Abstract
Computed tomographic angiography (CTA) has become a mainstay in preoperative perforator flap planning in the modern era of reconstructive surgery. However, the increased use of CTA does raise the concern of radiation exposure to patients. Several techniques have been developed to decrease radiation dosage without compromising image quality, with varying results. The most recent advance is in the improvement of image reconstruction using an adaptive statistical iterative reconstruction (ASIR) algorithm. We sought to evaluate the image quality of ASIR in preoperative deep inferior epigastric perforator (DIEP) flap surgery, through a direct comparison with conventional filtered back projection (FBP) images. A prospective review of 60 consecutive ASIR and 60 consecutive FBP CTA images using similar protocol (except for radiation dosage) was undertaken, analyzed by 2 independent reviewers. In both groups, we were able to accurately identify axial arteries and their perforators. Subjective analysis of image quality demonstrated no statistically significant difference between techniques. ASIR can thus be used for preoperative imaging with similar image quality to FBP, but with a 60% reduction in radiation delivery to patients.
Key words: Adaptive statistical iterative reconstruction, Computed tomographic angiography, Deep inferior epigastric artery, Perforator flap
Perforator flaps have become a mainstay in reconstructive surgery throughout the body, as they minimize donor site morbidity while allowing an almost infinite array of reconstructive options in terms of their vascular basis and surgical design. This is no more so than in breast reconstruction, with pioneering work since the early 1990s from surgeons such Robert Allen and others, expanding the use of abdominal wall tissues in free-tissue transfer to reconstruct breast tissue based on the deep inferior epigastric artery (DIEA) and its perforators.1 The DIEA perforator (DIEP) flap comprises the dissection of perforating branches of the DIEA and associated veins through the rectus abdominis muscle, while sparing both muscle and fascia from being harvested. The fine detail of this anatomy, including the course of the vasculature from source pedicle, through muscle, fascia, and fat, is complex and highly variable, with numerous variations in the vascular anatomy of the DIEA and its branches having been described in the literature.2–5 The ability to preoperatively define the location and diameter of perforating vessels has many advantages: reduced learning curve, operative times, and abdominal wall morbidity, as well as an increase in flap reliability and outcomes. For these reasons, preoperative imaging in the form of computed tomographic angiography (CTA) has been shown to be useful in assisting reconstructive surgeons in flap design and in identifying the vascular anatomy.6–9 CTA has been shown to have high accuracy and has now been adopted as part of the standard preoperative workup for DIEP free flap reconstructions by many (and anecdotally, most) international centers.
The increased use of preoperative CTA for DIEP flap reconstruction is not without implications though. There is a concern both in the literature and among clinicians regarding the magnitude of radiation exposure delivered in computed tomography (CT) and the potential for increasing the risk of radiation-induced carcinogenesis. The literature, although inconclusive, has reported a 0.6% to 3.2% increase in the cumulative risk of cancer until the age of 75, which has been attributed to diagnostic exposure in the developed world.10,11 In the United States, the estimated annual effective dose from medical radiation exposure per indivdual has increased sixfold over the past quarter century (from 0.5 mSv in 1980 to 3.0 mSv in 2006).12 This increase is largely attributable to the rapid and subsantial rise in CT utilization, and the growing concern for patient safety has driven the scientific community to reduce the radiation exposure with dose-reduction CT imaging. Several dose-reduction techniques have been implemented, such as tube current modulation, reduced tube voltage, use of a higher pitch, and noise reduction filters. These techniques have been shown to have variable degrees of success due to the depth of radiation penetration and image distortion. When using a standard filtered back projection (FBP) reconstruction alone, any reduction in radiation dosage will result in an increase of image noise that degrades image quality. This has substantially limited the implementation of such techniques. In preoperative perforator flap imaging with CTA, the image sharpness is critical for interpretation of the position, diameter, and course of the vascular anatomy, with vessels as small as 0.5 mm being analyzed for each of these variables.
The adaptive statistical iterative reconstruction (ASIR) technique, which has recently been incoporated into commercial CT scanners, allows for noise reduction with the potential for no interference in spatial resolution. It has been reported in the literature for identifying pathology within the chest, brain, cardiovascular, and abdominopelvic regions. Several manuscripts have reported a similar if not superior image quality when compared with conventional FBP technique.13–16 This has not been investigated or reported in the field of reconstructive surgery to the best of our knowledge. ASIR, however, is not free from unfavorable effects. Yanagawa et al15 and Hara et al17 have found that the technique can alter the texture of image noise and can yield a unusually homogenous image, which may not be immediately appealing to most radiologists usually accustomed to the FBP image. It is also known that an excessive degree of iterative reconstruction may obscure fine and subtle findings.15,17
In this study, we aim to analyze and subjectively assess the image quality of ASIR in preoperative perforator flap CTA of the abdominal wall, with a particular focus on DIEA perforators for DIEP flap breast-reconstruction planning. A comparison to conventional FBP is made as part of this assessment, with a view to assessing the quality and feasibility of the 2 techniques.
Materials and Methods
A quantitative and qualitative retrospective imaging study was undertaken, utilizing the scans of 120 consecutive abdominal-wall CTA images. All patients gave consent for undergoing CTA as per our institute protocol with no exclusions. This and other aspects of the study comply with the Declaration of Helsinki, 1995.
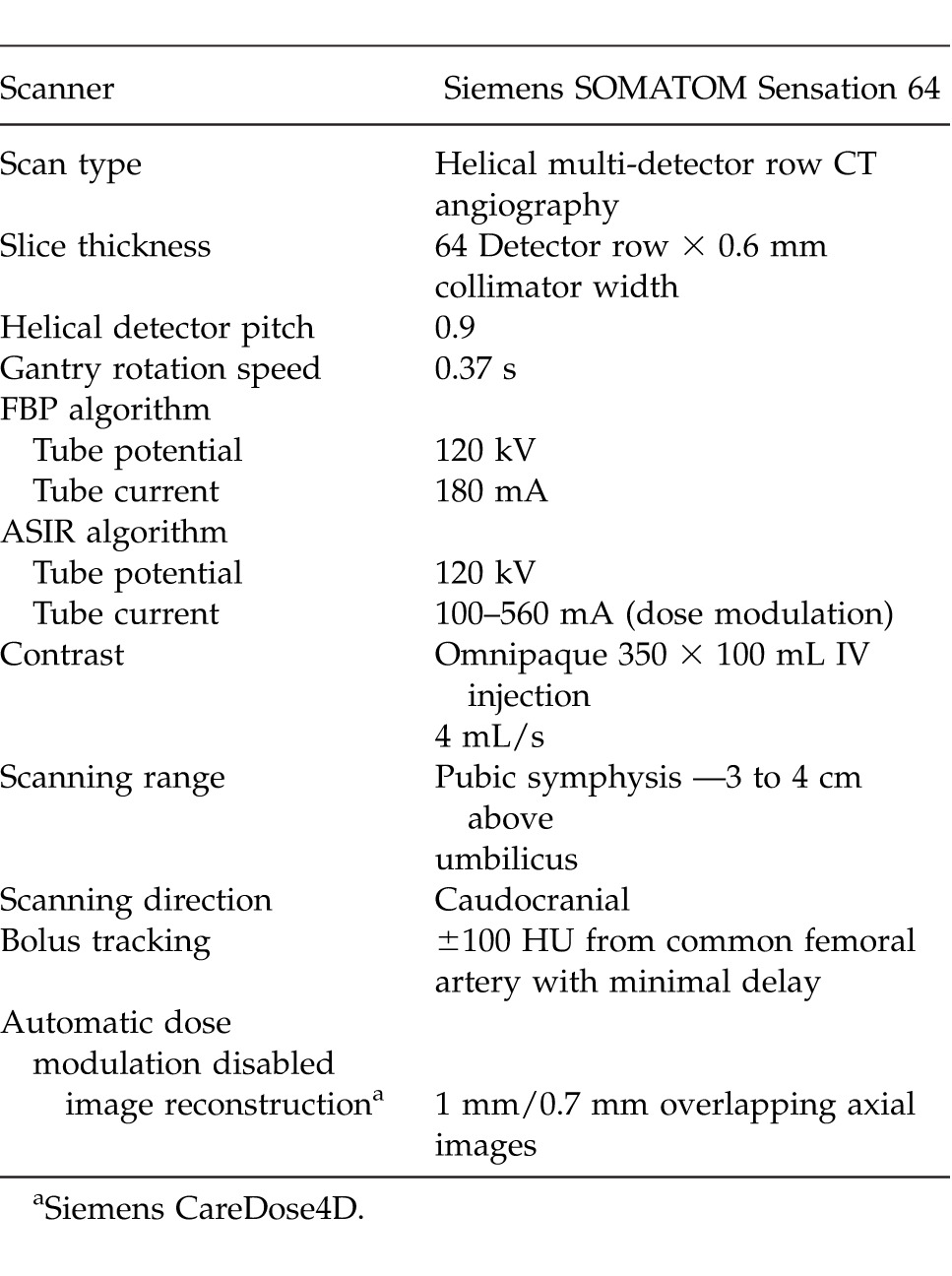
A retrospective analysis of prospectively collected data was undertaken from 3 imaging institutions with the same abdominal CTA protocol (Table 1). Both FBP and ASIR used the same protocol in obtaining the images except for the tube current and tube potential. In FBP, the tube current was 180 mA and tube potential was 120 kV. In the ASIR group, the tube current was 100 to 560 mA (dose modulated), and the tube potential was 120 kV. All scans were performed for preoperative imaging for DIEP free flap breast reconstruction between May 2011 and May 2013. All patients were female, spanned a range of body habitus types, and were 32 to 78 years of age. In each case, thin axial images were generated and reformatted into maximum intensity projection (MIP) and volume-rendered technique (VRT) reconstruction using commercially available software (Siemens Syngo InSpace; Version InSpace2004A_PRE_19, Siemens Australia, Victoria, Australia) and Osirix (Osirix Medical Imaging Software, GPL Licensing Open Source Initiative, http://www.osirix-viewer.com). The multiplanar 3-dimensional reconstructions enable visualization of the DIEA, its branching pattern, and communications.
Table 1.
CT scan parameters
Two investigators were used for image analysis: both had reported over 100 perforator-flap CTA scans to eliminate the influence of learning curve. The investigators independently evaluated the quality of each image in the following 2 aspects: the ability to identify axial vessels and their perforators, and the quality of the images. Both reviewers were blinded to the CTA techniques. Included in the analysis were the identification of major pedicles: each DIEA and each superficial inferior epigastric artery (SIEA). The course, branching pattern, and continuity of the vessels were recorded. The size of each of the DIEA perforators was also identified and recorded. In the analysis of image quality, the following 2 areas were analyzed: “noise” of the surrounding tissues and “image sharpness.” For comparison of image sharpness, the reviewers were asked to evaluate the sharpness of the axial vessels and the perforators, including the continuity of the course and distinction of the vessels from surrounding structures, using the Likert scale. This grading ranged from 1 = poor, 2 = fair, 3 = moderate, 4 = good, and 5 = excellent. Subjective image noise was also assessed by a 5-point scale: 1 = unacceptable image noise, 2 = less than average noise, 3 = average image noise, 4 = above average noise, and 5 = minimal image noise. Differences in diagnostic accuracy between the 2 reconstruction techniques were compared by creating matched sample tables and by using Student t test and λ2 test to calculate P values as appropriate.
Results
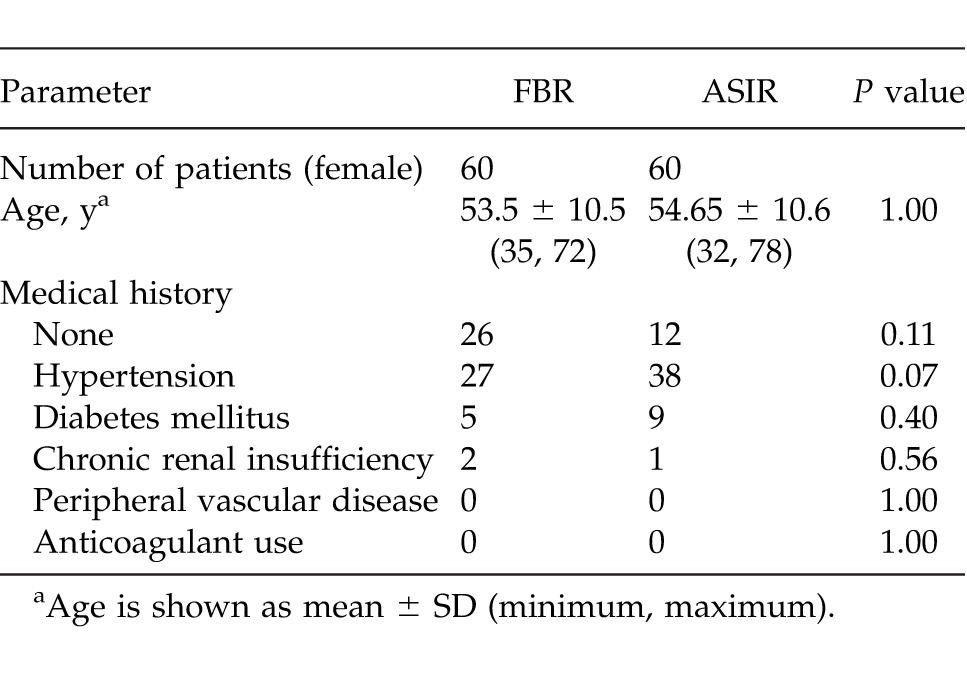
A total of 120 patients underwent preoperative CTA imaging prior to DIEA flap breast-reconstruction surgery. Of these, 60 patients (all scanned early in the series) had a conventional FBP, and 60 were scanned with ASIR technology. Between the 2 groups, there was no significant difference between presenting demographics, with all the patients female and no other significant differing factors. There was no statistically significant difference in the age of patients in either group: FBP, 53.5 ± 10.5 years (minimum age, 35; maximum, 72); ASIR, 54.65 ± 10.6 years (minimum age 32; maximum, 78). Between the 2 study groups, there was no significant difference in any of the following categories: medical history, hypertension, diabetes mellitus, anticoagulant use, chronic renal insufficiency, or peripheral vascular disease with all P values >0.05, as shown in Table 2.
Table 2.
Patients' demographics
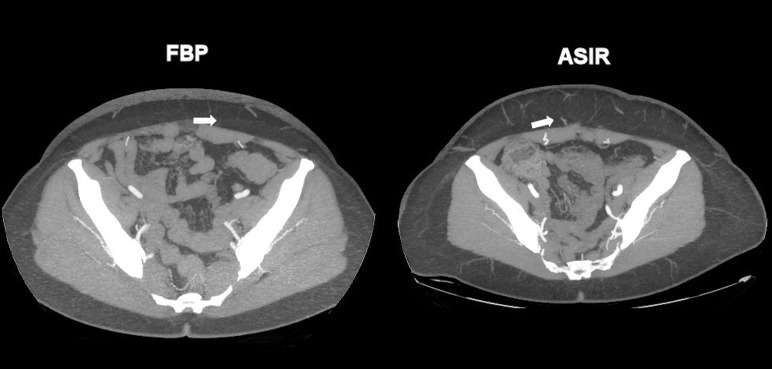
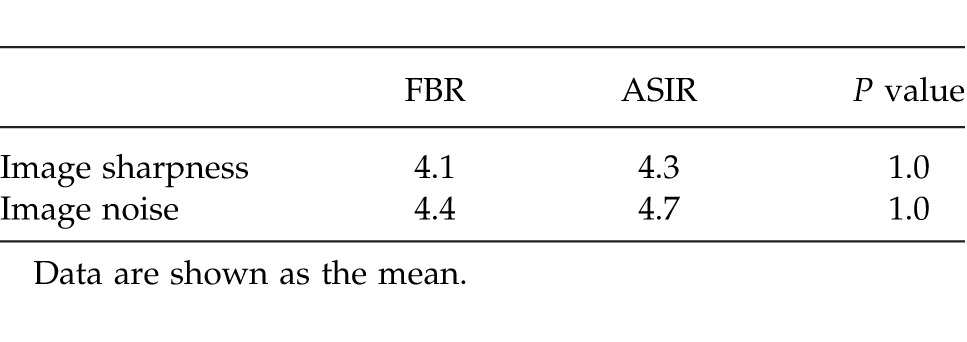
In all 120 CTA images, the course of the DIEAs were identified and found to be in continuity. Branching patterns of all DIEAs were identifiable by both methods. The intramuscular course of vessels was uniformly able to be traced within the rectus abdominis muscle. The location and size of both DIEAs and SIEAs were identifiable by both methods. The branching patterns of the DIEAs were similar in both groups, with a type II (bifurcating trunk) being the most common anatomic variation. Both FBP and ASIR algorithms were able to identify perforators as small as 0.7 mm in diameter within the anterior abdominal wall (smaller vessels were identifiable but not assessed). This finding is highlighted in Table 3 and Fig. 1. Overall image sharpness and noise was rated by the 2 independent readers to be similar by both methods, with a P value of 1.0 as shown in Table 4. The grading of the overall subjective and objective image quality revealed that reviewers' rankings showed no difference between the FBP data sets than they had for the ASIR data sets. The sharpness and noise difference for both techniques had a P value of 1.00 as shown in Table 4.
Table 3.
Identification analysis
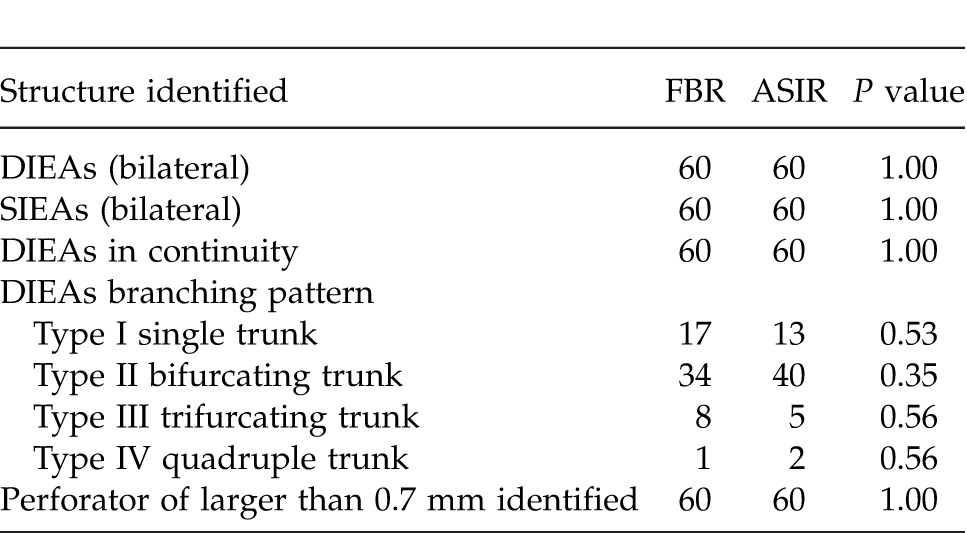
Fig. 1.
FBP and ASIR images identifying 0.7-mm perforator (white arrows).
Table 4.
Image quality analysis using Likert visual scale
Discussion
Previously, many reconstructive surgeons sought to improve outcomes in free or pedicle flap surgery by focusing on improving operative techniques and postoperative monitoring. With a growing population of patients undergoing this surgery, and the introduction of objective preoperative imaging with CTA, it has become evident that there is significant variability in vascular anatomy, particularly in the abdominal wall, which can substantially affect surgical approach and outcomes. With this awarensss, the use of preoperative CTA has since been shown to reduce flap-related complications, donor site morbidity, and operative stress for surgeons. There is also a decrease in operative times and a trend towards reduced length of hospital stay.18 CTA for preoperative imaging in DIEP-flap reconstruction has now been adopted by various centers around the world as routine. In addition to DIEP-flap surgery, other flaps have shown similar results, including the anterolateral thigh (ALT), deep superior epigastric artery (DSEA), and superior and inferior gluteal artery perforator (SGAP and IGAP) flaps. In many cases, there is also a desire to image the recipent sites because of the potential for anatomic variation or pathologic changes secondary to trauma or peripheral vascular disease. As such, there is an increased utilization of CTA and growing concern with regard to side effects of the imaging such as radiation exposure to the patient.
The desire for finer resolution, greater volume coverage, faster scan time, and concurrently lower radiation dosage has motivated efforts to improve performance of CT imaging and led to advances in imaging techniques. Several methods have been tried to address these issues, including lowering the tube current-time product, automatic exposure control, reducing the peak kilovoltage, using a higher pitch, and shielding radiosensitive organs such as the breast, thyroid, and lenses of the eye.19–21 Another successful technique has been to improve the image quality in low-radiation–dose CT. In this regard, image noise in low-dose CT can be decreased by using noise-reduction filters in FBP image domains or by using statistical iterative reconstruction techniques working with raw CT data.22 The introduction of the ASIR algorithm has presented one of the most promising innovations for radiation-dose reduction. The standard FBP algorithm operates on several fundamental assumptions about scanner geometry by way of compromising between reconstruction speed and image noise. In order to improve image quality (i.e., noise reduction and increased image sharpness), a more complex computational algorithm and combination of multiple iterations of reconstruction is required, which has become known as “statistical iterative reconstruction.” This technique may result in longer reconstruction times, but in substantially less image noise from the same raw data, achieved through more complex modeling of detector response and of statistical measurements. An adaptive iterative reconstruction bypasses the FBP reconstruction and can help shorten the longer reconstruction times of pure iterative reconstruction, while maintaining much lower image noise than if the same raw data were reconstructed with FBP alone.17,23,24
The first series of publications on adaptive statistical iterative reconstruction algorithms for abdominal CT demonstrated potential dose reduction of up to 65%.17,24 Since then, several papers have reported similar findings with the use of CT in several regions of the body including the lung to diagnose pulmonary embolism, in pediatric patients to investigate congenital cardiovascular pathology, and in the abdomen for intra-abdominal pathology.25–27 These studies have demonstrated that the ASIR alogrithm can be used successfully with similar or higher image quality when compared with conventional FBP and, in fact, has been shown in vascular imaging to be able to distinquish soft tissues and vascular structures effectively. Another aspect of CTA is concern with regard to decreased tissue penetration when using techniques to lower radiation dosage. This is particularly important in DIEP-flap reconstruction, with the cohort of patients selected for this surgery known to have a somewhat thick abdominal pannus, in order to make them eligible for breast reconstruction. These techniques to reduce radiation dosage can therotically reduce the image quality, which may lead to substantially increased image noise and compromised diagnostic confidence for vascular anatomy detection. However, as a study by Singh et al26 has highlighted, the image quality of the ASIR approach was no different to FBP for patients of <90 kg body weight and was in fact better for patients >90 kg. This finding suggests that ASIR techniques can be used regardless of patient body habitus.
In this prospective study of 120 patients, the use of ASIR was evaluated in relation to image quality and diagnostic performance. We also compared ASIR with the conventional FBP CTA images for DIEP-flap preoperative planning, in an approach that saw almost 63% less radiation dosage delivered to patients. We have demonstrated there were no significant differences in image quality, in regards to image sharpness or noise, and no significant differences between ASIR and FBP with regards to diagnostic acceptability. To the best of our knowledge, this is the first prospective clinical study in the evaluation of preoperative CTA for DIEP free flap reconstruction between the 2 methods of ASIR and FBP. These findings are consistent with the results from previous reports in other fields. We were able to demonstrate the accuracy for identifying perforators as small as 0.7 mm and in analyzing the fine details of the anatomy of vessels of this size.
Conclusions
The use of ASIR has a clear benefit in radiation reduction when compared with FBP and can do so without severely compromising image quality. The technique can be used for preoperative CTA imaging in DIEP-flap reconstruction with high image quality and no detriment to preoperative planning. It can therefore be concluded that ASIR is a suitable technique in preoperative CTA imaging that should be sought as standard.
Acknowledgments
Some of this work was presented at the 38th International College of Surgeons (ICS) World Congress in Brisbane, Australia in November 2012. Warren Rozen undertook this work while a scholarship recipient of the AVANT Doctors in Training Research Scholarship. There were no other grants, research scholarships, or financial support provided for the study. There is no conflict of interest on preparation of this manuscript.
References
- 1.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Heitmann C, Felmerer G, Durmus C, Matejic B, Ingianni G. Anatomical features of perforator blood vessels in the deep inferior epigastric perforator flap. Br J Plast Surg. 2000;53(3):205–208. doi: 10.1054/bjps.1999.3257. [DOI] [PubMed] [Google Scholar]
- 3.Heo C, Yoo J, Minn K, Kim S. Circummuscular variant of the deep inferior epigastric perforator in breast reconstruction: importance of preoperative multidetector computed tomographic angiography. Aesthetic Plast Surg. 2008;32(5):817–819. doi: 10.1007/s00266-008-9219-6. [DOI] [PubMed] [Google Scholar]
- 4.Lasso JM, Sancho M, Campo V, Jimenez E, Perez CR. Epiperitoneal vessels: more resources to perform DIEP flaps. J Plast Reconstr Aesthet Surg. 2008;61(7):826–829. doi: 10.1016/j.bjps.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Rozen WM, Houseman ND, Ashton MW. The circummuscular or paramuscular variants of deep inferior epigastric perforators detected with CTA: should these be called variants at all? Aesthetic Plast Surg. 2009;33(1):119–120. doi: 10.1007/s00266-008-9252-5. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, Cano D, Martinez-Cuesta A, Pina LJ. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multi-slice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg. 2006;59(6):585–593. doi: 10.1016/j.bjps.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Rozen WM, Ashton MW. Modifying techniques in deep inferior epigastric artery perforator flap harvest with the use of preoperative imaging. ANZ J Surg. 2009;79(9):598–603. doi: 10.1111/j.1445-2197.2009.05013.x. [DOI] [PubMed] [Google Scholar]
- 8.Rozen WM, Garcia-Tutor E, Alonso-Burgos A, Acosta R, Stillaert F, Zubieta JL, et al. Planning and optimizing DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconst Aesth Surg. 2010;63(2):289–297. doi: 10.1016/j.bjps.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Rozen WM, Phillips TJ, Stella DL, Ashton MW. Preoperative CT angiography for DIEP flaps: 'must-have' lessons for the radiologist. J Plast Reconstr Aesthet Surg. 2009;62(12):e650–651. doi: 10.1016/j.bjps.2008.11.039. http://www.jpras.com Accessed Nov 1, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Mettler FA, Jr, Bhargavna M, Faulkner K, Giley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253(2):520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 11.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363(9406):345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 12.Schauer DA, Linton OW. NCRP. Report No. 160, ionizing radiation exposure of the population of the United States, medical exposure—are we doing less with more, and is there a role for health physicists? Health Phys. 2009;97(1):1–5. doi: 10.1097/01.HP.0000356672.44380.b7. [DOI] [PubMed] [Google Scholar]
- 13.Pontana F, Duhamel A, Pagniez J, Flohr T, Faivre JB, Hachulla AL, et al. Chest computed tomography using iterative reconstruction vs filtered back projection (part 2): image quality of low-dose CT examinations in 80 patients. Eur Radiol. 2011;21(3):636–643. doi: 10.1007/s00330-010-1991-4. [DOI] [PubMed] [Google Scholar]
- 14.Prakash P, Kalra MK, Digumarthy SR, Hsieh J, Pien H, Singh S, et al. Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr. 2010;34(1):40–45. doi: 10.1097/RCT.0b013e3181b26c67. [DOI] [PubMed] [Google Scholar]
- 15.Yanagawa M, Honda O, Yoshida S, Kikuyama A, Inoue A, Sumikawa H, et al. Adaptive statistical iterative reconstruction technique for pulmonary CT: image quality of the cadaveric lung on standard- and reduced-dose CT. Acad Radiol. 2010;17(10):1259–1266. doi: 10.1016/j.acra.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, et al. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology. 2010;254(1):145–153. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 17.Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol. 2009;193(3):764–771. doi: 10.2214/AJR.09.2397. [DOI] [PubMed] [Google Scholar]
- 18.Rozen WM, Anavekar NS, Ashton MW, Stella DL, Grinsell D, Bloom RJ, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery. 2008;28(7):516–523. doi: 10.1002/micr.20526. [DOI] [PubMed] [Google Scholar]
- 19.Cohnen M, Fischer H, Hamacher J, Lins E, Kötter R, Mödder U. CT of the head by use of reduced current and kilovoltage: relationship between image quality and dose reduction. AJNR Am J Neuroradiol. 2000;21(9):1654–1660. [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzo S, Kalra M, Schmidt B, et al. Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR Am J Roentgenol. 2006;186(3):673–679. doi: 10.2214/AJR.04.1513. [DOI] [PubMed] [Google Scholar]
- 21.Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology. 2007;245(2):577–583. doi: 10.1148/radiol.2452061919. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Kalra MK, Gilman MD, Hsieh J, Pien HH, Digumarthy SR, et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology. 2011;259(2):565–573. doi: 10.1148/radiol.11101450. [DOI] [PubMed] [Google Scholar]
- 23.Flicek KT, Hara AK, Silva AC, et al. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: a pilot study. AJR Am J Roentgenol. 2010;195(1):126–131. doi: 10.2214/AJR.09.3855. [DOI] [PubMed] [Google Scholar]
- 24.Marin D, Nelson RC, Samei E, Paulson EK, Ho LM, Boll DT, et al. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection—initial clinical experience. Radiology. 2009;251(3):771–779. doi: 10.1148/radiol.2513081330. [DOI] [PubMed] [Google Scholar]
- 25.Sato J, Akahane M, Inano S, Terasaki M, Akai H, Katsura M, et al. Effect of radiation dose and adaptive statistical iterative reconstruction on image quality of pulmonary computed tomography. Jpn J Radiol. 2012;30(2):146–153. doi: 10.1007/s11604-011-0026-7. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Kalra MK, Hsieh J, Licato PE, Do S, Pien HH. Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology. 2010;257(2):373–383. doi: 10.1148/radiol.10092212. [DOI] [PubMed] [Google Scholar]
- 27.Tricarico F, Hlavacek AM, Schoepf UJ, Ebersberger U, Nance JW, Jr, Vliegenthart R, et al. Cardiovascular CT angiography in neonates and children: image quality and potential for radiation dose reduction with iterative image reconstruction techniques. Eur Radiol. 2013;23(5):1306–1315. doi: 10.1007/s00330-012-2734-5. [DOI] [PubMed] [Google Scholar]