Abstract
The blood-testis barrier (BTB) is one of the tightest blood-tissue barriers in mammals including rodents and humans. It is used to sequester meiosis I and II, postmeiotic spermatid development via spermiogenesis and the release of sperm at spermiation from the systemic circulation, such that these events take place in an immune-privileged site in the adluminal (apical) compartment behind the BTB, segregated from the host immune system. Additionally, drug transporters, namely efflux (e.g., P-glycoprotein) and influx (e.g., Oatp3) pumps, many of which are integral membrane proteins in Sertoli cells at the BTB also work cooperatively to restrict the entry of drugs, toxicants, chemicals, steroids and other xenobiotics into the adluminal compartment. As such, the BTB that serves as an important physiological and selective barrier to protect germ cell development also poses a “hurdle” in male contraceptive development. For instance, adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, a potential nonhormonal male contraceptive that exerts its effects on germ cell adhesion, most notably at the Sertoli cell-spermatid interface, to induce “premature” germ cell loss from the seminiferous epithelium mimicking spermiation, has a relatively poor bioavailability largely because of the BTB. Since male contraceptives (e.g., adjudin) will be used by healthy men for an extended period of his life span after puberty, a better understanding on the BTB is necessary in order to effectively deliver drugs across this blood-tissue barrier in particular if these compounds exert their effects on developing germ cells in the adluminal compartment. This can also reduce long-term toxicity and health risk if the effective dosing can be lowered in order to widen the margin between its safety and efficacy. Herein, we summarize latest findings in this area of research, we also provide a critical evaluation on research areas that deserve attention in future studies.
Introduction
The blood-testis barrier (BTB) and the blood-brain barrier (BBB) are considered to be two of the tightest blood-tissue barriers in the mammalian body.1-5 This consideration is based on studies performed more than a century ago when dyes were administered to laboratory animals, only the testis and the brain were found to remain unstained,1 illustrating the presence of a barrier in these two organs. Structurally, however, the BTB and the BBB are quite different. For instance, the BTB is constituted by specialized junctions between adjacent Sertoli cells near the basement membrane of the seminiferous tubules in which microvessels located in the interstitium contribute relatively little permeability barrier function to the BTB. For the BBB, it is almost exclusively constituted by endothelial tight junction (TJ) in the capillaries near the base of the brain, with minor structural contributions by pericytes.1,6 While TJ is also found at the Sertoli-Sertoli cell interface at the BTB, the typical TJ ultrastructure is being reinforced by co-existing basal ectoplasmic specialization [basal ES, a testis-specific adherens junction (AJ) type] limited only to the BTB in the mammalian testis, such as in rats.1,7 At the BTB, tightly packed actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane are sandwiched between cisternae of endoplasmic reticulum and the two apposing Sertoli cell plasma membranes.1,7 Besides TJ, basal ES also coexists with gap junction. Collectively, these junctions coupled with desmosome constitute the BTB. While these unusual ultrastructural features serve well to protect the events of meiosis I and II, postmeiotic spermatid development and spermiation from drugs, chemicals and toxicants that may be found in the systemic circulation, they also pose a significant barrier to male contraceptives and/or therapeutic agents if these drugs exert their effects primarily behind the BTB.1,8 Recent studies have shown that this is largely due to the presence of multiple drug transporters found in the testis, mostly restricted to the Sertoli cells that constitute the BTB, but also in different germ cell types ranging from spermatogonia, spermatocytes, spermatids and spermatozoa, to limit the entry and/or the distribution of drugs behind the BTB.1,9 Herein, we summarize some recent findings in the field based on studies on adjudin, a potential male contraceptive, regarding its tissue distribution and bioavailability following its administration in rats. These findings are helpful in future studies to design better compounds and/or formulations to improve the bioavailability and distribution in the testis behind the BTB.
Adjudin and its Bioavailability in the Testis
When [3H]-adjudin was administered to adult rats via gavage and the distribution of [3H]-adjudin in different organs, including the testis, was monitored thereafter, it was found that few than 1% of administered adjudin was uptaken by the testis,10 even though it was found to be a very potent drug to induce germ cell loss from the seminiferous epithelium in different treatment regimens performed over at least a 5-year period.10,11 Also, adjudin was found to be rapidly metabolized and cleared from the host body, such as in rats, usually within 20-24 hours either administered orally10 or through i.v.12 These findings thus illustrate that the narrow margin that was observed between its efficacy and safety in a subchronic toxicity study in rats could be “widened” if adjudin and other male contraceptives under development in the field, could be reformulated to improve their tissue distribution and thus bioavailability in the testis.8
Drug Transporters and Blood-Testis Barrie R (Btb) Dynamics
For virtually any drugs, unless they belong to the classes of compounds that can bind to specific receptors, such as antagonists or agonists of cytokines (e.g., TGF-β and TNFα), hormones (e.g., FSH) and/or cytokines per se that can competitively bind onto the corresponding receptors of hormones (e.g., FSH receptors) or cytokines (e.g., TGF-β receptors, TNF receptors) in Sertoli cells, they are restricted from entering the adluminal compartment behind the BTB since one of the major functions of the BTB is to restrict paracellular flow of substances.1-4,9 However, many of these compounds, including drugs, can penetrate the BTB to gain access to the adluminal compartment via drug transporters utilizing the transcellular route. However, premeiotic (e.g., spermatogonia, early spermatocytes) and postmeiotic (e.g., late primary and secondary spermatocytes, spermatids and sperm) germ cells are also equipped with efflux pump transporters to actively pump harmful, but also therapeutic, drugs and substances out of the adluminal compartment.1,9,13 At present, ∼800 drug transporters are known to date found in different epithelial/endothelial cells including cancer cells;14-16 as well as Sertoli cells and almost all classes of germ cells in the testis.1,9,17 Unfortunately, virtually all the reports found in the literature regarding the study of drug transporters in the testis were limited to cellular distribution/localization studies, investigating the cellular expression of different drug transporters in the testis without any pertinent functional studies in particular the transport of drugs across the BTB.9,13,17
In the testis, drug transporters can be broadly classified into two categories: namely the ATP-binding cassette (ABC) drug transporters and the SoLuteCarrier (SLC) drug transporters. ABC drug transporters in the testis are efflux pumps which either prevents drugs (e.g., adjudin) from entering the adluminal compartment or pumps drugs out of the adluminal compartment should they enter the immune-privileged site via influx pumps, and these drug transporters are ATP-dependent drug pumps. The best studied ABC transporters in the testis are: (1) P-glycoprotein (a multidrug resistance protein, also known as Mdr, or Abcb1), (2) multidrug resistance-related protein (Mrp) and (3) breast cancer resistance protein (Bcrp), all of which are found in Sertoli cells.9,13 For SLC drug transporters in the testis, they are mostly influx pumps which pump drugs (e.g., adjudin) ATP-independently into the adluminal compartment behind the testis, and the energy required for transport is coming from gradient generated by a primary active transport system or drugs can pass through a SLC transporter through the built-in “pores”.1 The best studied SLC transporters in the testis are: (1) organic anion transporting polypeptide (OATP) transporters (e.g., Oatp3) and (2) organic anion transporter (OAT)/organic cation transporter (OCT)/organic cation/carnitine transporter (OCTN) (e.g., Slc22a5 also known as OCTN2, a SLC organic cation transporter family member 5 and also known as Na+-dependent organic cation/carnitine transporter 2; Slco6b1, a SLC organic anion transporter family member 6b1 also known as a testis-specific transporter-1 (TST-1); Slco6c1, a SLC organic anion transporter family member 6c1, also known as TST-2].1 Study has shown that [3H]-adjudin enters the adluminal compartment via these influx pumps as demonstrated in an in vitro system by culturing Sertoli cells on Matrigel-coated bicameral units with negligible germ cell contamination which formed a functional TJ-barrier that mimicked the Sertoli BTB in vivo.13 For instance, a knockdown of either Oatp3 by RNAi or a knockdown of all four influx pumps: Oatp3, Slc22a5, Slco6b1 and Slco6c1 by RNAi using specific corresponding siRNA duplexes versus nontargeting control siRNA duplexes was found to significantly impede the flow of [3H]-adjudin from the basal to the apical compartment.13 In short, the distribution of [3H]-adjudin is the net result of interactions of multiple drug transporters (perhaps in the 100s), both influx and efflux drug transporters, in which adjudin serves as a substrate to these drug pumps. These findings also illustrate the complexity of drug designs for male contraceptive development.
P-Glycoprotein Regulates the Bioavailability of Adjudin in the Testis
P-glycoprotein is one of the best studied efflux pumps in the testis.9,13 A recent study has shown that P-glycoprotein is localized predominantly at the BTB in the seminiferous epithelium of adult rat testes in all stages of the seminiferous epithelium cycle.18 Its expression at the BTB is noted to be the highest at Stages IV-VII of the seminiferous epithelial cycle, diminished somewhat at Stages VIII-IX18 when the BTB undergoes restructuring to accommodate the transit of preleptotene spermatocytes at the site to enter the adluminal compartment for further development, suggesting P-glycoprotein may possibly play a role in regulating BTB dynamics. In studies based on the use of co-immunoprecipitation coupled with dual-labeled immunofuorescence analysis, P-glycoprotein was found to structurally interact with TJ-integral membrane proteins occludin, claudin-11 and JAM-A,18 as well as FAK.19 This latter observation is important since FAK is an important nonreceptor protein tyrosine kinase known to be involved in integrin-based signaling20,21 and plays a crucial role in conferring junction permeability.22,23 In fact, FAK was recently shown to be an integrated component of the occludin-ZO-1 protein complex at the BTB,24 and it plays a critical role in regulating the Sertoli cell TJ-permeability barrier function.25 Thus, the structural association of P-glycoprotein and FAK seemingly suggests that this efflux drug transporter may be functioning in some other ways via its interactions with the occludin-ZO-1-FAK protein complex besides being served as a drug transporter. Indeed, it was found that the silencing of P-glycoprotein in Sertoli cell epithelium in vitro, in which the Sertoli cell epithelium had a functional TJ-barrier with ultrastructures of TJ, basal ES, gap junction and desmosome,26,27 led to a disruption of the TJ-barrier function,19 that is, making the TJ-barrier “leaky” when p-glycoprotein was knockdown. Interestingly, pharmacokinetics analysis of the flux of [3H]-adjudin across the Sertoli cell BTB has shown that the silencing of P-glycoprotein by RNAi did not render the Sertoli cell BTB to become completely “disrupted” and “freely” permeability to the transport of [3H]-adjudin across the barrier, perhaps due to the presence of other efflux pumps at the site, such as Mrp and Bcrp, which are known to be present at the Sertoli cell BTB.18,28,29 Interestingly, in a pharmacokinetics study to assess the flux of [3H]-adjudin from apical to basal (A to B) or basal to apical (B to A) compartment using Sertoli cells cultured on bicameral units having the establishment of a functional TJ-barrier, it was found that while the silencing of P-glycoprotein by RNAi affected the flux of [3H]-adjudin from B to A (i.e., making the BTB “leaky”), it failed to perturb the A to B flux illustrating while P-glycoprotein guards the entry of [3H]-adjudin to the adluminal compartment of the epithelium, it did not “eliminate” adjudin that was already “admitted” to the adluminal compartment via influx pump transporters.19,30 These findings thus illustrate the intriguing role of P-glycoprotein in regulating the distribution adjudin in the testis. Of course, it remains to be determined if other efflux drug transporters, such as Mrp and Bcrp, are effective to pump adjudin that has reached the adluminal compartment out of the BTB, or how these three drug efflux pumps work cooperatively to regulate the distribution/bioavailability of adjudin in the adluminal compartment behind the BTB.
Interactions of Adjudin With P-Glycoprotein in Rats and Humans: a Molecular Modeling Analysis
Introduction
In order to better understand the structural interactions between adjudin and P-glycoprotein so that second generation analogs of adjudin can be synthesized to improve their transport across the BTB to enter the adluminal compartment, so that they can induce “premature” germ cell depletion from the epithelium more effectively than adjudin, we sought to perform molecular modeling analysis to identify the putative interacting domain and/or amino acid residues between the two molecules.
Tertiary Structure Prediction for P-Glycoprotein of Rat and Human
The amino acid sequences of P-glycoprotein of Rattus norvegicus (UniProtKB ID: P43245) and Homo sapiens (UniProtKB ID: P08183) were retrieved from UniProt (http://www.uniprot.org). A BLASTp search31 was carried out to find appropriate proteins with significant amino acid sequence and structural similarity to P-glycoprotein by searching the Protein Data Bank (PDB) database at http://www.rscb.org/pdb/.32 The search was refined to find a suitable structural homolog for the modeling of P-glycoprotein in rat and human. The amino acid sequence of these two proteins and their template sequences were aligned using the Align Sequence to template, a sequence alignment tool in Accelrys Discovery Studio 3.1(DS3.1), (Accelrys Software, http://accelrys.com/). Based on the alignment generated, the tertiary structure of P-glycoprotein of rat and human were predicted using Build Homology Models tool from DS3.1. Discrete Optimized Protein Energy (DOPE) and Modeller Objective Function (MOF) scores of the resulting models were used to select the most reliable model. The predicted structures were energy minimized by Smart Minimizer algorithm in Discovery Studio 3.1. The minimization was carried out in 500 steps by applying CHARMm force field and then subjected to validation. Backbone conformation was evaluated by examining the Psi/Phi interactions in Ramachandran Plot, obtained from PROCHECK.33 Based on the plot, residues in the disallowed regions were refined using Loop Refinement (MODELER) module, from DS3.1. The final refined model was tested for their stability and reliability using ERRAT.34
Molecular Docking of Rat and Human P-Glycoprotein with Adjudin
Active sites of proteins are often associated with structural pockets in the protein. The identification of such substrate binding sites in enzymes helps us to understand their binding interactions with substrates and other small molecules. The drug binding site of P-glycoprotein was taken from the template structure that was used for homology modeling. The docking simulation tool, Glide (Schrödinger, Inc.) was used to perform docking with each of the two proteins and adjudin. The modeled proteins were prepared using the Protein Preparation Wizard, a workflow in the Schrödinger Suite of programs. Using this tool, all hydrogen atoms were added to the proteins, the protonation states for histidine residues were optimized and the entire protein was minimized using OPLS-2005 force field. The ligand, adjudin was prepared using Schrödinger Ligprep tool (version 2.3, Schrödinger, LLC, New York, NY, 2009). LigPrep was used to find stereoisomers and to perform energy minimization using OPLS_2005 force field. The binding site was defined in terms of two concentric cubes: the bounding box, which contains the center of any acceptable ligand pose and the enclosing box, which contains all ligand atoms of an acceptable pose. The prepared ligand was docked flexibly to the two P-glycoproteins using Simple Precision mode (SP) mode in Glide (Version 5.5, Schrödinger, LLC, New York, NY). To soften the potential for nonpolar parts of the ligand, the vdW (van der Waal's) radii of ligand atoms were scaled with partial atomic charge (absolute value) less than the specified cutoff. Default scaling factor was 0.8 and the partial cutoff value was 0.15. The Glide docking algorithm generated 5000 poses per ligand for the initial phase of docking and restricted to 400 poses for energy minimization. Upon completion of each docking calculation, the best docked structure was chosen using Glidescore (Gscore) function, a modified and extended version of the empirically based Chemscore function.
Homology Modeling
The amino acid sequence of P-glycoprotein of rat and human was used to find a suitable structural template using BLASTp against PDB database. The best homolog was selected based on similarity score and crystal structures with better resolution. The template crystal structure of multidrug resistance protein (PDB ID: 3G60), namely P-glycoprotein, from mouse35 showed 82% and 87% identity with rat and human P-glycoprotein sequence, respectively. The structure of P-glycoprotein of rat and human was modeled and the best reliable models were subjected to loop refinement by DS3.1 and validation by ProCheck.
Validation for Modeled Structure of Rat and Human P-Glycoprotein
The empirical distribution of data points observed in rat (Fig. 1) and human (Fig. 2) P-glycoprotein were subjected to Ramachandran plot analysis (see Figs. 1 and 2) for structure validation, illustrating the theoretically “favored”, “allowed” and “generously allowed” regions as defined by PROCHECK. The validation of the two modeled P-glycoprotein structures shows that the stereochemical geometry as well as the overall structural geometry of the models is good. For rat and human P-glycoprotein, the number of residues in most favored regions, additional allowed regions, generously allowed regions and disallowed regions are: 967 (86.6%), 114 (10.2%), 36 (3.2%) and 0 (0%); and 909 (87.7%), 107 (10.3%), 21 (2%) and 0 (0%), respectively. The secondary structure of the two modeled proteins was also generated by PDBsum (Fig. 3A,B),36 in which the structure of P-glycoprotein of rat (Fig. 3A) and human (Fig. 3B) represents secondary structure elements (alpha-helices and beta-sheets) together with various structural motifs, such as beta- and gamma-turns, and beta-hairpins. The 3D model of P-glycoprotein of rat and human shows an inward-facing conformation closely representing a two-fold symmetry. The nucleotide binding domains (NBDs) are separated and the inward facing conformation is formed from two bundles of six helices. This results in a large internal cavity open to both the cytoplasm and the inner leaflet (see Figs. 4 and 5). The modeled structure for rat (Fig. 4) and human (Fig. 5) P-glycoprotein is consistent with the template crystal structure of mouse P-glycoprotein.35 Thus, these modeled structures can be further used to study interactions with small molecules, such as adjudin.
Figure 1.
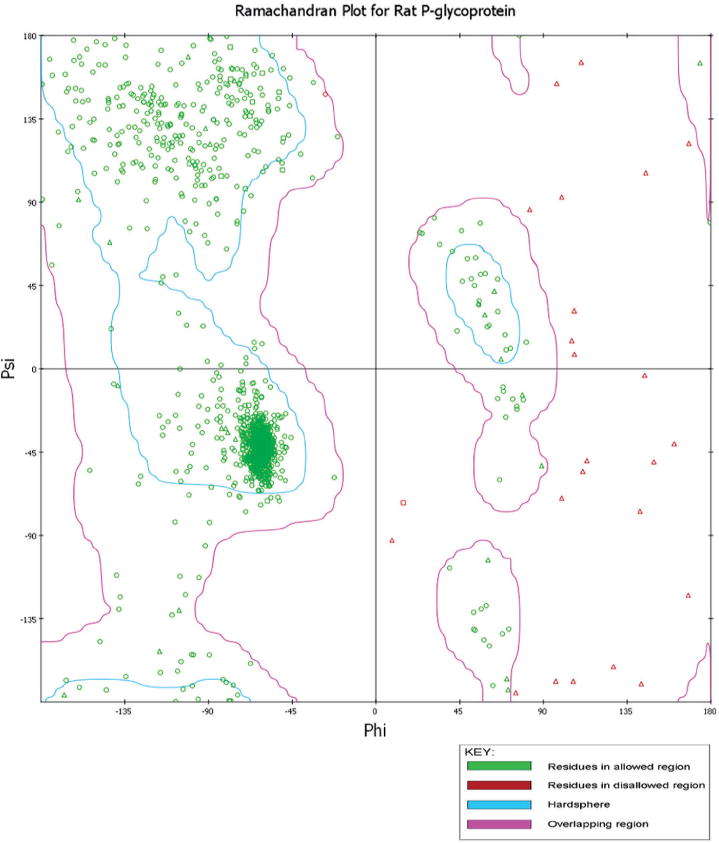
Ramachandran plot for the modeled rat P-glycoprotein.
Figure 2.
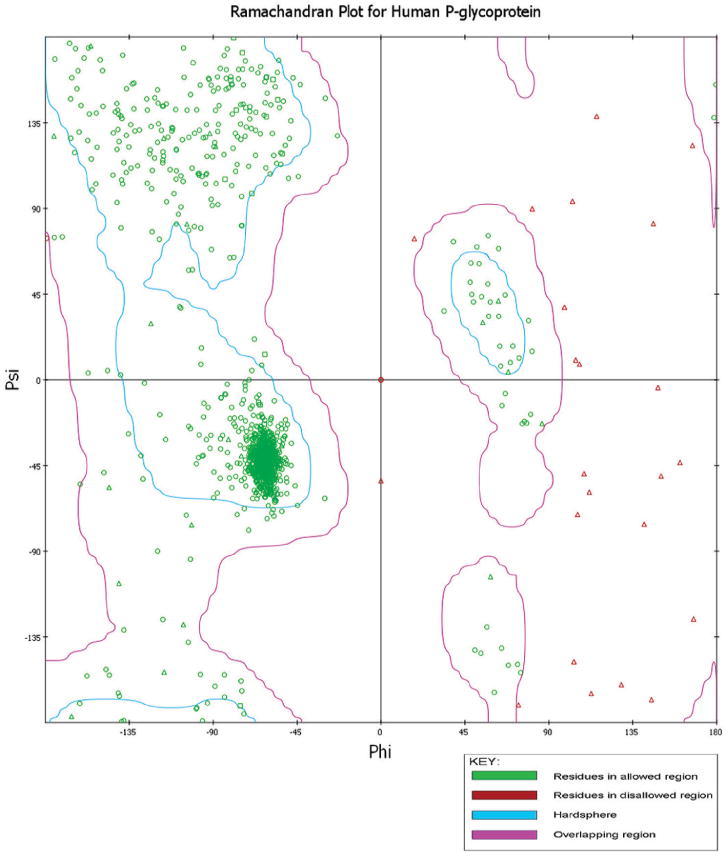
Ramachandran plot for the modeled human P-glycoprotein.
Figure 3.


A) Secondary structure representation for modeled structures of rat P-glycoprotein.
B) Secondary structure representation for modeled structures of human at P-glycoprotein
Figure 4.
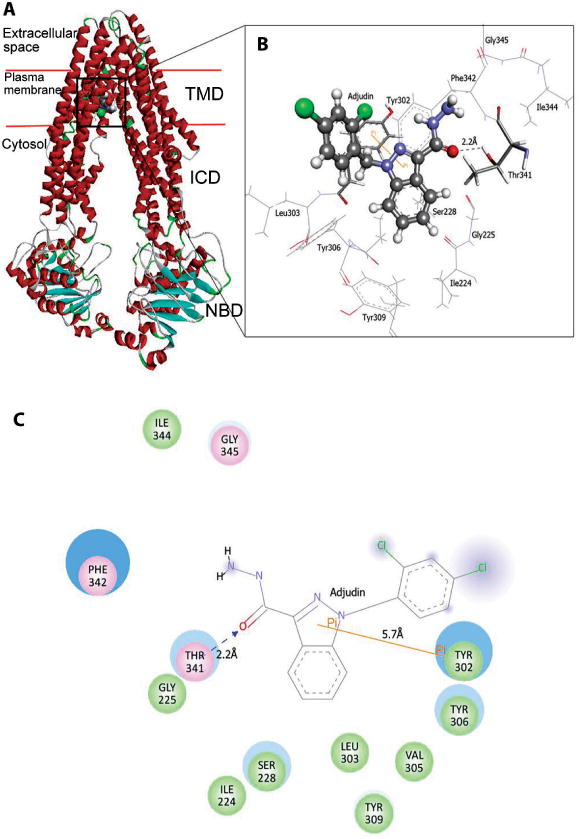
Docked complex of rat P-glycoprotein with adjudin. A) This depicts the 3-D modeled rat P-glycoprotein in ribbon format, docked with adjudin and its relative configuration across the Sertoli cell plasma membrane. TMD (transmembrane domain), ICD (intracellular domain) and NBD (nucleotide-binding domain where ATP binds). B) This represents an enlarged view of the interacting residues in CPK 3D conformation (CPK is a coloring scheme named after the CPK molecular models designed by Robert Corey and Linus Pauling and improved by Walter Koltun: “white” for hydrogen; “black” for carbon; “blue” for nitrogen; “red” for oxygen; “green” for chloride). C) Two-dimension representation of molecular interactions between adjudin and the rat P-glycoprotein. “Green” circles represent residues involved in van der Waals interactions; “Pink” circles represent residues involved in hydrogen bond, polar or charge interactions; “Blue” halo around residues represent solvent accessible surface of an interacting residue. “Orange” Line represents Pi-Pi interaction (Pi stacking interaction refers to noncovalent interactions between aromatic rings) between Tyr302 and adjudin and “blue” dotted line represents hydrogen bond formation between side chain of Thr341 and donor oxygen atom of adjudin. GLY (Gly, G), Glycine; ILE (Ile, I), Isoleucine; LEU (Leu, L), Leucine; PHE (Phe, F), Phenylalanine; SER (Ser, S), Serine; THR (Thr, T), Threonine; TYR (Tyr, Y), Tyrosine; VAL (Val, V), Valine. A color version of this fgure is available online at www.landesbioscience.com/curie.
Figure 5.

Docked complex of human P-glycoprotein with adjudin. A) The 3-D modeled human P-glycoprotein in ribbon format, docked with adjudin and its relative configuration across the Sertoli cell plasma membrane. TMD (transmembrane domain), ICD (intracellular domain) and NBD (nucleotide-binding domain where ATP binds). B) Enlarged CPK view of the docking site in (A) (“white” for hydrogen; “black” for carbon; “blue” for nitrogen; “red” for oxygen; “green” for chloride). Adjudin is depicted in scaled ball-and-stick model and interacting residues are in stick model and their interactions in its 3D conformation. C) Two-dimension representation of molecular interactions between adjudin and the human P-glycoprotein. “Green” circles represent residues involved in van der Waals interactions; “Pink” circles represent residues involved in hydrogen bond, polar or charge interactions; “Blue” halo around residues represent solvent accessible surface of an interacting residue. “Orange” lines represent Pi-Pi and Pi-cation interactions between Phe732 and adjudin. “Green” dotted line represents hydrogen bond formation with main chain of Leu975 with an estimated bond length of 1.90. ALA (Ala, A), Alanine; GLN (Gln, Q), Glutamine; LEU (Leu, L), Leucine; MET (Met, M), Methionine; PHE (Phe, F), Phenylalanine; SER (Ser, S), Serine; TYR (Tyr, Y), Tyrosine; VAL (Val, V), Valine. A color version of this figure is available online at www.landesbioscience.com/curie.
Molecular Docking Analysis
Molecular-docking was performed on the 3D model of P-glycoprotein, built by the homology modeling method (see above). The presumptive drug binding pocket of P-glycoprotein comprises mostly hydrophobic and aromatic residues.35 The modeled structure reveals that the inward facing conformation is competent to bind drugs. The common residues involved in the drug binding cavity of inward facing crystal structure of mouse P-glycoprotein and the outward facing homology model from Sav1866 structure (note: Sav1866 is the first high-resolution structure of an ABC exporter of Sav1866 from Staphylococcus aureus)37-39 was used in defining the binding site for docking with adjudin. Since P-glycoproteins from rat and human share significant similarity in their sequence and structure, the same binding pocket residues with slight variation corresponding to their sequence position were specified for docking (Figs. 4 and 5). Many of these residues face the drug binding pocket and are highly conserved, suggesting a common mechanism of poly-specific drug recognition. The drug binding site of P-glycoprotein resides in the cell membrane and is formed by TM helices.40
Docking of P-Glycoprotein With Adjudin
The docking simulation of P-glycoprotein with adjudin shows that adjudin binds to rat P-glycoprotein with a docking score of -5.497kcal/mol and human P-glycoprotein with a docking score of -7.496 kcal/mol. The docking energy and van der Waal's energy involved in docking are tabulated in Table 1. The docked complex of rat P-glycoprotein illustrates that adjudin forms hydrogen bond with Thr341 and forms a Pi-Pi interaction with Tyr302 (Fig. 4A,B). The docked complex of human P-glycoprotein shows that Leu975 forms hydrogen bond with adjudin. Phe732 plays a major role in forming Pi interactions with two Pi-Pi interactions and a Pi-cation interaction with the ligand (Fig. 5A,B). Two dimension plot created using DS3.1 for both the docked complexes illustrates a detailed view of the types of molecular interactions between adjudin and P-glycoprotein of rat and human (Figs. 4C and 5C).
Table 1. Molecular interactions of P-glycoprotein with adjudin.
| Receptor | Docking Score (kcal/mol) | Docking Energy (kcal/mol) | Vander Waal's Energy (kcal/mol) | Hydrogen Bond Interacting Residues | Vander Waal's Interaction Residues | Pi Stacking Interaction Residues |
|---|---|---|---|---|---|---|
| Human P-glyco-protein | −7.49 6 | -38.798 | -38.170 | Leu975 (1.90Å) | Met69, Phe72, Tyr307, Tyr310, Leu332, Phe336, Ala729, Phe732, Ser733, Val982 | Phe732 |
| Rat P-glyco-protein | -5.497 | -31.679 | -31.483 | Thr341 (2.2Å) | Ile224, Gly225, Ser228, Tyr302, Leu303, Val305, Tyr306, Tyr309, Ile344 | Tyr302 |
Conclusion
As reported in two recent studies illustrating that the entry of drugs (e.g., [3H]-adjudin) to the adluminal compartment behind the BTB is mediated via the combined effects of influx (e.g., Oatp3, OCTN2, TST-1, TST-2)30 and efflux (e.g., P-glycoprotein)19 drug transporters since the silencing of these genes via the use of specific siRNA duplexes without any detectable off-target effects would impede the transport of [3H]-adjudin across the Sertoli cell BTB. These findings also illustrate the significance of understanding the regulation of the BTB and how drug transporters regulate the barrier function via its interactions with FAK and some of the adhesion protein complexes, such as occluding-ZO-1 and JAM-A-ZO-1, at the BTB. More important, preliminary molecular modeling studies, such as those summarized above, have demonstrated the presence of putative interacting domains and the involving amino acid residues within the 3D structure of P-glycoprotein, both in rats and humans, that structurally engage adjudin via hydrogen bonds and van der Waal's force (see Figs. 4 and 5). This information should be helpful to design other analogs of adjudin that can have “lesser” interactions with efflux pumps but “better” interactions with influx pumps to optimize their transport across the BTB and their retainment in the adluminal compartment. In short, molecular modeling can be a powerful tool to design male contraceptive drugs based on some existing known molecular entitites, in particular those that have poor bioavailability and distribution due to their interactions with drug transporters at the BTB.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5 to CYC; R01 HD056034 to CYC) and the Government of India, Department of Biotechnology (BT/BI/03/015/2002 to PPM) and Department of Information Technology (DIT/R & D/BIO/15(9)/2007 to PPM).
Footnotes
NOTE ADDED IN PROOF: An important recent paper from Reina Bendayan's Laboratory has demonstrated that ABC efflux drug transporters (e.g., P-glycoprotein) are also important to regulate the transport of antiretroviral drugs (e.g., atazanavir, mitoxantrone) across the BTB using TM4 cells (a Sertoli cell line)41 as the study model, which is in agreement with our latestfindings.19
References
- 1.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:A–AW. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franca LR, Auharek SA, Hess RA, et al. Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. In: Cheng CY, editor. Biology and Regulation of Blood-Tissue Barriers. Austin, TX: Landes Bioscience and Springer Science + Business Media, LLC; http://www.landesbioscience.com/curie/chapter/5148/2011. [PubMed] [Google Scholar]
- 5.Easton AS. Regulation of permeability across the blood-brain barrier. In: Cheng CY, editor. Biology and Regulation of Blood-Tissue Barriers. Austin TX: Landes Bioscience and Springer Science Business Media, LLC; 2011. (in press) [Google Scholar]
- 6.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mruk DD, Cheng CY. Delivering nonhormonal contraceptives to men: advances and obstacles. Trends Biotechnol. 2008;26:90–99. doi: 10.1016/j.tibtech.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 10.Cheng CY, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CY, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- 12.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kis O, Robillard K, Chan GN, et al. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.DeGorter MK, Xia CQ, Yang JJ, et al. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxciol. 2012 doi: 10.1146/annurev-pharmtox-010611-134529. in press. [DOI] [PubMed] [Google Scholar]
- 16.Mandery K, Glaeser H, Fromm M. Interaction of innovative small molecule drugs used for cancer theraphy with drug transporters. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01618.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su L, Mruk DD, Lui WY, et al. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA 2011. doi: 10.1073/pnas.1111414108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CY, Mruk DD. Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK) An unexpected turn of events Cell Cycle. 2009;8:3493–3499. doi: 10.4161/cc.8.21.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belvitch P, Dudek SM. Role of FAK in S1P-regulated endothelial permeability. Microvasc Res. 2011 doi: 10.1016/j.mvr.2011.08.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinnell KL, Harrington EO. Microvasc Res. doi: 10.1016/j.mvr.2011.04.005. in press. Interplay between FAK, PKCd and p190RhoGAP in the regulation of endothelial barrier function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siu ER, et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu ER, Wong EWP, Mruk DD, et al. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siu MKY, Wong CH, Lee WM, et al. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 27.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 28.Melaine N, et al. Multidrug resistance genes and P-glycoprotein in the testis of the rat, mouse, guinea pig and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- 29.Bart J, et al. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Su L, Mruk DD, Lee WM, et al. Drug transporters and blood-testis barrier function. J Endocrinol. 2011;209:337–351. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman HM, et al. The Protein Data Bank. Nucleic Acid Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskowski RA, Rullmann JAC, MacArthur MW, et al. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 34.Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laskowski RA. PDBsum new things. Nucleic Acid Res. 2009;37:D355–D359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Globisch C, Pajeva IK, Wiese M. Identification of putative binding sites of P-glycoprotein based on its homology model. ChemMedChem. 2008;3:280–295. doi: 10.1002/cmdc.200700249. [DOI] [PubMed] [Google Scholar]
- 38.Dawson RJ, Locher KP. Structure of the multidrug ABC tarnsporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 39.Pohl A, Devaux PF, Herrmann A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro AB, Ling V. Extraction of Hoechst 33342 and the cytoplasmic leafet of the plasma membrane by P-glycoprotein. Eur J Biochem. 1997;250:122–129. doi: 10.1111/j.1432-1033.1997.00122.x. [DOI] [PubMed] [Google Scholar]
- 41.Robillard KR, Hoque MdT, Bendayan R. Expression of ATP binding cassette membrane transporters in rodent and human Sertoli cells: Relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.186916. In press. [DOI] [PubMed] [Google Scholar]


