Abstract
Although salvage esophagectomies are widely performed, reports on salvage lymphadenectomy (SL) are few. We review our SL cases to clarify the indications. Fifty-five patients with esophageal cancer underwent chemoradiotherapy or radiotherapy, including 3 patients with single lymph node (LN) recurrences and one with allochronic double cervical node recurrence. Our department removed 5 recurrent LNs from these 4 patients. In Case 1, right supraclavicular LN was judged to be metastatic and R0 resection was carried out; he is alive without recurrence. In Case 2, we found, allochronically, metastases in his left cervical paraesophageal LN and left supraclavicular LN; residual tumors were R1 in both lesions. He is alive despite esophageal recurrence. In Case 3, a lymphadenectomy was performed on his thoracic para-aortic LN; however, tumor was removed incompletely, and he died 4 months after SL from disease progression. In Case 4, a subcarinal LN was thought to be metastatic, and was removed but no malignant tissues detected. He died 17 months after SL from pneumonia. Our experiences suggest that some patients survive relatively long with SL. Moreover, molecular examination of resected lesions could guide subsequent therapies. SL might be more widely used for these patients if not otherwise contraindicated.
Key words: Esophageal cancer, salvage lymphadenectomy, Salvage surgery, Esophagectomy, Chemoradiotherapy
Esophageal cancer is the eighth most common form of cancer worldwide, and is one of the most difficult malignancies to cure.1 Excluding cases with severe concomitant diseases, surgery is the best modality to cure esophageal cancer.2 However, many patients with esophageal cancer have concomitant diseases that are associated with alcohol and tobacco consumption, such as chronic obstructive pulmonary disease, liver cirrhosis, and synchronous cancers of the lung or head and neck region.3 For patients with such concomitant diseases, chemoradiotherapy (CRT) is usually performed to cure esophageal cancer. For unresectable advanced-stage tumors, CRT is also used, and sometimes has favorable results. The Radiation Therapy Oncology Group trial (RTOG 85-01) has established CRT without surgery as one standard for definitive treatment.4 Many patients and oncologists have accepted the nonsurgical approach with CRT as definitive therapy for esophageal carcinoma. Although complete response (CR) rates are high and short-term survival is favorable after definitive CRT, locoregional disease persists or recurs in 40–60% of patients.5 From Japan, a phase II study of CRT for Stage II–III esophageal squamous cell carcinoma (JCOG9906)6 found a CR rate of 62.2%, with 34.2% patients having residual or locoregional recurrence without distant metastasis after CRT.
For resectable residual or recurrent lesions after definitive CRT, surgical excision is the only curative modality. Therefore, such operations are called salvage surgery. In Japan, salvage surgery is defined as a procedure for recurrent or residual cancer after definitive CRT (RT > 50 Gy)7 and thought to be the only curative method. Conversely, salvage surgery is widely considered elsewhere to be a type of palliative surgery—the excision of tissue to reduce the risk of death due to physiologic derangement. Although salvage esophagectomy is performed in many institutions in Japan,8–13 reports on salvage lymphadenectomy (SL) are still few.14,15 In this article, we review our SL cases, and examine indications for this kind of surgery.
Patients and Methods
Patients
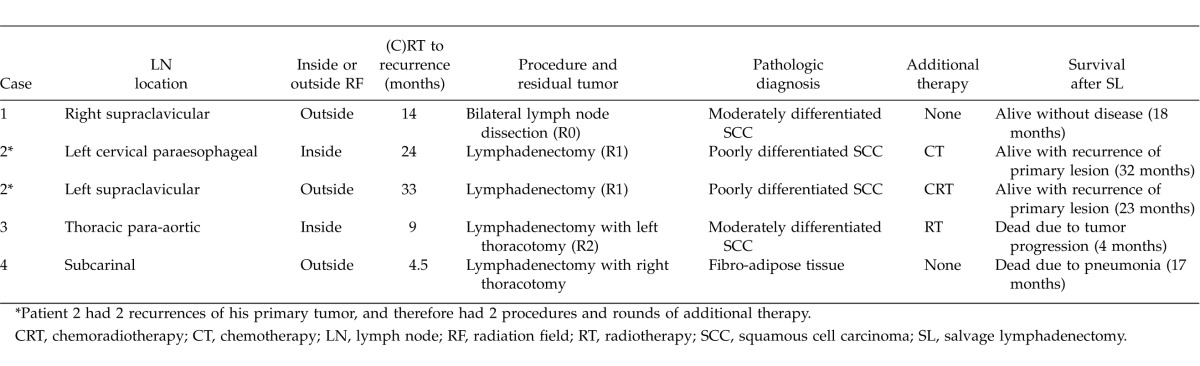
From May 2009 to July 2012, 55 esophageal cancer patients underwent CRT or RT (RT ≥ 50 Gy) at Dokkyo Medical University Hospital. Among them, 36 patients underwent CRT as initial therapy, another 15 patients did so for recurrent disease, and the remaining 4 patients because of microscopic positive disease after esophagectomy. Therapeutic effect was evaluated according to the criteria of the Japan Esophageal Society.16 Response evaluation of 51 patients with target lesions (the 4 patients with microscopic disease were excluded) was 27 patients (52.9%) with CR; 15 (29.5%) with partial response (PR); 5 (9.8%) with progressive disease (PD); and 4 (7.8%) with stable disease (SD). Among 27 patients who were evaluated with CR, 10 had recurrences; among these 10 patients, 6 had hematologic or multiple recurrent lesions. Subsequently, CRT revealed single lymph node recurrence in 4 patients (7.8%), including 1 with allochronic double cervical node recurrence. Finally, 5 recurrent lymph nodes were removed surgically from 4 patients in our department (Table 1). Among these patients, 2 patients had cervical node recurrence, and 2 suffered from mediastinal node recurrent disease. These 4 patients, with recurrence limited to the cervical or mediastinal nodes, were investigated in this study.
Table 1.
Patients receiving salvage lymphadenectomies
Diagnosis of recurrences
Recurrences were diagnosed by physical examinations and imaging studies including computed tomography (CT) and 18F-fluorodeoxy glucose positron emission tomography (FDG-PET), supplemented by use of tumor markers (CEA, SCC, and CYFRA).
Surgical procedures
All 4 patients underwent salvage lymph node (LN) dissection. The range of the dissection included lymphatic stations containing the metastatic nodes, and other stations if imaging studies showed any suspicious nodes. In the 3 cases of cervical LN dissection, the cervical approach with collar incision was used; for a case of thoracic para-aortic LN dissection, left 5th intercostal thoracotomy was used, and for a case of subcarinal LN dissection, right 4th intercostal thoracotomy was performed (Table 2).
Table 2.
Results and clinical courses of salvage lymphadenectomy cases
Follow-up
All patients were followed up as outpatients at least every month. The median follow-up period was 17.8 months.
Results
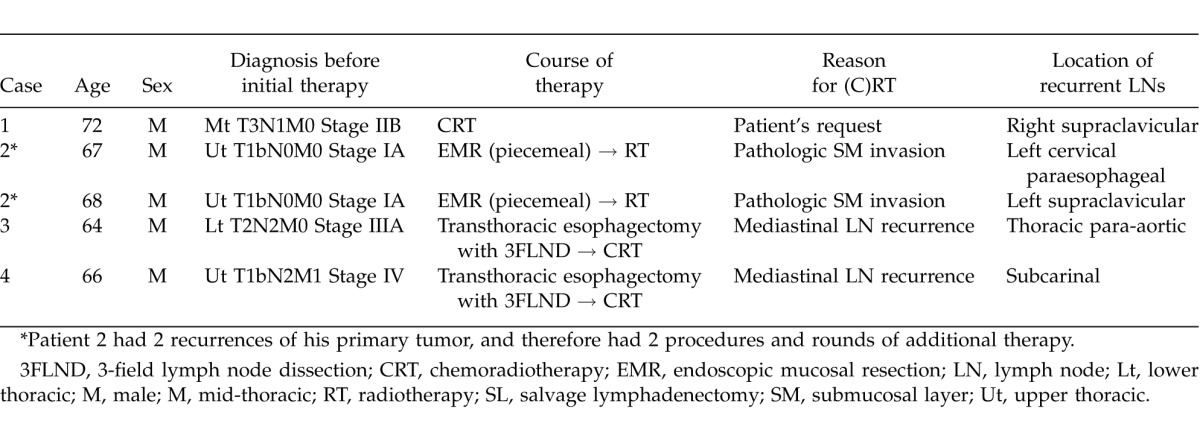
Table 1 shows patients' backgrounds and their reasons for undergoing (C)RT. Clinical stages at the time of initial therapy varied from I to IVa, according to the UICC (7th ed.) TNM classification. Table 2 shows the SL results. Clinical outcomes are summarized in Fig. 1.
Fig. 1.
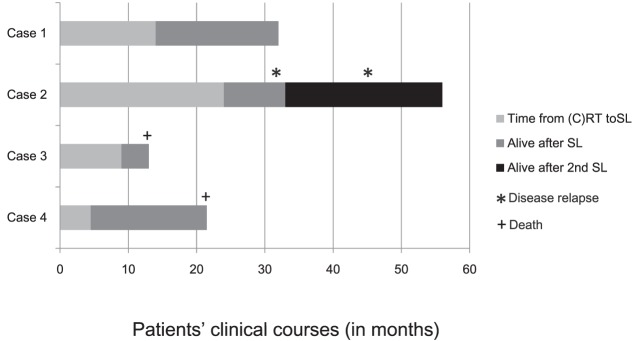
Clinical course for patients who underwent salvage lymphadenectomy after definitive chemoradiotherapy. Case 1: This patient is alive without recurrent disease. Case 2: This patient underwent salvage surgery twice. He is still alive despite recurrence of the primary lesion. Case 3: Salvage lymphadenectomy resulted in R2 resection. This patient underwent additional radiotherapy but died of disease progression. Case 4: Pathology findings were negative for malignant tissues. However, the patient died of pneumonia.
Case 1 underwent concurrent CRT at patient's request. The site of recurrence and removed lymph node after (C)RT by SL was right supraclavicular LN, which was located outside the radiation field. Pathologic examination of excised tumor showed squamous cell carcinoma (SCC). The excision has no residual tumor (R0), and no additional therapies were carried out after SL. He is still alive with no recurrent disease at 18 months after the SL.
Case 2 underwent an endoscopic mucosal resection; pathology findings showed tumor invasion to submucosal depth, for which radiotherapy was selected as definitive therapy. The recurrent and removed LNs were the left cervical paraesophageal LN (within the radiation field) and left supraclavicular LN (outside the radiation field). SCC was confirmed pathologically in both removed nodes; both lesions had microscopic residual tumor (R1). For the left cervical paraesophageal area, 2 courses of chemotherapy (cisplatin and 5-fluorouracil) were used; but CRT was used for the left supraclavicular area as it was located out of the irradiated area for the first CRT. Although this patient had an esophageal relapse 24 months after his first SL, which was 13 months after his second SL, he is still alive 8 months later, with recurrent disease.
Case 3 underwent transthoracic esophagectomy with 3-field lymph node dissections, using the McKeown technique, and later underwent CRT for thoracic para-aortic LN recurrence; however, viable tumor was evident after CRT, which pathologists found to be SCC in removed LN by the SL, and to have macroscopic residual tumor (R2). Although this patient underwent additional radiotherapy (30 Gy), residual tumor had progressed and multiple liver metastases appeared; as a result, he died 4 months after the SL.
Case 4 underwent transthoracic esophagectomy with 3-field lymph node dissections, using the McKeown technique. Thereafter, he underwent CRT for right recurrent nerve LN recurrence. The extracted site that was thought to be a recurrence was the subcarinal LN, which was located inside the radiation field. Although the resected tumor was not malignant; an obstinate pleural effusion appeared 12 months after the salvage surgery, and the patient suffered thereafter from repeated pneumonia, from which he died 17 months after the surgery. As of his death, no recurrent disease had been confirmed.
Discussion
In this article, we reviewed 4 patients and 5 lesions treated with SL after definitive CRT for esophageal cancer. CRT is the standard for unresectable esophageal cancer and could also be considered as an option for resectable tumors. For patients who are medically or technically inoperable, concurrent CRT should be the standard of care.5 For recurrent or residual tumors after CRT, salvage esophagectomy has recently become an important option, especially in Japan.8–13,17,18 However, no evidence seems to confirm the efficacy of SL after definitive CRT, although we found 2 case reports.14,15
The absence of systematic articles may have several causes. First, resectable recurrent lesions are relatively uncommon. For example, simultaneous multiple lymphadenopathy, simultaneous hematologic metastasis, and invasion to adjacent organs are not conducive to curative resections. Second, as sophisticated surgical technique is required, excision tends to be avoided. Third, prognoses after salvage surgery are often pessimistic.
In this series, 3 cervical lesions were removed from 2 patients, leading to R0 or R1 results. Although observation times are not yet long enough, SL for cervical lesions may be effective because cervical lymph nodes are easy to access and remove. Watanabe et al reported salvage cervical lymphadenectomy is a safe and effective treatment for patients with cervical node recurrence after esophagectomy.19 Their study had 8 patients with cervical lymph node recurrence; salvage cervical lymphadenectomy was performed for 5 patients among them, leading to R0 results in 4 patients. Lymphadenectomy performed after CRT makes LN removal more difficult as the radiation induces tissue fibrosis. However, salvage cervical lymphadenectomy must be valuable to prolong patients' survival.
Removal of 2 mediastinal lymph nodes in 2 patients in this series resulted in an R2 resection in one case, and the extraction of nonmalignant cells in the other case. Both of these 2 cases involved removal of recurrent mediastinal lesions after esophagectomy and CRT, and—unusually—transthoracic lymphadenectomy alone without esophagectomy for residual or recurrent lesion after definitive CRT. Although we could find no report on salvage transthoracic mediastinal lymphadenectomy, we did find some reports with regard to the difficulty of mediastinal lymphadenectomy after curative esophagectomy.20,21 In our cases, in addition to the status after esophagectomy, radiation-induced fibrosis made it difficult to remove the lesions completely. Moreover, a false-positive node was removed in one patient; worse, an obstinate pleural effusion appeared 12 months after the salvage surgery, thereafter, the patient suffered from repeated pneumonia, from which he died 17 months after the salvage surgery. Salvage lymphadenectomy may have adversely affected his condition. In this case, the indication of lymphadenectomy was decided by the finding of FDG-PET/CT. FDG-PET is very useful for detecting recurrent or residual lesions after CRT.22,23 After CRT, FDG is often taken up, due to inflammation. Although inflammation complicates differential diagnosis of malignant activity, accurate diagnosis is obviously required. In any case, the need for salvage mediastinal lymphadenectomy should be discussed carefully.
Salvage abdominal lymphadenectomy was not used in this series. However, Nakamura et al stated that lymphadenectomy might occasionally be indicated for patients with abdominal para-aortic node recurrence because dissection of para-aortic nodes requires distal pancreatosplenectomy and postoperative complications, including pancreatic fistula and intestinal adhesion, occurred frequently.20 In post-CRT lesions, such risk could be increased by radiation-induced tissue sclerosis, salvage abdominal lymphadenectomy might be appropriate in limited cases.
Indications for salvage surgery are often unclear, particularly as it is widely considered to be a palliative, rather than therapeutic, treatment. However, in Japan, salvage surgery is viewed and practiced as only method to cure residual or recurrent lesions after CRT for esophageal cancer. Although establishing a policy of salvage lymphadenectomy may be difficult, it offers a chance to prolong some patients' lives, as with the cases we present here. Moreover, molecular analysis of extracted LNs may guide subsequent therapies. SL may thus be a practicable expedient for patients with low surgical risk.
Conclusion
Effectiveness of SL after CRT is unclear because of insufficient evidence. However, our few experiences indicate that some patients can live longer through SL. Although futile surgery must be avoided, patients who would benefit from SL might be identified by accurate examination. Moreover, subsequent therapies may be found out by molecular examination of removed lesions could lead to improved subsequent therapies. SL could be more widely used where not contraindicated.
Acknowledgments
The authors have no conflicts of interest to report.
References
- 1.Kato H, Fukuchi M, Miyazaki T, Nakajima M, Tanaka N, Inose T, et al. Surgical treatment for esophageal cancer. Current issues. Dig Surg. 2007;24(2):88–95. doi: 10.1159/000101894. [DOI] [PubMed] [Google Scholar]
- 2.Kuwano H, Fukuchi M, Kato H. Thoracoscopic surgery for esophageal cancer. Ann Thorac Cardiovasc Surg. 2006;12(5):305–307. [PubMed] [Google Scholar]
- 3.Natsugoe S, Matsumoto M, Okumura H, Ishigami S, Uenosono Y, Owaki T, et al. Multiple primary carcinomas with esophageal squamous cell cancer: clinicopathologic outcome. World J Surg. 2005;29(1):46–49. doi: 10.1007/s00268-004-7525-y. [DOI] [PubMed] [Google Scholar]
- 4.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81(3):684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus. 2009;6(1):1–25. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tachimori Y. Role of salvage esophagectomy after definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg. 2009;57(2):71–78. doi: 10.1007/s11748-008-0337-5. [DOI] [PubMed] [Google Scholar]
- 9.Miyata H, Yamasaki M, Takiguchi S, Nakajima K, Fujiwara Y, Nishida T, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 2009;100(6):442–446. doi: 10.1002/jso.21353. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi H, Saikawa Y, Oyama T, Ozawa S, Suda K, Wada N, et al. Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg. 2010;34(2):277–284. doi: 10.1007/s00268-009-0331-9. [DOI] [PubMed] [Google Scholar]
- 11.Morita M, Kumashiro R, Hisamatsu Y, Nakanishi R, Egashira A, Saeki H, et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol. 2011;46(11):1284–1291. doi: 10.1007/s00535-011-0448-0. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. 2013;61(6):330–335. doi: 10.1007/s11748-013-0246-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother. 2013;14(10):1345–1354. doi: 10.1517/14656566.2013.801454. [DOI] [PubMed] [Google Scholar]
- 14.Doki Y, Yasuda T, Miyata H, Fujiwara Y, Takiguchi S, Yamasaki M, et al. Salvage lymphadenectomy of the right recurrent nerve node with tracheal involvement after definitive chemoradiation therapy for esophageal squamous cell carcinoma: report of two cases. Surg Today. 2007;37(7):590–595. doi: 10.1007/s00595-006-3447-7. [DOI] [PubMed] [Google Scholar]
- 15.Tada H, Shiozaki A, Fujiwara H, Ichikawa D, Okamoto K, Komatsu S, et al. Lymphadenectomy via a cervical approach for upper mediastinal lymph node recurrence of esophageal cancer: report of a case. Surg Today. 2011;41(11):1562–1566. doi: 10.1007/s00595-010-4521-8. [DOI] [PubMed] [Google Scholar]
- 16.Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: parts II and III. Esophagus. 2009;6(2):71–94. [Google Scholar]
- 17.Schieman C, Wigle DA, Deschamps C, Nichols FC, III, Cassivi SD, Shen KR, et al. Salvage resections for recurrent or persistent cancer of the proximal esophagus after chemoradiotherapy. Ann Thorac Surg. 2013;95(2):459–463. doi: 10.1016/j.athoracsur.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Yoo C, Park JH, Yoon DH, Park SI, Kim HR, Kim JH, et al. Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg. 2012;94(6):1862–1868. doi: 10.1016/j.athoracsur.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Nishida K, Kimura Y, Miyazaki M, Baba H. Salvage lymphadenectomy for cervical lymph node recurrence after esophagectomy for squamous cell carcinoma of the thoracic esophagus. Dis Esophagus. 2012;25(1):62–66. doi: 10.1111/j.1442-2050.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Ota M, Narumiya K, Sato T, Ohki T, Yamamoto M, et al. Multimodal treatment for lymph node recurrence of esophageal carcinoma after curative resection. Ann Surg Oncol. 2008;15(9):2451–2457. doi: 10.1245/s10434-008-0016-x. [DOI] [PubMed] [Google Scholar]
- 21.Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Uenosono Y, et al. The role of salvage surgery for recurrence of esophageal squamous cell cancer. Eur J Surg Oncol. 2006;32(5):544–547. doi: 10.1016/j.ejso.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Kato H, Fukuchi M, Miyazaki T, Nakajima M, Tanaka N, Inose T, et al. Prediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomography. Anticancer Res. 2007;27(4C):2627–2633. [PubMed] [Google Scholar]
- 23.Duong CP, Hicks RJ, Weih L, Drummond E, Leong T, Michael M, et al. FDG-PET status following chemoradiotherapy provides high management impact and powerful prognostic stratification in oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33(7):770–778. doi: 10.1007/s00259-005-0040-z. [DOI] [PubMed] [Google Scholar]