDE1 inhibitor IC86340 [39], it inhibits PDE1 with IC50 values 0.06–0.44 μM. However, the IC50 values for other PDE family members are greater than 100 μM, except for PDE11 (11.30 μM for PDE11). Given that PDE11 is very limited in these cells (Fig. S1), IC86340 should primarily target the PDE1 family at the concentrations used in this study. We found that IC86340 significantly reduced Ang II-stimulated cardiac fibroblast activation measured by α-SMA promoter-driven luciferase activity (Fig. 3a). In order to delineate PDE1A specific effects, we utilized an adenovirus expressing a short hairpin RNA (shRNA) against rat PDE1A to downregulate PDE1A expression. As shown in Fig. 3b, PDE1A shRNA attenuated Ang II-stimulated α-SMA-luciferase activity in cardiac fibroblasts. In addition, both the PDE1 inhibitor and Ad-PDE1A shRNA also suppressed Ang II induced collagen synthesis, assessed by [3H]-proline incorporation (Fig. 3c and d). Similar to Ang II, TGF-β stimulated α-SMA promoter activity was also attenuated by IC86340 and PDE1A downregulation (Fig. S3a and S3b).
Fig. 3.
PDE1 inhibitors and PDE1A knockdown attenuates myofibroblast activation, ECM synthesis and pro-fibrotic gene expression. a Cardiac fibroblast activation assessed by α-SMA promoter luciferase activity in rat cardiac fibroblasts pre-treated with vehicle or IC86340 (30 μmol/L) prior to Ang II (100 nmol/L) stimulation for 24 h, and normalized to β-galactosidase activity. b Cardiac fibroblasts transduced with 100 MOI Ad-LacZ shRNA or Ad-PDE1A shRNA and stimulated as described above. c Collagen synthesis measured via of [3H]-proline incorporation in cardiac fibroblasts pre-treated with vehicle or IC86340 prior to Ang II stimulation for 48 h, and normalized to total protein levels. d Cardiac fibroblasts transduced with Ad-LacZ shRNA or Ad-PDE1A shRNA and stimulated as described above. Values represent mean ± SD from three independent experiments performed in triplicates. *P < 0.05 versus Ctrl (Vehicle or LacZ shRNA), †P < 0.05 versus Ang II alone. e Representative RT-PCR results showing effects of the PDE1 inhibitor IC86340 on Ang II stimulated pro-fibrotic marker gene expression, including type I collagen (Col1a), fibronectin (Fn), plasminogen activator inhibitor 1 (PAI-1), and α-SMA. Similar results were observed from at least three independent experiments. f RT-PCR results showing effects of PDE1A shRNA versus LacZ shRNA on Ang II stimulated pro-fibrotic gene expression in cardiac fibroblasts. Similar results were observed from at least two independent experiments
We further demonstrated that the PDE1 inhibitor IC86340 or Ad-PDE1A-shRNA significantly blocked the induction of other ECM and pro-fibrotic markers, including collagen I, fibronectin (Fn) and plasminogen activator inhibitor (PAI-I) (Fig. 3e, f). This is in line with previous findings that cGMP [47] and cAMP [57] signaling negatively regulate expression of these genes. Levels of α-SMA mRNA were also markedly reduced, consistent with changes in α-SMA-luciferase activity. Together these results support a critical role for PDE1A in modulating stress induced myofibroblast formation, ECM synthesis and pro-fibrotic gene expression. As expected, PDE1C shRNA did not block Ang II-induced collagen synthesis and other ECM/pro-fibrotic marker expression levels (Fig. S3c). The specificity of the PDE1A and PDE1C shRNA on PDE1A and PDE1C expression was demonstrated in Fig. S3e–g, consistent with previous findings in rat cardiomyocytes [38] and vascular smooth muscle cells (VSMCs) [11]. These results suggest that the effects of IC86340 on ECM synthesis in cardiac fibroblasts are primarily mediated by PDE1A.
PDE1 inhibition attenuates ISO-induced cardiac fibrosis and myofibroblast formation in vivo
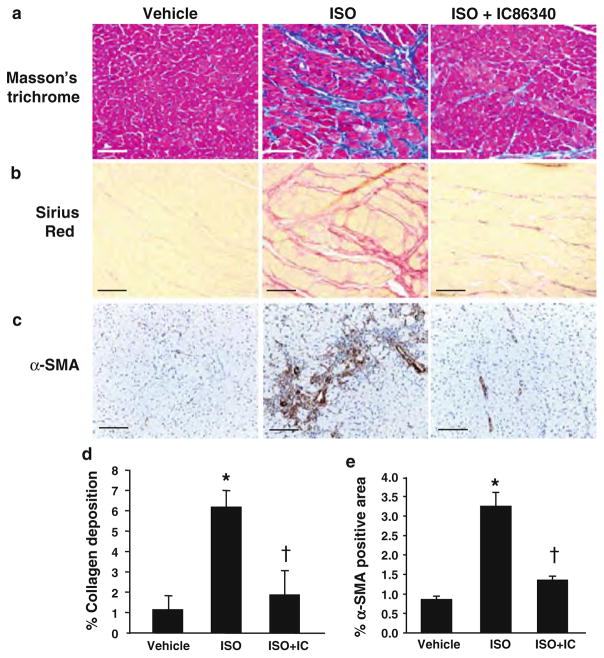
To investigate the role of PDE1 in cardiac fibrotic remodeling in vivo, we examined the effects of the PDE1 inhibitor IC86340 in response to chronic isoproterenol infusion in mice. Total collagen deposition was evaluated by different histological methods, including Masson’s trichrome (Fig. 4a) and picro-sirius red (Fig. 4b). We found that ISO infusion increased interstitial collagen deposition (middle panels) in the endomyocardium compared with vehicle control (left panels), which was significantly attenuated in IC86340 treated mice (right panels). We also assessed the degree of cardiac fibroblast activation and differentiation to myofibroblasts by α-SMA immunostaining. As predicted, ISO infusion triggered an increase in interstitial α-SMA staining in the myocardium, however this response was abrogated in animals treated with IC86340 (Fig. 4c). Quantitated measurements of picro-sirius red and α-SMA staining demonstrated a significant reduction of total collagen deposition (interstitial and perivascular collagen) (Fig. 4d) and α-SMA positive area in the myocardium (Fig. 4e). We have previously reported that IC86340 attenuated cardiac hypertrophy and myocyte enlargement induced by chronic ISO infusion [38]. Thus, the anti-hypertrophic effect of IC86340 is likely attributed to both reducing fibrosis and myocyte hypertrophic growth.
Fig. 4.
PDE1 inhibition attenuates collagen deposition and myofibroblast formation in response to ISO induced cardiac remodeling in vivo. Mice at age of 10–12 weeks were infused with 30 mg/kg/day for 1 week and treated with vehicle or IC86340 (3 mg/kg/day, i.p.). a, b Representative images of collagen deposition in left ventricle of mouse hearts depicted by Masson’s trichrome staining (a) or picro-sirius red staining (b) of vehicle control (left panel), ISO (middle panel) and ISO + IC86340 (right panel) treatment groups. Scale bar = 30 μm (Masson’s trichrome staining) or = 20 μm (picro-sirius red). c Effects of IC86340 treatment on myofibroblast formation assessed by α-SMA immunostaining. Scale bar = 20 μm. d, e Quantitated results of percentage area of picro-sirius staining in B, of background subtracted interstitial and perivascular collagen (including left and right ventricle and intraventricular septum) and percentage area of α-SMA staining in whole myocardium for each treatment group (n = 9–12). Values represent mean ± SD. *P < 0.05 versus Vehicle, †P < 0.05 versus ISO
Role of cGMP-PKG in PDE1-inhibition mediated attenuation of ECM synthesis
Activation of cGMP-dependent protein kinase (PKG I) by ANP was previously shown to inhibit collagen synthesis in cardiac fibroblasts [35] and PDE1A has been previously shown to regulate intracellular cGMP levels [38]. Therefore, we first evaluated the contribution of PKG on PDE1 regulation of collagen synthesis in cardiac fibroblasts. We found that the effects of IC86340 or PDE1A knock down on suppressing collagen synthesis were partially blocked in the presence of the selective PKG inhibitor, DT-2[18] and Rp-8-Br-PET-cGMPS [10] (Fig. 5a), as well as partially blocked by PKG I downregulation via PKG I shRNA (Fig. 5b and S3a). To determine the efficacy of PKG I inhibition by PKG inhibitors or shRNA, we measured PKG-dependent VASP Ser239 phosphorylation in cardiac fibroblasts (Fig. 5c and 5d). We found that PKG inhibitors and PKG shRNA caused ~50% reduction of PKG-dependent VASP phosphorylation via a cGMP analog, comparable to the extent of blocking PDE1 inhibitor-mediated suppression of collagen synthesis. These observations indicate that the partial effects of PKG inhibition on collagen synthesis are very likely due to the partial inhibition of PKG I function by PKG inhibitors and PKG shRNA, and suggest a critical role of PKG in PDE1 regulated collagen synthesis in cardiac fibroblasts.
Fig. 5.
PKG-I dependent effects of PDE1 inhibition on collagen synthesis. a Collagen synthesis measured via [3H]-proline incorporation in cardiac fibroblasts treated with vehicle or the PDE1 inhibitor IC86340 (30 μmol/L) in the presence of vehicle or PKG-I inhibitors, DT-2 (250 nmol/L) or Rp-8-Br-PET-cGMPS (20 μmol/L) and stimulated with Ang II (100 nmol/L) for 48 h, and normalized to total protein levels. Values represent mean ± SD from a typical experiment performed in triplicate. Similar results were observed from n = 3 independent experiments. *P < 0.05 versus Ang II + Vehicle, †P < 0.05 versus Ang II + DT-2 + Vehicle. b Collagen synthesis in cardiac fibroblasts transduced with adenovirus expressing LacZ shRNA (Ad-LacZ shRNA) or PKG I (Ad-PKG I shRNA) prior to IC86340 treatment and Ang II stimulation. Values represent mean ± SD from a typical experiment performed in triplicate. Similar results were observed from n = 3 independent experiments. *P < 0.05 versus Ang II + LacZ shRNA, †P < 0.05 versus Ang II + PKG shRNA + LacZ shRNA. c, d Effects of PKG I inhibitors and PKG I shRNA on PKG-dependent Ser239 VASP phosphorylation. Cardiac fibroblasts were pre-treated with DT-2 or Rp-8-Br-PET-cGMPS for 30 min or transduced with Ad-LacZ shRNA or Ad-PKG shRNA for 48 h prior to stimulation with 8-pCPT-cGMP (100 μmol/L) for 30 min. Western blots were performed using a monoclonal antibody recognizing phosphorylated Ser239 VASP or polyclonal antibody against total VASP. The ratio of phosphorylated VASP to total VASP shown below each blot was determined via densitometry in a linear range
A previous study has shown that the activation of PKG I upon ANP stimulation inhibits myofibroblast transformation via inhibiting Smad2 and Smad3 nuclear translocation [35]. Therefore, we examined the effects of PDE1 inhibition on Smad activation. As expected, TGF-β stimulated Smad-dependent reporter luciferase activity, however this was unaltered by PDE1 inhibition (Fig. S4b). In addition, we found that TGF-β stimulated phosphorylated Smad2/3 nuclear translocation as expected, however, PDE1 inhibition did not change nuclear pSmad2/3 accumulation (Fig. S4c and S4d). These results suggest that in contrast to ANP-cGMP-PKG signaling, PDE1A-cGMP-PKG does not regulate Smad-dependent transcriptional activity.
Role of cAMP-PKA and cAMP-Epac-Rap1 in PDE1 inhibition-mediated reduction of ECM synthesis
Given the inhibitory role of cAMP signaling on collagen synthesis [69] and the dual substrate specificity of PDE1A, we also evaluated the potential involvement of cAMP signaling in PDE1 inhibition-mediated regulation of collagen synthesis. To determine the direct involvement of PKA in PDE1A-mediated regulation of collagen synthesis, we used an adenovirus expressing the selective inhibitor of PKA (Ad-PKI). We found that treatment with Ad-PKI had no measureable effect on IC86340 mediated reduction of collagen synthesis (Fig. S5a). However, PKA-dependent VASP Ser157 phosphorylation was significantly blocked by PKI, which confirmed the function of PKI (Fig. S5b).
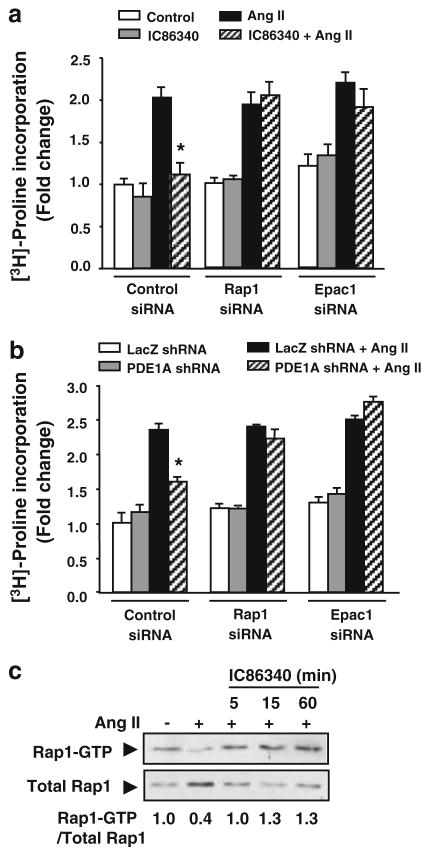
We next assessed the involvement of Epac-Rap1 signaling during PDE1 inhibition on collagen synthesis. Interestingly, knockdown of both Epac1 and Rap1 with siRNA (Fig. S5c) completely abolished the effects of the PDE1 inhibitor IC86340 on collagen synthesis (Fig. 6a). Similar results were observed in the presence of PDE1A shRNA (Fig. 6b), strongly suggesting Epac1-Rap1 mediates the effects of PDE1A on collagen synthesis. To determine whether PDE1 inhibition modulates Epac1-Rap1 signaling, we measured the effects of IC86340 on Rap1 activation using GST-RalGDS-RBD to pull-down activated Rap1. As shown in Fig. 6c, Ang II stimulation markedly reduced Rap1-GTP, consistent with prior reports that Ang II and TGF-β inhibit Rap1 activity [69]. Interestingly, IC86340 blocked the inhibitory effects of Ang II on Rap1-GTP (Fig. 6c). These observations suggest that cAMP-dependent Epac1-Rap1 activation is also involved in PDE1A modulated pro-fibrotic cardiac fibroblast function likely via cAMP-dependent Epac1-Rap1 activation.
Fig. 6.
Role of Epac1-Rap1 signaling in mediating effects of PDE1 inhibition. a Collagen synthesis measured via [3H]-proline incorporation in cardiac fibroblasts transfected with siRNA against non-targeting control, Rap1, or Epac1 followed by treatment with vehicle or the PDE1 inhibitor IC86340 (30 μmol/L) and Ang II (100 nmol/L) stimulation for 48 h, and normalized to total protein levels. b Collagen synthesis in cardiac fibroblasts transduced with Ad-Lacz shRNA or PDE1A shRNA for 48 h followed by Ang II stimulation, and normalized to total protein levels. Values represent mean ± SD of triplicates from n = 3 independent experiments. *P < 0.05 versus Ang II or LacZ shRNA + Ang II. c Rap1 activity determined using GST-RalGDS-RBD to pulldown Rap1-GTP in cardiac fibroblasts stimulated with Ang II (100 nmol/L) for 24 h prior to treatment with PDE1 inhibitor IC86340 (30 μmol/L) for the indicated times. Rap1-GTP levels shown below each blot were quantitated by densitometry in a linear range and normalized to total Rap1 levels. Values (normalized to serum-free control) are averaged from two independent experiments
Since cGMP may also increase intracellular cAMP through cGMP-inhibited PDE3 [1], we evaluated the involvement of PDE3 using PDE3 inhibitors milrinone or cilostamide. We found that both PDE3 inhibitors only caused a minor reduction of collagen synthesis in cardiac fibroblasts (Fig. S5d), suggesting that the effect of PDE1 inhibition is unlikely through indirect cGMP-mediated inhibition of PDE3 to elevate cAMP.
PDE1-regulated cGMP responses in activated cardiac fibroblasts
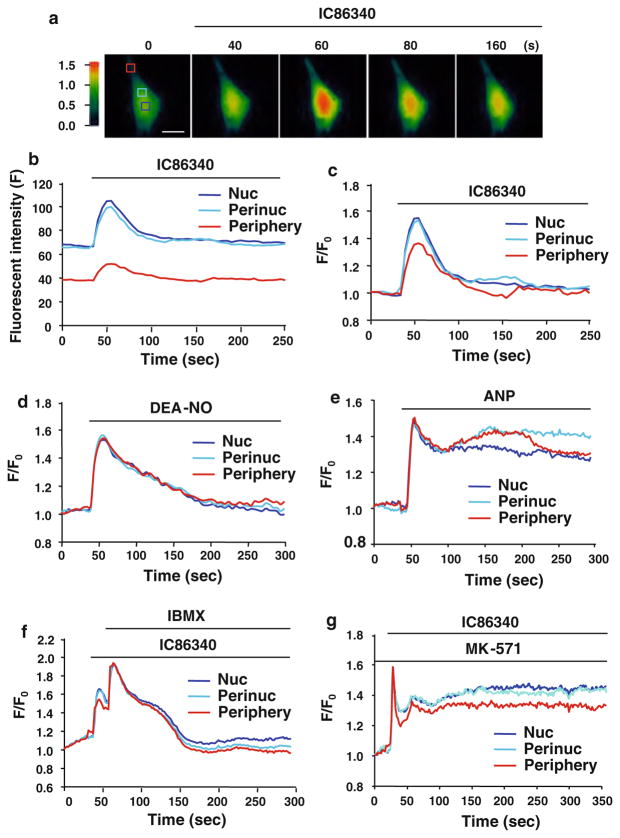
To determine the role of PDE1 in controlling cGMP changes in activated cardiac fibroblasts, we used a GFP-based cGMP sensor, δ-FlincG that was previously shown to detect rapid and physiologically relevant concentrations of NO and ANP evoked cGMP dynamics in vascular smooth muscle cells (VSMC)[41] or cell lines exogenously expressing pGC or cGMP-PDE [3]. Transduction of cardiac fibroblasts with an adenovirus encoding δ-FlincG generated a uniform GFP distribution throughout the nucleus and cytoplasm (Fig. 7a). Cardiac fibroblasts were activated in medium containing 5% serum for 48 h, which induced both α-SMA and PDE1A expression, while maintaining expression of the cGMP sensor (data not shown). Treatment with the PDE1 inhibitor IC86340 caused a rapid but transient elevation of cGMP in activated cardiac fibroblasts (Fig. 7a), with larger increases in nuclear and perinuclear regions (approximately 1.5-fold at the peak) compared to the peripheral region (around 1.35-fold at the peak) (Fig. 7b, c).
Fig. 7.
Effects of PDE1 inhibition on spatial and temporal cGMP changes in activated cardiac fibroblasts. a Pseudo-colored images depict the relative PDE1 inhibition-induced cGMP changes measured by GFP-based cGMP sensor δ-FlincG in primary cardiac fibroblasts activated in the presence of 5% serum. Activated cardiac fibroblasts transduced with adenovirus δ-FlincG were treated with the PDE1 inhibitor IC86340 (15 μmol/L) and the fluorescence intensities (excitation at 485 nm and emission at 510 nm) were captured. Boxes denote regions of interests in the nucleus (Nuc), perinucleus (Perinuc), and peripheral compartments. Scale bar = 25 μm. b Fluorescence intensity traces from the above boxed regions after IC86340 treatment with background fluorescence subtracted. c Background subtracted intensity traces normalized to the fluorescence intensity at 0 s (ΔF/F0) for each compartment. Results are typical from n = 10. d Activated cardiac fibroblasts expressing Ad-δ-FlincG and treated with the NO donor DEA-NONOate (2.5 nmol/L) elicits a transient cGMP response in each region of interests. Results are typical from n = 12. e ANP (30 nmol/L) treatment results in sustained cGMP response in each region of interests. Results are typical from n = 8. f Effects of global PDE inhibition via IBMX (100 μmol/L) on PDE1 inhibitor induced transient cGMP response. Results are typical from n = 8. g Effects of cGMP efflux transporter MRP4/5 inhibitor MK-571 (20 μmol/L) on PDE1 inhibitor induced transient cGMP response. Results are typical from n = 6
To compare the cGMP response elicited by PDE1 inhibition to other cGMP elevators, we monitored cGMP changes treated with NO donors or ANP in activated cardiac fibroblasts where the dynamic cGMP responses have not been characterized. We found that the NO donor DEA-NO (Fig. 7d) elicited a transient and global cGMP response (with equivalent cGMP elevation in all three regions), similar to previous observations in VSMCs [41]. However, ANP resulted in a sustained but global cGMP response (Fig. 7e), distinct from the sustained membrane-restricted cGMP elevation seen in VSMC [41]. It has been shown that this membrane-restricted ANP induced cGMP response in VSMCs is largely controlled by PDE5 in VSMCs, since PDE5 inhibitor sildenafil resulted in global cGMP elevation [41]. Therefore, the global cGMP increases observed in ANP treated cardiac fibroblasts may be attributed to low PDE5A expression in these cells (Fig. S1), or due to activation of intracellular ANP receptor guanylyl cyclase-A (GC-A) [46].
To elucidate the underlying mechanism(s) responsible for the transient cGMP elevation upon PDE1 inhibition, we first tested the possible involvement of other PDEs. We found that IBMX treatment further increases but does not fully sustain the IC86340-mediated cGMP response (Fig. 7f). Eflux transporters may also rapidly deplete intracellular cGMP levels [2]. Indeed, we found that in the presence of an inhibitor of efflux transporters such as multidrug resistance-associated proteins (MRPs), MK-571, the IC86340-elicited cGMP response was sustained (Fig. 7g), suggesting that this transient nature of cGMP elevation may be controlled by cGMP efflux. Of course the precise role and mechanism of MRP in regulating PDE1-controlled cGMP deserves further investigation.
PDE1-regulated cAMP responses in activated cardiac fibroblasts
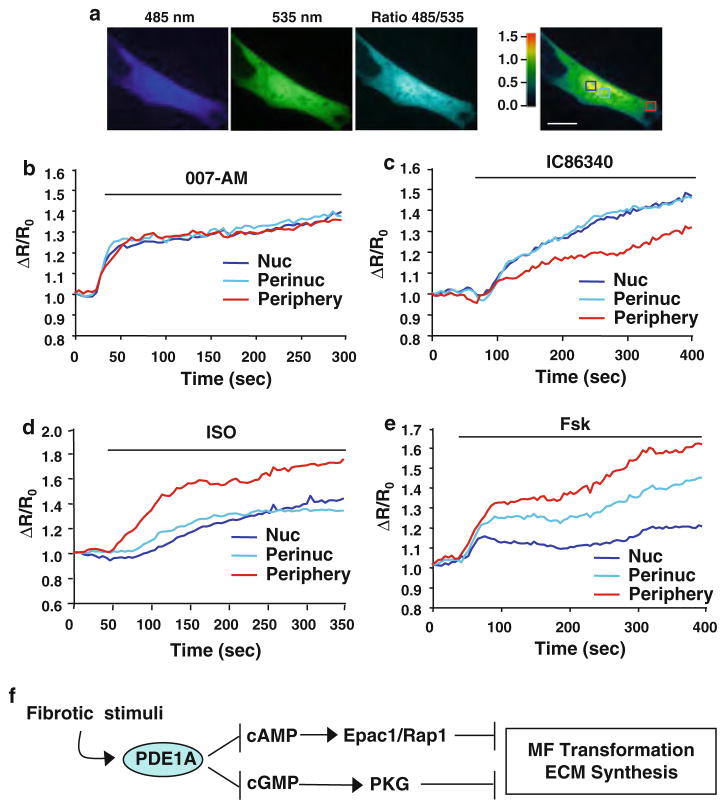
To determine the role of PDE1 in modulating cAMP changes in activated cardiac fibroblasts, we used the established single chain Epac1-camps FRET sensor [42]. In order to simultaneously assess both nuclear and cytosolic cAMP changes, we co-transfected the Epac1-camps sensor (cytosolic restricted) and a nuclear-targeted Epac1-camps based sensor (H30-NLS)[59]. Co-expression of these molecules generated an even distribution of YFP and CFP expression in the nucleus and cytosol of activated cardiac fibroblasts (Fig. 8a). We first verified the functionality of the sensors using the Epac1 agonist (007-AM), which elicited a rapid and uniform increase in background-subtracted CFP/YFP FRET fluorescence ratio (485/535 nm) over time (Fig. 8b), consistent with previous reports [65]. Interestingly, treatment with IC86340 caused a gradual and predominant increase in CFP/YFP FRET (increased [cAMP]i) in the nuclear and perinuclear regions (1.26 ± 6 and 1.35 ± 3 fold, respectively) compared to that in the peripheral region (1.15 ± 6) (Fig. 8c). In contrast, the cAMP elevating stimuli adenylyl cyclase activators for-skolin (Fsk) and ISO elicited greater cAMP elevation at the peripheral region than the nuclear and perinuclear regions (Fig. 8d, e). This is in line with observations that the stimulation of β-adrenergic receptor (β-AR) or membrane adenylyl cyclase generates cAMP proximate to the cell membrane [17, 50]. The predominant elevation of cAMP by PDE1 inhibition in the nuclear and perinuclear regions is unlikely due to different saturable properties of the sensors since the cAMP analog 007-AM shows equivalent responses in all three regions, whereas ISO elicits a predominant cAMP response in the peripheral region, as expected. Together these findings suggest that PDE1 regulates distinct pools of cAMP and cGMP in activated cardiac fibroblasts, which may selectively target cyclic nucleotide effectors during ECM remodeling.
Fig. 8.
Effects of PDE1 inhibition on spatial and temporal cAMP changes in activated cardiac fibroblasts. a Primary rat cardiac fibroblasts (activated in the presence of 5% serum) were co-transfected with Epac1-camps and the nuclear targeted Epac1-H30-NLS which resulted in a relatively uniform distribution of the cAMP sensor throughout the cytosol, nucleus, perinucleus, and cell periphery regions. Pseudo-colored fluorescence intensity is shown for the FRET donor, CFP (485 nm), the acceptor YFP (535 nm), corrected FRET ratio 485/535 nm. Boxes denote regions of interests in the nucleus (Nuc), perinucleus (Perinuc), and peripheral compartments. Scale bar = 10 μm. b Stimulation of activated cardiac fibroblasts with the membrane permeable Epac-selective agonist, 007-AM (1 μmol/L) generates a rapid and uniform increase in 485/535 confirming the functionality of the sensor. Data are represented as the fluorescence intensity changes in the FRET donor/acceptor ratio 485/535 normalized to the baseline ratio at 0 s (ΔR/R0), for each regions of interest. c Effects of the PDE1 inhibitor IC86340 (15 μmol/L) on 485/535 which elicits changes in [cAMP]i preferentially in the nucleus (Nuc) and perinucleus (Perinuc) compared to the cell periphery (Periphery). d Stimulation of activated cardiac fibroblasts with ISO (0.1 μmol/L) generates an increase in cAMP predominately in the cell periphery. e Stimulation with the AC activator Forskolin (Fsk; 10 μmol/L) elicits an increase in intracellular cAMP, differentially in the regions of interests, preferentially at the cell periphery. Results are typical from n = 6–10 per treatment. f Proposed model of PDE1A-dependent dual regulation of cAMP-Epac1-Rap1 and cGMP-PKG signaling to suppress myofibroblast (MF) transformation and ECM synthesis during pathological cardiac remodeling
Discussion
The notable findings of this study include that (1) PDE1A is one of the major PDE isoforms that are highly induced in activated cardiac myofibroblasts compared to normal quiescent cardiac fibroblasts in vitro and in vivo within fibrotic regions of mouse, rat, and human diseased hearts. To our knowledge, this is the first PDE isoform reported to be specifically induced in cardiac fibroblasts of diseased hearts. (2) PDE1A plays a critical role in cardiac fibroblast activation, ECM synthesis and the expression of various pro-fibrotic marker genes. This suggests that PDE1A induction in activated cardiac fibroblasts is causative to the pathological fibrotic remodeling. (3) PDE1A modulates both cAMP-Epac-Rap1 and cGMP-PKG signaling in cardiac fibroblasts, which mediates the effects of PDE1A in cardiac fibroblasts. Based on our observations, PDE1A appears to hydrolyze both cAMP and cGMP in intact cardiac fibroblasts. This is the first evidence that PDE1A functions as a dual-esterase in vivo although PDE1A is capable of hydrolyzing both cAMP and cGMP in a cell-free system [52]. Collectively, the findings from this study and our previous report that PDE1A mediates cardiomyocyte hypertrophy [38], suggest that PDE1A is highly induced in both cardiomyocytes and activated fibroblasts to modulate pathological cardiac remodeling. Selective inhibition of PDE1A may represent a novel approach to mitigate pathological remodeling underlying the progression of cardiac disease.
Regulation of ECM such as collagen is a dynamic process involving equilibrium between biosynthesis and degradation. Studies have shown that chronic Ang II or TGF-β stimulation shifts the balance toward excess collagen synthesis and accumulation [13, 32]. Excess interstitial collagen contributes to myocardial stiffness leading to impaired diastolic function [7]. The degradation of structural collagen may also lead to systolic or diastolic dysfunction [28]. We recently demonstrated PDE1C but not PDE1A regulates lysosomal-dependent degradation of the basal type I collagen in VSMCs [11]. Herein we show that PDE1A regulates agonist stimulated collagen synthesis through changes in gene expression, suggesting two distinct mechanisms of collagen homeostasis regulated by different PDE1 isozymes. This is supported by the lower expression of PDE1C observed in stimulated rat cardiac fibroblasts (Fig. 1b), which is in line with lower PDE1C in activated mouse cardiac fibroblasts relative to cardiac myocytes [36]. We have previously reported that PDE1A expression is upregulated by TGF-β in vascular adventitial fibroblasts, and PDE1A-PKC signaling mediates TGF-β-stimulated myofibroblast activation (α-SMA expression) in vascular adventitial fibroblasts [72]. Therefore, our current findings of the underlying mechanisms of PDE1A in regulating cardiac fibroblast activation and ECM production may represent common pathways of fibroblast activation in other tissues. Other PDE isoforms such as PDE5A have been identified in mouse cardiac myofibroblasts [36], however, PDE5A expression was relatively low in rat cardiac myofibroblasts (unpublished observations). The potential contribution of myofibroblast PDE5A in regulating cardiac remodeling deserves further investigation.
Studies have shown that cGMP elevating agents, such as nitric oxide donors or ANP, inhibit cardiac fibroblast functions including ECM synthesis and proliferation [12, 29]. These effects often correlate with cGMP-mediated reduction of cardiomyocyte hypertrophy [30, 38, 62]. In fibroblasts, PKG Iα activation by ANP was shown to phosphorylate Smad3 and prevent its nuclear translocation, which is believed to inhibit TGF-β induced myofibroblast transformation [35]. Herein we show that PKG is also responsible for the effects of PDE1 inhibition on collagen synthesis in cardiac fibroblasts (Fig. 5), however PDE1 inhibition does not alter TGF-β-stimulated Smad transcriptional activity or Smad2/3 nuclear translocation (Fig. S4). These data indicate that PDE1 regulates ECM synthesis via a different mechanism from ANP-cGMP signaling. Indeed we observed that the dynamic cGMP responses elicited by PDE1 inhibition and ANP are different in activated cardiac fibroblasts (Fig. 7), suggesting that PDE1 and ANP regulate distinct pools of cGMP. Selective activation of Epac1 by an Epac-selective cAMP analog was shown to elicit anti-fibrotic functions such as inhibiting collagen synthesis in adult rat cardiac fibroblasts [69]. However, the specific PDE isoform that selectively targets this pool of cAMP has not been described. Our findings suggest that PDE1A represents the primary PDE isoform modulating collagen synthesis through cAMP-Epac1-Rap1 signaling in cardiac fibroblasts (Fig. 6). Taken together, these findings reveal a previously undescribed PDE1A-cAMP-Epac1-Rap1 signaling mechanism underlying myofibroblast activation during pathological cardiac remodeling (Fig. 8f).
Emerging evidence suggest multiple mechanisms are involved in the compartmentation of cAMP and more recently cGMP, which involve cyclases, PDEs, anchoring proteins, kinases and physical barriers such as caveolae [21]. Using an innovative GFP-based cGMP sensor Nausch et al. [41] demonstrated distinct regulation of pGC and sGC cGMP in VSMCs, which were shown to involve PDE5A. Recent evidence in cardiac myocytes also demonstrated differential feedback control of PKG on pGC and sGC induced cGMP concentration at the sarcolemmal membrane [14], where PDE5A may control sGC-cGMP-PKG signaling [33]. However, studies on PDE1 mediated cGMP compartmentation are limited. Our findings that PDE1 inhibition elicits transient cGMP elevation in activated cardiac fibroblasts is distinct from the sustained cGMP response of PDE5 inhibition by sildenafil in VSMCs [41], suggesting these PDEs control temporally distinct pools of cGMP. The transient nature of cGMP elevation by PDE1 inhibition might be attributed to cGMP efflux through MRP (Fig. 7g) although the role of MRP in regulating cyclic nucleotide signaling in cardiac fibroblasts has not been characterized. However, a recent study has shown that in related growing VSMCs, MRP4 inhibition increases cAMP and cGMP and activates the PKA/CREB pathway, which blocks VSMC proliferation and prevents neointimal hyperplasia in the injured rat carotid artery [53]. PDE1 also preferentially elevated nuclear and perinuclear cGMP, which is consistent with the endogenous PDE1A localization (Fig. 2). It is feasible that the PDE1 inhibitor IC86340 primarily elevates nuclear and perinuclear cGMP levels, and the elevation of peripheral cGMP levels occurs through rapid intracellular diffusion [31]. While the functional relevance of nuclear/perinuclear cGMP is not well described, a recent study suggests nuclear cGMP/PKG modulates gene expression via recruitment of a histone deacetylase complex [24]. These data together add to our understanding of tightly regulated and compartmentalized cGMP signaling by multiple PDEs within a given cell.
Interestingly, we found PDE1 inhibition also preferentially elevated nuclear and perinuclear cAMP. This is the first observation of localized and endogenous PDE1-mediated cAMP dynamics in PDE1A-enriched cells. In contrast to the PDE1 inhibitor, cAMP-elevating agonists, ISO and Fsk triggered cAMP more at the peripheral regions (Fig. 8). This is in line with activation of membrane AC-cAMP proximate to the cell membrane. The spatial and temporal control of cAMP gradients in cardiomyocytes have been well documented [21]. Interestingly, ISO-stimulated cAMP responses are often rapid and transient in cardiomyocytes [20, 71], which is different from the gradual and sustained cAMP increase in cardiac fibroblasts (Fig. 8). These dynamic variations might be due to distinct cyclases and/or PDE isoforms coupling to the receptors in different cell types, or due to the nature of the cAMP sensor utilized. For instance, CNG-based sensors detect submembrane-restricted sub-cellular cAMP pools compared to the more uniformly distributed Epac-based sensors [43]. Previously, we failed to detect cAMP changes upon PDE1 inhibition in VSMCs [38] as well as in cardiomyocytes [38] that highly express PDE1A using traditional radioimmunoassay (RIA) methods. This might also be due to differences among the methodologies or cell types examined. Therefore, it will be of great interest to determine if PDE1A regulates cAMP in VSMCs and cardiomyocytes using more sensitive FRET based approaches. It will also be important to extrapolate these findings to human cardiac tissue, given that PDE isoforms may control different cyclic nucleotide patterns across different species [51].
The molecular mechanism of how rapid changes in cyclic nucleotide levels elicit long-term changes in gene expression remains elusive. It seems likely that distal gene programs regulating growth and differentiation are controlled by a summation of cyclic nucleotide signals, involving multiple tightly coupled effectors (i.e. Epac, PKG), and feedback regulation. The induction of immediate-early genes such as Egr-1, c-fos, c-jun is a characteristic response of Ang II stimulated cardiac fibroblasts [58]. The Smad-independent early growth response-1 (Egr-1) transcription factor was shown to critically regulate myofibroblast activation and tissue fibrosis, both in vivo and in vitro [40, 67]. Interestingly, we found Egr-1 expression was significantly downregulated in proliferative VSMCs treated with the PDE1 inhibitor IC86340 (unpublished observations). In contrast, global cAMP or cGMP elevation has minimal effects on Ang II mediated Egr-1 induction in VSMCs [22] or cardiac fibroblasts [58], respectively. Our findings that PDE1 controls unique spatial and temporal cAMP and cGMP dynamics may implicate differential regulation of stress responsive genes in activated cardiac fibroblasts.
Supplementary Material
Acknowledgments
We thank Dr. Soyeon Lim, Dr. Nhat-Tu Le, and Dr. Yuichiro Takei (University of Rochester, USA) for providing cardiac fibroblasts. We thank Dr. Kees Jalink (The Netherlands Cancer Institute, The Netherlands) for providing the permission to use the Epac1-H30-cyto construct. We thank Dr. Rajesh Kukreja (Virginia Commonwealth University, USA) for providing Ad-PKG I shRNA. We thank Dr. Jian-Dong Li (University of Rochester, USA) for providing the Smad-binding element-luciferase reporter construct and plasmids encoding Smad2 and Smad3. This work was supported by an American Heart Association Established Investigator Award 0740021N (to C.Y.), NIH grants HL088400 and HL077789 (to C.Y.), American Heart Association Predoctoral Fellowship 0815730D (to C.L.M.), This work was also supported by NIH HL68891 (to W.R.D.) and the Totman Trust for Biomedical Research (to W.R.D.) and by the British Heart Foundation PG/07/091/23698 (to M.Z.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-011-0228-2) contains supplementary material, which is available to authorized users.
Contributor Information
Clint L. Miller, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA
Yujun Cai, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA.
Masayoshi Oikawa, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA.
Tamlyn Thomas, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA.
Wolfgang R. Dostmann, Department of Pharmacology, University of Vermont College of Medicine, Burlington, VT 05405, USA
Manuela Zaccolo, Institute of Neuroscience and Psychology, University of Glasgow, Glasgow, UK.
Keigi Fujiwara, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA.
Chen Yan, Email: Chen_Yan@urmc.rochester.edu, Department of Pharmacology and Physiology, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642, USA.
References
- 1.Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res. 2003;93:406–413. doi: 10.1161/01.RES.0000091074.33584.F0. [DOI] [PubMed] [Google Scholar]
- 2.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147:3435–3445. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor AM, Bartus K, Reynell C, Constantinou S, Halvey EJ, Held KF, Dostmann WR, Vernon J, Garthwaite J. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc Natl Acad Sci USA. 2010;107:22060–22065. doi: 10.1073/pnas.1013147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bax NA, van Oorschot AA, Maas S, Braun J, van Tuyn J, de Vries AA, Groot AC, Goumans MJ. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFbeta-signaling and WT1. Basic Res Cardiol. 2011;106:829–847. doi: 10.1007/s00395-011-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 6.Beavo JA, Hardman JG, Sutherland EW. Hydrolysis of cyclic guanosine and adenosine 3′, 5′-monophosphates by rat and bovine tissues. J Biol Chem. 1970;245:5649–5655. [PubMed] [Google Scholar]
- 7.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode DC, Kanter JR, Brunton LL. Cellular distribution of phosphodiesterase isoforms in rat cardiac tissue. Circ Res. 1991;68:1070–1079. doi: 10.1161/01.res.68.4.1070. [DOI] [PubMed] [Google Scholar]
- 9.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 10.Butt E, Pohler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol. 1995;116:3110–3116. doi: 10.1111/j.1476-5381.1995.tb15112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, Miller CL, Nagel DJ, Jeon KI, Lim S, Gao P, Knight PA, Yan C. Cyclic nucleotide phosphodiesterase 1 regulates lysosome-dependent type I collagen protein degradation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:616–623. doi: 10.1161/atvbaha.110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castoldi G, Di Gioia CR, Pieruzzi F, D’Orlando C, Van De Greef WM, Busca G, Sperti G, Stella A. ANG II increases TIMP-1 expression in rat aortic smooth muscle cells in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H635–H643. doi: 10.1152/ajpheart.00986.2001. [DOI] [PubMed] [Google Scholar]
- 14.Castro LR, Schittl J, Fischmeister R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:1232–1240. doi: 10.1161/circresaha.110.226712. [DOI] [PubMed] [Google Scholar]
- 15.Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, Frey N. FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010;105:301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 16.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci USA. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle DD, Upshaw-Earley J, Bell EL, Palfrey HC. Natriuretic peptide receptor-B in adult rat ventricle is predominantly confined to the nonmyocyte population. Am J Physiol Heart Circ Physiol. 2002;282:H2117–H2123. doi: 10.1152/ajpheart.00988.2001. [DOI] [PubMed] [Google Scholar]
- 20.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 21.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 22.Giasson E, Servant MJ, Meloche S. Cyclic AMP-mediated inhibition of angiotensin II-induced protein synthesis is associated with suppression of tyrosine phosphorylation signaling in vascular smooth muscle cells. J Biol Chem. 1997;272:26879–26886. doi: 10.1074/jbc.272.43.26879. [DOI] [PubMed] [Google Scholar]
- 23.Hammoud L, Lu X, Lei M, Feng Q. Deficiency in TIMP-3 increases cardiac rupture and mortality post-myocardial infarction via EGFR signaling: beneficial effects of cetuximab. Basic Res Cardiol. 2011;106:459–471. doi: 10.1007/s00395-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 24.Hao Y, Xu N, Box AC, Schaefer L, Kannan K, Zhang Y, Florens L, Seidel C, Washburn MP, Wiegraebe W, Mak HY. Nuclear cGMP-dependent kinase regulates gene expression via activity-dependent recruitment of a conserved histone deacetylase complex. PLoS Genet. 2011;7:e1002065. doi: 10.1371/journal.pgen.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haworth RS, Cuello F, Avkiran M. Regulation by phosphodiesterase isoforms of protein kinase A-mediated attenuation of myocardial protein kinase D activation. Basic Res Cardiol. 2011;106:51–63. doi: 10.1007/s00395-010-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon KI, Jono H, Miller CL, Cai Y, Lim S, Liu X, Gao P, Abe J, Li JD, Yan C. Ca2+/calmodulin-stimulated PDE1 regulates the beta-catenin/TCF signaling through PP2A B56 gamma subunit in proliferating vascular smooth muscle cells. FEBS J. 2010;277:5026–5039. doi: 10.1111/j.1742-4658.2010.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 28.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D’Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–866. doi: 10.1172/jci8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim NN, Villegas S, Summerour SR, Villarreal FJ. Regulation of cardiac fibroblast extracellular matrix production by bradykinin and nitric oxide. J Mol Cell Cardiol. 1999;31:457–466. doi: 10.1006/jmcc.1998.0887. [DOI] [PubMed] [Google Scholar]
- 30.Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londono JE, Baba HA, Abramowitz J, Birnbaumer L, Penninger JM, Pongs O, Kuhn M. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol. 2010;105:583–595. doi: 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutalos Y, Nakatani K, Yau KW. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995;68:373–382. doi: 10.1016/s0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 33.Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leineweber K, Bohm M, Heusch G. Cyclic adenosine mono-phosphate in acute myocardial infarction with heart failure: slayer or savior? Circulation. 2006;114:365–367. doi: 10.1161/circulationaha.106.642132. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 36.Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci USA. 2010;107:5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuyama H, Tsuruda T, Kato J, Imamura T, Asada Y, Stasch JP, Kitamura K, Eto T. Soluble guanylate cyclase stimulation on cardiovascular remodeling in angiotensin II-induced hypertensive rats. Hypertension. 2006;48:972–978. doi: 10.1161/01.HYP.0000241087.12492.47. [DOI] [PubMed] [Google Scholar]
- 38.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C. Role of Ca2 +/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2 +/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98:777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura H, Isaka Y, Tsujie M, Rupprecht HD, Akagi Y, Ueda N, Imai E, Hori M. Introduction of DNA enzyme for Egr-1 into tubulointerstitial fibroblasts by electroporation reduced interstitial alpha-smooth muscle actin expression and fibrosis in unilateral ureteral obstruction (UUO) rats. Gene Ther. 2002;9:495–502. doi: 10.1038/sj.gt.3301681. [DOI] [PubMed] [Google Scholar]
- 41.Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci USA. 2008;105:365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 43.Nikolaev VO, Lohse MJ. Monitoring of cAMP synthesis and degradation in living cells. Physiology (Bethesda) 2006;21:86–92. doi: 10.1152/physiol.00057.2005. [DOI] [PubMed] [Google Scholar]
- 44.Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP. Active Wnt signaling in response to cardiac injury. Basic Res Cardiol. 2010;105:631–641. doi: 10.1007/s00395-010-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, Insel PA, Brunton LL, Meszaros JG. Angiotensin II enhances adenylyl cyclase signaling via Ca2 +/calmodulin. Gq-Gs crosstalk regulates collagen production in cardiac fibroblasts. J Biol Chem. 2003;278:24461–24468. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- 46.Pandey KN. Ligand-mediated endocytosis and intracellular sequestration of guanylyl cyclase/natriuretic peptide receptors: role of GDAY motif. Mol Cell Biochem. 2010;334:81–98. doi: 10.1007/s11010-009-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–1046. doi: 10.1161/01.res.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 48.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Rich TC, Xin W, Mehats C, Hassell KA, Piggott LA, Le X, Karpen JW, Conti M. Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells. Am J Physiol Cell Physiol. 2007;292:C319–C331. doi: 10.1152/ajpcell.00121.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M. Conserved expression and functions of PDE4 in rodent and human heart. Basic Res Cardiol. 2011;106:249–262. doi: 10.1007/s00395-010-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 53.Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Cohen Aubart F, Russel FG, Mougenot N, Vrignaud C, Lechat P, Lompre AM, Hulot JS. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe J. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/circresaha.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder PB, Florio VA, Ferguson K, Loughney K. Isolation, expression and analysis of splice variants of a human Ca2 +/calmodulin-stimulated phosphodiesterase (PDE1A) Cell Signal. 1999;11:535–544. doi: 10.1016/s0898-6568(99)00027-3. (S0898-6568(99)00027-3[pii]) [DOI] [PubMed] [Google Scholar]
- 56.Sonnenburg WK, Seger D, Beavo JA. Molecular cloning of a cDNA encoding the “61-kDa” calmodulin-stimulated cyclic nucleotide phosphodiesterase. Tissue-specific expression of structurally related isoforms. J Biol Chem. 1993;268:645–652. [PubMed] [Google Scholar]
- 57.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takizawa T, Gu M, Chobanian AV, Brecher P. Effect of nitric oxide on DNA replication induced by angiotensin II in rat cardiac fibroblasts. Hypertension. 1997;30:1035–1040. doi: 10.1161/01.hyp.30.5.1035. [DOI] [PubMed] [Google Scholar]
- 59.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiede K, Melchior-Becker A, Fischer JW. Transcriptional and posttranscriptional regulators of biglycan in cardiac fibroblasts. Basic Res Cardiol. 2010;105:99–108. doi: 10.1007/s00395-009-0049-8. [DOI] [PubMed] [Google Scholar]
- 61.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 62.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 64.Villarreal F, Epperson SA, Ramirez-Sanchez I, Yamazaki KG, Brunton LL. Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3 K. Am J Physiol Cell Physiol. 2009;296:C1178–C1184. doi: 10.1152/ajpcell.00291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Hayn K, Werthmann RC, Nikolaev VO, Hommers LG, Lohse MJ, Bunemann M. Gq-mediated Ca2+ signals inhibit adenylyl cyclases 5/6 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C324–C332. doi: 10.1152/ajpcell.00197.2009. [DOI] [PubMed] [Google Scholar]
- 66.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 67.Wu M, Melichian DS, de la Garza M, Gruner K, Bhattacharyya S, Barr L, Nair A, Shahrara S, Sporn PH, Mustoe TA, Tourtellotte WG, Varga J. Essential roles for early growth response transcription factor Egr-1 in tissue fibrosis and wound healing. Am J Pathol. 2009;175:1041–1055. doi: 10.2353/ajpath.2009.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA. 2008;105:6386–6391. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 72.Zhou HY, Chen WD, Zhu DL, Wu LY, Zhang J, Han WQ, Li JD, Yan C, Gao PJ. The PDE1A-PKCalpha signaling pathway is involved in the upregulation of alpha-smooth muscle actin by TGF-beta1 in adventitial fibroblasts. J Vasc Res. 2010;47:9–15. doi: 10.1159/000231716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.