Abstract
We describe the case of a patient with a diaphragmatic hernia associated with radiofrequency ablation for hepatocellular carcinoma who was successfully treated by laparoscopic surgery. A 62-year-old man with a long history of hepatitis C-induced liver cirrhosis was admitted to our institution because of recurrent postprandial periumbilical pain. Eight years earlier, he had undergone radiofrequency ablation for hepatocellular carcinoma at hepatic segment VIII. Computed tomography, gastrografin enema examination revealed transverse colon obstruction because of a diaphragmatic hernia. We diagnosed diaphragmatic hernia associated with the prior radiofrequency ablation treatment. The patient underwent laparoscopic repair of the diaphragmatic hernia. Though the patient experienced the recurrence once, relaparoscopic treatment has improved the patient's conditions. Thus, diaphragmatic hernia can develop as a complication of radiofrequency ablation treatment. A laparoscopic approach is safe, feasible, and minimally invasive, even in patients with cirrhosis who develop iatrogenic diaphragmatic hernia as a complication of radiofrequency ablation treatment.
Key words: Diaphragmatic hernia, Radiofrequency ablation, Complication, Laparoscopic surgery
Although surgery is accepted as the first-line treatment for hepatocellular carcinoma (HCC) and colorectal metastases that are limited in number, radiofrequency ablation (RFA) is an effective treatment option for patients with primary and metastatic liver tumor, who are not surgical candidates because of tumor location, poor hepatic reserve, or advanced age.1,2,3 Chen et al conducted a prospective randomized trial comparing RFA with hepatectomy; however, they were not able to determine whether on treatment alternative was superior to the other.4 RFA treatment is the best option among the locoregional treatments for HCC.5,6 According to the HCC treatment algorithm in the National Comprehensive Cancer Network guideline, RFA treatment should be chosen as a locoregional therapy depending on the degree of liver damage. Tumors ≤ 3 cm are optimally treated with ablation.7 RFA for hepatic tumors is a relatively safe modality with a reported overall complication rate of 7.1% and a very low mortality rate (0.3%);8 however, the guideline reinforces awareness of the major vessels, major bile ducts, diaphragm, and other intra-abdominal organs.7
Diaphragmatic hernia is defined as out-pocketing of abdominal contents into the thoracic cavity, through a defect in the diaphragm. However, most of the acquired diaphragmatic hernias are caused by penetrating or blunt traumatic injury and are rarely caused by surgical procedures such as gastric banding or abdominal surgeries (e.g., nephrectomy).4,9 Especially, the appearance of a diaphragmatic hernia after RFA treatment is quite rare and clinically unrecognized as a complication of RFA.10 Surgical intervention is the best single treatment for the permanent cure of a diaphragmatic hernia. Among the surgical procedures, open laparotomy for diaphragmatic hernia has been widely accepted; however, only 1 case of liver cirrhosis and HCC has been reported, in which a laparoscopic approach was used to treat the diaphragmatic hernia associated with RFA treatment.10 We report the case of a patient with a diaphragmatic hernia caused by RFA treatment for HCC with cirrhosis, who was successfully treated with laparoscopic surgery.
Case Report
A 62-year-old man with cirrhosis caused by chronic hepatitis C had been regularly visiting the internal medicine department of our institution for 20 years. The patient had previously received interferon therapy 3 times; however, the treatments were ineffective. In 2005, follow-up CT revealed a solitary liver tumor measuring 21 mm at segment VIII, just below the right diaphragm. The serum level of alpha-fetoprotein was elevated (41.8 μg/L; normal range: < 10 μg/L), and, pretreatment diagnosis by liver biopsy confirmed well-differentiated HCC. At the time, RFA was preferred rather than surgical treatment, considering the decreased hepatic reserve. Subsequently, he underwent RFA for HCC. RFA was performed under ultrasonographic guidance using the Cool-tip RF ablation system (Covidien, Mansfield, Massachusetts, US). The mass was approached by a transthoracic route without artificial pleural effusion or ascites, avoiding contact with lung parenchyma to prevent pneumothorax as a complication. A 7-gauge needle with an exposed tip 3 cm in length was used (Covidien, Mansfield, Massachusetts), with the generator switched on and set at 100 W. Three sessions were performed for 9.5 minutes in total. At completion of 3 sessions of ablation, the temperature of the ablated tissue had increased to 75°C. Eight years later and 4 weeks before admission for a hernia repair, he presented with recurrent and acute-onset postprandial periumbilical pain. There was no history of trauma. Afterwards, the patient reported symptoms consistent with ileus, such as nausea, belching, and evacuation difficulty. Subsequently, he was hospitalized, and we searched for the cause of the symptoms. Physical examination revealed mild tenderness around the umbilicus with no peritoneal signs. Laboratory data on admission showed normal level of white blood cells (4.4 × 109/L; normal range: 3.3–9.0 × 109/L). Regarding hepatic functions, the patient could be classified as Child-Pugh Class C with mild ascites, hypoalbuminemia (2.8 g/dL; normal range: 3.8−5.2 g/dL), prolonged prothrombin time (49.6%; normal range: 80−120%), mild elevation of total serum bilirubin level (2.3 mg/dL; normal range: 0.2−1.0 mg/dL), and without hepatic encephalopathy. Indocyanine green retention rate at 15 minutes was 46% (normal range: <10%), which suggested severe liver cirrhosis. To make a definite diagnosis, the patient underwent colonoscopic screening at first; however, the endoscope could not pass into the ascending colon. Subsequently, gastrografin enema showed that the right colon was highly compressed and obstructed due to out-pocketing into the thoracic cavity at the hepatic flexure (Fig. 1). The definitive diagnosis was further confirmed by CT showing a right diaphragmatic hernia. The right colon with the greater omentum deviated into the thoracic cavity (Fig. 2). Review of the radiograph obtained on admission showed deviation of the right colon across the right diaphragm, which suggested the possibility of partial rupture of the right diaphragm (Fig. 3). These findings led us to a preoperative diagnosis of diaphragmatic hernia. We considered that repairing the hernia laparoscopically, which reduces postoperative complications in patients with severe liver cirrhosis, would be the most appropriate treatment option. Under general anesthesia, the patient was placed in the supine position. The first 12-mm port was introduced above the umbilicus, by using the open laparotomy method. After creating a pneumoperitoneum by carbon dioxide insufflation, we inserted the ports in a similar fashion as a standard laparoscopic cholecystectomy (Fig. 4). Intraoperatively, the colon was excessively invaginated into the thoracic cavity through the diaphragmatic defect. The colon was bluntly separated from the diaphragmatic defect (Fig. 5). The size of the hernia orifice was estimated about 4 cm in diameter, the primary closure without application of tension-free mesh would be valid intraoperatively. The defect was completely repaired using 2-0 nonabsorbable monofilament polypropylene sutures (PROLENE; Ethicon Inc., Somerville, New Jersey) in an interrupted fashion (Fig. 6). Bowel resection was not required because intestinal blood flow was not disturbed. We did not place drain tubes to confirm the absence of bleeding, and all small wounds were closed subcutaneously. The operation time was 131 min and the estimated blood loss was 200 mL. No perioperative complications were encountered, including postoperative ascites predicted preoperatively. The patient received a normal diet on the first 3 postoperative days, and was discharged on the eighth postoperative day. The patient had been quite well since discharge; however, contrary to our expectation, the patient began to complain of dyspnea suddenly. Diaphragmatic hernia relapsed in the first 4 months after the surgery, and the patient underwent more relaparoscopic treatment (Fig. 7a). The diaphragmatic defect was repaired using tension-free Parietex Composite Open Skirt Mesh (Covidien, Mansfield, Massachusetts) with a relaxation suture in the middle of the defect (Fig. 7b). The mesh was fixed by Multifire Endo Hernia stapler (Covidien, Mansfield, Massachusetts). The main reason for using a tension-free mesh was that the recurrence was raised because of the enlarged hernia orifice and poor wound healing due to liver cirrhosis. No postoperative adhesion could be observed, and good intraoperative view could be realized as a result of the first laparoscopic treatment. The patient's conditions have improved since relaparoscopic surgery. Postoperative radiologic work-up showed no evidence of recurrence during the 4-month follow-up period.
Fig. 1.
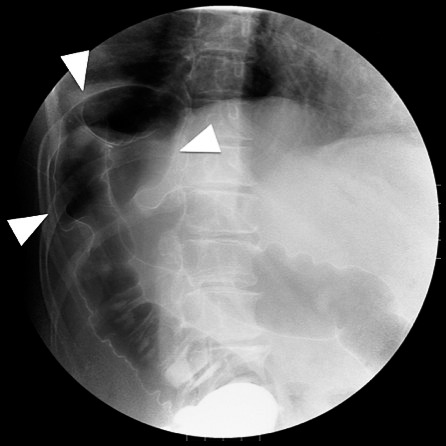
A gastrographin enema showed that the right colon was highly compressed and obstructed due to out-pocketing into the thoracic cavity at the hepatic flexure (white arrows).
Fig. 2.
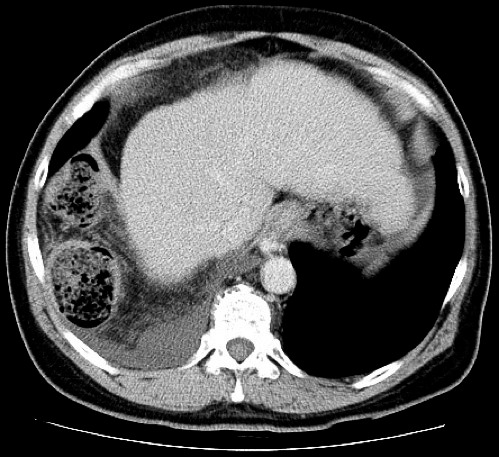
The definitive diagnosis was further confirmed by CT showing a right diaphragmatic hernia. The right colon with the greater omentum deviated into the thoracic cavity.
Fig. 3.
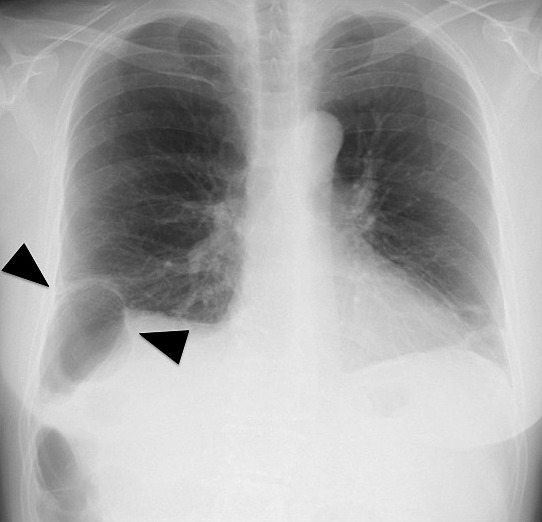
Review of the radiograph obtained on admission showed deviation of the right colon across the right diaphragm, which suggested the possibility of partial rupture of the diaphragm (black arrows).
Fig. 4.
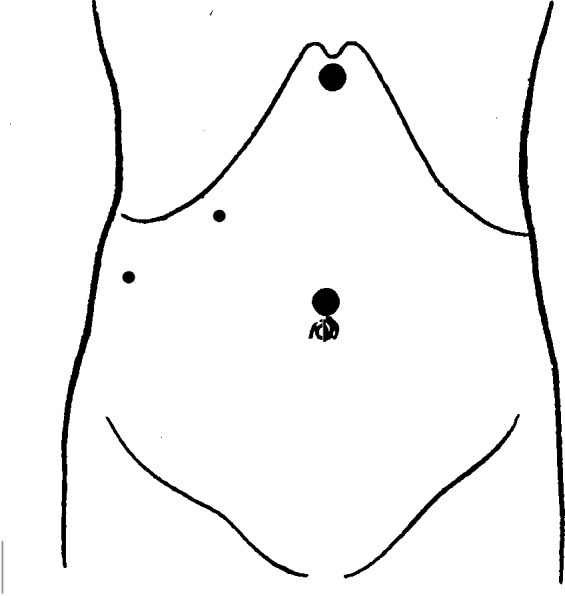
Placement of ports: umbilicus (12-mm), epigastric region (12-mm), right subcostal area (5-mm), and right flank region (5-mm).
Fig. 5.

Intraoperatively, the colon was excessively invaginated into the thoracic cavity through the diaphragmatic defect.
Fig. 6.
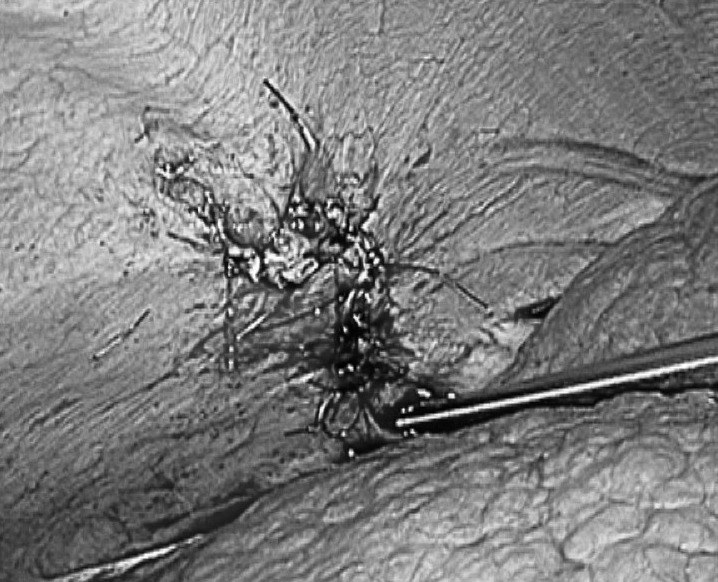
The defect was completely repaired using 2-0 nonabsorbable monofilament polypropylene sutures in an interrupted fashion.
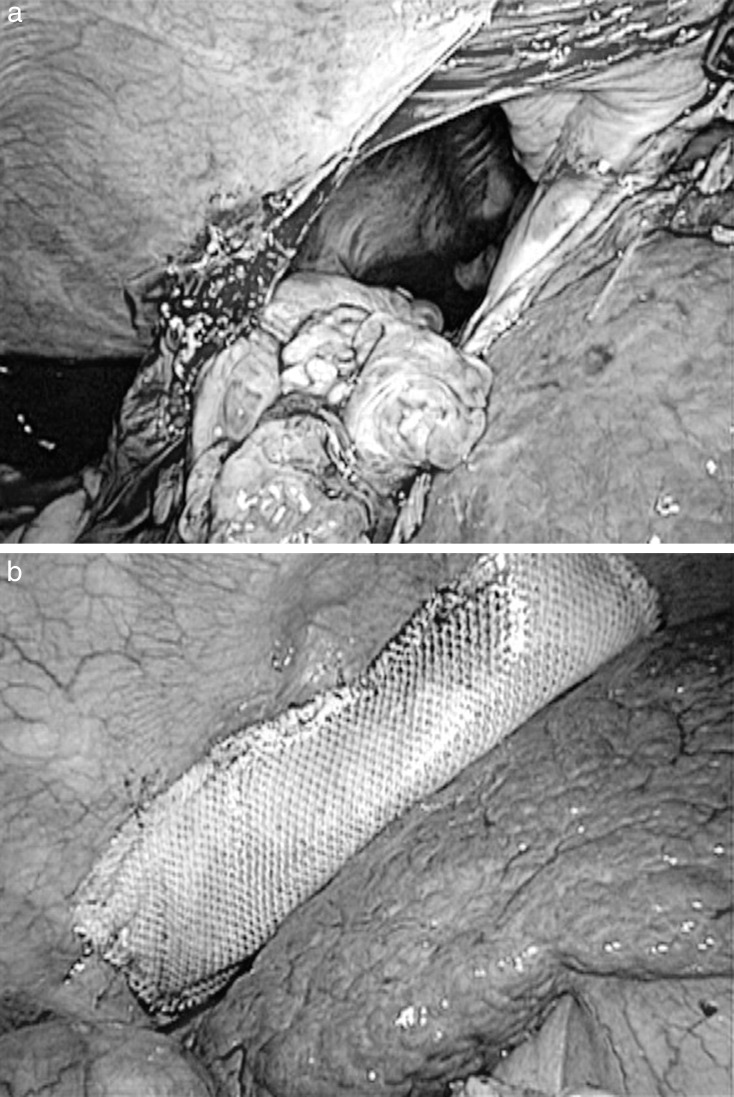
Fig. 7.
The big diaphragmatic defect was clearly visible again by laparoscope (a). The defect was repaired by tension-free mesh fixed by laparoscopic stapler (b).
Discussion
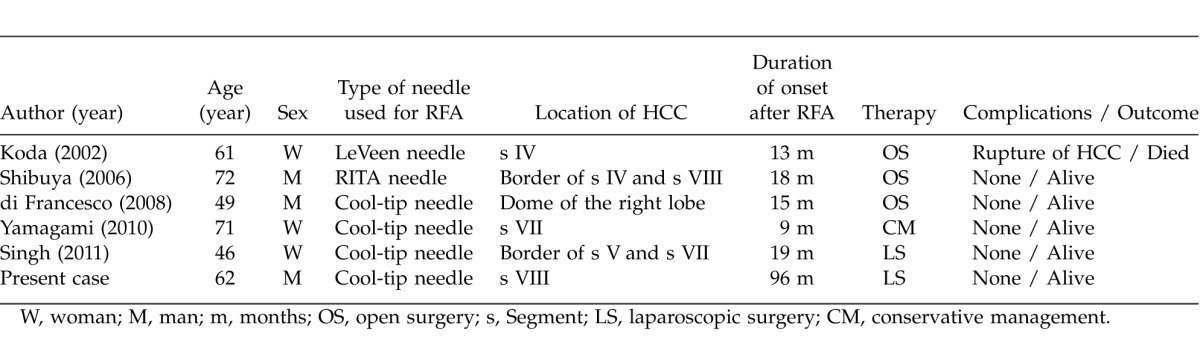
There are 2 main types of hepatic RFA-related complications. The first type consists of electrode placement-related complications, such as bleeding, vascular complications, pneumothorax, hemobilia, and ground pad burns. The other is thermal treatment-related injury to nontargeted organs including the bowel, gallbladder, bile ducts, and diaphragm.11 Nontargeted thermal injury to the diaphragm is well described in the literature, with a prevalence of approximately 17%.11,12 Since Shibuya et al reported the first case of diaphragmatic hernia as a complication of RFA,13 there have been only four reported cases of RFA-associated iatrogenic diaphragmatic hernia.13–16 Table 1 shows an overview of the English literature. Since the currently available literature on the subject is limited, physicians providing RFA treatment are unfamiliar with diaphragmatic hernia as a treatment-related complication. In the present case, it is noteworthy that the diaphragmatic hernia developed after more than 8 years. This delay is much greater than those described in other reports. As shown in Table 1, the onset of the defect occurs from 9–18 months after RFA treatment with a mean of 13.6 months. We speculated that the delayed appearance of the diaphragmatic hernia in the present case occurred mainly because of the progression of liver atrophy due to cirrhosis. The most probable pathogenesis of the present case occurred as follows. First, iatrogenic diaphragmatic injury occurred after RFA treatment 8 years previously. Then, the thermally-damaged diaphragm adhered with the liver. As the liver cirrhosis and atrophy progressed through the years, the adhesion between the injured right diaphragm and the surface of the liver began to be released and to buckle under the pressure of the abdomen. Finally, the injured diaphragm turned into a diaphragmatic hernia, pushing the right colon into the thoracic cavity. Chilaiditi's syndrome should remain as a differential diagnosis in diaphragmatic hernia; however, CT indicated incarceration of the right colon into the thoracic cavity, which ruled out Chilaiditi's syndrome.
Table 1.
Diaphragmatic hernia after the treatment of RFA
The only option to treat a diaphragmatic hernia is surgical intervention. However, liver cirrhosis limits this possibility. For the surgical treatment of patients with severe cirrhosis, interventional options should be carefully assessed. Kanazawa et al showed the importance and feasibility of laparoscopic hepatectomy compared with the conventional laparotomy method, considering postoperative complications. They indicated that intractable ascites can be notably reduced by using a laparoscopic approach.17 Likewise, we believe that a laparoscopic approach should be used for repairing diaphragmatic hernias. In the present case, we considered that a laparoscopic approach was safer and more feasible than open laparotomy. We arrived at this decision after carefully considering the possibility of postoperative complications, such as intractable pleural effusion and ascites followed by reduced collateral circulation in the abdominal wall and ligaments surrounding the liver. Once intractable pleural effusion and ascites develop, the patients could require prolonged hospitalizations and have increased risk of fatal liver failure in the worst cases. Kanazawa et al reported a decrease in postoperative morbidity, such as surgical site infection and intractable ascites, with the introduction of laparoscopic liver resection in patients with liver cirrhosis.17 Most importantly, our patient developed no complications, such as pleural effusion, ascites, or hepatic failure, and showed less postoperative pain, rapid recovery, and reduction of hospital stay. Furthermore, a laparoscopic approach provides a better intraoperative view than that provided by the traditional open procedure. The conventional laparotomy method is generally thought to be more invasive, because a larger skin incision and wound are needed to acquire a better intraoperative view. It is also a fact that the patient experienced the recurrence in the first 4 months after the first surgery; however, the prior laparoscopic procedure reduced as much as possible the degree of difficulty in the following relaparoscopic treatment, which realized the better intraoperative view. Moreover, adhesion formation could not be observed, synechiotomy was not required. Thus far, only 1 case of cirrhosis with HCC has been reported, in which the patient underwent a laparoscopic approach for the treatment of a diaphragmatic hernia that appeared after RFA treatment.10 Considering the fact that HCC usually develops in the presence of liver cirrhosis, careful selection of therapeutic options is required when treating HCC. If RFA is chosen as a treatment for HCC, unexpected diaphragmatic injury should be kept in mind. There were 3 lessons learned from the present case. First, we should have performed hydrodissection using artificial pleural effusion or ascites during the RFA treatment. Tatli et al recommend the use of hydrodissection to displace the diaphragm and prevent diaphragmatic injury during the ablation of tumors in the liver dome.1,18 Hydrodissection with artificial ascites can both reduce thermal damage of the diaphragm by a cooling effect and avoid contact with adjacent visceral organs such as the small bowel and colon.19–21 Second, we overlooked preoperative chest radiography findings. We should have suspected diaphragmatic hernia after observing partial elevation of the right diaphragm. Third, we should have considered diaphragmatic hernia as a differential diagnosis since the patient had undergone RFA treatment, even if the procedure had been performed more than 1−2 years earlier. Since RFA is becoming a more available treatment option, reports of RFA-associated diaphragmatic hernia are predicted to increase in number. Therefore, therapeutic options are important for RFA-associated diaphragmatic hernia, since this is an iatrogenic complication. Minimally invasive surgery should be especially indicated for treatment-associated complications. In conclusion, diaphragmatic hernia should be recognized as one of the early or late critical complications of RFA treatment, and a laparoscopic approach, which is technically feasible and safe, should be considered even for patients with severe liver cirrhosis.
Acknowledgments
The authors have no conflicts of interest to disclose, and the authors state that they declare to disclose any sponsorship or funding arrangement relating to this research.
References
- 1.Tatli S, Tapan U, Morrison PR, Silverman SG. Radiofrequency ablation: technique and clinical applications. Diagn Interv Radiol. 2012;18(5):508–516. doi: 10.4261/1305-3825.DIR.5168-11.1. [DOI] [PubMed] [Google Scholar]
- 2.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137(4):422–426. doi: 10.1001/archsurg.137.4.422. discussion 427. [DOI] [PubMed] [Google Scholar]
- 3.Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2008;18(4):672–677. doi: 10.1007/s00330-007-0811-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7(4):350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 8.Boyce S, Burgul R, Pepin F, Shearer C. Late presentation of a diaphragmatic hernia following laparoscopic gastric banding. Obes Surg. 2008;18(11):1502–1504. doi: 10.1007/s11695-008-9515-x. [DOI] [PubMed] [Google Scholar]
- 9.de Meijer VE, Vles WJ, Kats E, den Hoed PT. Iatrogenic diaphragmatic hernia complicating nephrectomy: top-down or bottom-up? Hernia. 2008;12(6):655–658. doi: 10.1007/s10029-008-0377-x. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Singh G, Pandey A, Cha CH, Kulkarni S. Laparoscopic repair of iatrogenic diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41(11):1132–1136. doi: 10.1111/j.1872-034X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 11.Mendiratta-Lala M, Brook OR, Midkiff BD, Brennan DD, Thornton E, Faintuch S, et al. Quality initiatives: strategies for anticipating and reducing complications and treatment failures in hepatic radiofrequency ablation. Radiographics. 2010;30(4):1107–1122. doi: 10.1148/rg.304095202. [DOI] [PubMed] [Google Scholar]
- 12.Head HW, Dodd GD, 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243(3):877–884. doi: 10.1148/radiol.2433060157. [DOI] [PubMed] [Google Scholar]
- 13.Shibuya A, Nakazawa T, Saigenji K, Furuta K, Matsunaga K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186(5 Suppl):S241–243. doi: 10.2214/AJR.04.0931. [DOI] [PubMed] [Google Scholar]
- 14.Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180(6):1561–1562. doi: 10.2214/ajr.180.6.1801561. [DOI] [PubMed] [Google Scholar]
- 15.di Francesco F, di Sandro S, Doria C, Ramirez C, Iaria M, Navarro V, et al. Diaphragmatic hernia occurring 15 months after percutaneous radiofrequency ablation of a hepatocellular cancer. Am Surg. 2008;74(2):129–132. [PubMed] [Google Scholar]
- 16.Yamagami T, Yoshimatsu R, Matsushima S, Tanaka O, Miura H, Nishimura T. Diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2011;34(Suppl 2):S175–177. doi: 10.1007/s00270-010-9832-z. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, et al. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27(7):2592–2597. doi: 10.1007/s00464-013-2795-9. [DOI] [PubMed] [Google Scholar]
- 18.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190(1):91–98. doi: 10.2214/AJR.07.2384. [DOI] [PubMed] [Google Scholar]
- 19.Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology. 2002;222(3):819–823. doi: 10.1148/radiol.2223001805. [DOI] [PubMed] [Google Scholar]
- 20.Raman SS, Aziz D, Chang X, Ye M, Sayre J, Lassman C, et al. Minimizing diaphragmatic injury during radiofrequency ablation: efficacy of intraabdominal carbon dioxide insufflation. AJR Am J Roentgenol. 2004;183(1):197–200. doi: 10.2214/ajr.183.1.1830197. [DOI] [PubMed] [Google Scholar]
- 21.Mendiratta-Lala M, Brook OR, Midkiff BD, Brennan DD, Thornton E, Faintuch S, et al. Quality initiatives: strategies for anticipating and reducing complications and treatment failures in hepatic radiofrequency ablation. Radiographics. 2010;30(4):1107–1122. doi: 10.1148/rg.304095202. [DOI] [PubMed] [Google Scholar]