Abstract
Background
It has become increasingly evident that disease flares in the human inflammatory bowel diseases (IBD) are influenced by life stress. It is known that life stress can trigger disturbances in intestinal barrier function and activate proinflammatory signaling pathways, which are important contributors to intestinal inflammation and clinical disease; however, the exact mechanisms of stress-induced IBD exacerbations remain to be elucidated. Here we present a model of early life stress-induced exacerbation of colitis in IL-10-/- mice.
Methods
C57Bl/6 wild type and IL-10-/- mice were exposed to neonatal maternal separation (NMS) stress on postnatal days 1-18 and reared under normal conditions until 10-12 weeks of age. At this time, histopathology, colitis scores, intestinal barrier function, proinflammatory cytokine expression and mast cell activity were evaluated.
Results
NMS increased the severity of colitis IL-10-/- mice indicated by greater colitis scores and colonic proinflammatory cytokine concentrations. NMS and IL-10-/- increased colonic permeability; however, NMS alone did not induce colitis. Increased mast cell activation and colonic tryptase release were observed in IL-10-/- mice exposed to NMS, indicating mast cell activation.
Conclusions
This study demonstrates that colitis in IL-10-/- mice can be exacerbated by NMS stress. The precise mechanisms of enhanced colitis severity in NMS IL10-/- mice are unclear but persistent defects in intestinal barrier function likely play a contributing role. NMS serves as a novel model to investigate the mechanisms by which early life stress influences the development and course of IBD in adulthood.
Keywords: Psychological stress, neonatal maternal separation (NMS), inflammatory bowel disease (IBD), colitis, animal models of IBD, barrier function
Introduction
The pathogenesis of IBD is multifactorial in nature, involving host genetic susceptibility, dysregulated immune responses to the enteric microbiota, and environmental influences. Life stress has been studied as a possible environmental trigger of IBD exacerbations. In one analysis, 45% of UC patients reported a stressful life event within 12 months of diagnosis compared to only 11% of matched hospital controls.1 Other studies have corroborated these results, linking coping behavior, chronic stress, and relapse in IBD.2-6 Although epidemiological analyses demonstrate a link between stress and IBD, the pathophysiological mechanisms through which stress impacts the onset and clinical course of IBD are not understood. Early life stressful events, including inadequate parental bonding and low maternal care have become increasingly scrutinized as a possible risk factor for the development of IBD later in life, but the biologic link between early life stress and development of IBD remains poorly understood.7-9 Infancy, childhood, and adolescence are critical periods in development that are characterized by increased vulnerability to stressors; therefore, exposure of the developing brain and intestine to adverse environmental factors may have long-lasting effects on the individual that may influence disease development and activity in adulthood.7 Neonatal maternal separation (NMS) stress is a documented model of early life stress which results in long-term alterations in behavior as a consequence.10, 11
The pathogenesis of IBD includes loss of intestinal epithelial barrier function and a consequent increase in intestinal permeability. These factors allow excessive translocation of luminal bacteria and antigens into subepithelial tissues, triggering a vicious cycle of chronic intestinal inflammation and further intestinal barrier injury.12, 13 Several animal studies have demonstrated that psychological stress negatively influences intestinal barrier function.14-16 In rodent and porcine animal models, early life stress resulted in permanent alterations in central adaptive stress responses17 and chronic disturbances in intestinal barrier function.18-20 Thus, intestinal barrier disturbances may serve as an important link between stress and IBD flare-ups. The exact role of stress-induced barrier disruption causing the exacerbation of IBD has not been investigated in animal models of naturally occurring colitis.
The IL-10-/- mouse develops spontaneous colitis and therefore serves as a model commonly utilized to study IBD. Conventionally reared IL-10-/- mice on the C57Bl/6 background develop a mild, patchy colitis with incomplete disease penetrance;21 therefore, this represents an ideal model to study the role of stress on the initiation and exacerbation of colitis. The objective of this study was to investigate whether early life stress (NMS) influences the development of colitis in IL-10-/- mice. We hypothesized that NMS would induce chronic disturbances in barrier function and increase colonic inflammation in IL-10-/- mice.
Materials and Methods
Mice
Wild type and IL-10-/- mice on a C57Bl/6 background were obtained from the Jackson Laboratories, Bar Harbor, ME, and were maintained in a specific pathogen free environment at the University of North Carolina, Chapel Hill. Litters were randomly assigned to one of two groups: neonatal maternal separation stress (NMS) or control (normally reared). All animals were housed in accordance with guidelines from the American Association for Laboratory Animal Care and Research Protocols and experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
Neonatal Maternal Separation
NMS was performed as previously described by Chung.22 Briefly, pups in the NMS group were removed from dams and placed in isolated warmed cages for 180 minutes daily for postnatal days 1-18 (Figure 1). Following isolation, pups were returned to the cage containing their dam and littermates and were left undisturbed other than routine bedding changes.
Figure 1.
Experimental timeline.
Sample collection
Mice were sacrificed at 10 - 12 weeks of age and colonic samples were immediately collected and divided for Ussing chamber experiments, histopathology, and colonic explant cultures.
Histology
Colonic tissue sections were fixed in 10% buffered formalin, embedded in paraffin, and 4-μm-thick sections were stained with hematoxylin and eosin using standard techniques. Spontaneous colitis scoring was adapted from the criteria reported by Berg et al, as previously described.21 All histological scores were determined by a single pathologist (LB) who was blinded to the experimental protocols. Mucosal hypertrophy score was assigned as follows: a clinical light microscope (Olympus BX45) equipped with a high resolution digital camera (Olympus DP2-BSW version 2.2) was used to measure the colonic epithelial thickness in microns. Well oriented crypts from 5 separate colon sections per mouse (n=5-6 mice/experimental group) and 6 randomly selected mucosal areas within each section were measured from the basement membrane of the crypt base to the mucosal section using the arbitrary line tool and the 20× objective. The average mucosal thickness of each of the 5 colon sections was expressed as a percentage of wild type colon epithelial thickness. The percentages were then re-coded into a mucosal hypertrophy score (0 to 4) as follows: 0 = 90-125%; 1 = 125-150%; 2= 151-175%; 3= 176-200% and 4= >200 and summed for final mucosal hypertrophy score. Inflammation was characterized for cell types as follows: Additional sections were stained following standard immunohistochemical procedures for CD3 as previously described23 and areas of inflammatory infiltrate, when available, were targeted for examination in each of the 5 colon sections. At high magnification (40× objective), 100 cell count differentials were performed with CD3 positive lymphocytes counted as T. Macrophages, neutrophils and eosinophils were identified based on morphology. Cell types were represented as percentage of inflammatory cells present.
Cytokine analysis
Intestinal tissue explant cultures
Sections of the colon were collected and processed for IL-12 p40 as previously described,24 using a cytokine specific ELISA immunoassay kit according to manufacturer's instructions (BD Biosystems, Franklin Lakes, NJ).
Quantitative real-time PCR, using SYBR Green Master Mix (Applied Biosystems, Bedford, MA), was performed on an HT-7900 (Applied Biosystems, Bedford, MA), as previously published.25
The following primer sequences were used: β-actin: forward, 5′-AGCCATGTACGTAGCCATCCAG-3′, reverse, 5′-TGGCGTGAGGGAGAGCATAG-3′; Ifng: forward, 5′-CTTCCTCATGGCTGTTTCTG-3′, reverse, 5′-ACGCTTATGTTGCTGATGG-3′; Il12b: forward, 5′- CGCAAGAAAGAAAAGATGAAGGAG-3′, reverse, 5′-TTGCATTGGACTTCGGTAGATG-3′; Il17a: forward, 5′-AACCGTTCCACGTCACCCTGGA-3′, reverse, 5′-TGGTCCAGCTTTCCCTCCGCA-3′; tnf: forward, 5′-TGAGATCCATGCCGTTGG-3′, reverse, 5′- ACCCTCACACTCAGATCATCTTCTC-3′; Il6: forward, 5′-GAAATGATGGATGCTACCAAACTG-3′, reverse, 5′-CTCTCTGAAGGACTCTGGCTTTG-3′; Il1b: forward, 5′-CTCAATGGACAGAATATCAACCAAC-3′, reverse, 5′-GGCTGTGCCGTCTTTCATTAC-3′
Intestinal Barrier Function
Ussing chamber experiments
Segments of proximal colon were harvested and immediately placed in oxygenated (95% O2-5% CO2) Ringer solution. Tissues were then mounted in 0.3 cm2 aperture Ussing chambers (Physiologic Instruments, Inc, San Diego, CA) as described previously.26 After a 15-minute equilibration period on chambers, short circuit current (Isc), an index of electrogenic ion transport, was monitored by a multichannel voltage–current clamp (model VCC MC6, Physiologic Instruments, San Diego, CA) at 1 minute intervals over a 60-minute period and expressed as microamperes (μA) per square centimeter. Mucosal-to-serosal flux of (FITC)-dextran (4 kDa; Sigma-Aldrich, St. Louis, MO) (FD4) were performed as an index of paracellular permeability. After a 15 min equilibration, FD4 (0.25 mM) was added to the mucosal side of Ussing chamber-mounted tissues. The FD4 was allowed to equilibrate for 15 min, after which 100 μL samples (in triplicate) were collected from the serosal side of tissues at 15 minute intervals and transferred into a 96 well assay plate. The presence of FD4 was assayed by measurement of fluorescence intensity using an fMax Fluorescence Microplate Reader (Molecular Devices, Sunnyvale, CA) and concentrations were determined from standard curves generated by serial dilution of FD4. FD4 flux across colonic tissues was determined by dividing the final concentration of FD4 in the serosal chamber in the by the initial FD4 concentration of the mucosal chamber and was expressed as % FD4 permeability.
Mast cell counts and assessment of mast cell product tryptase
Formalin-fixed, paraffin-embedded tissue (n=4 mice/experimental treatment) was mounted on slides and stained with the mast cell granule stain toluidine blue. Lamina propria mast cell counts were performed by an investigator blinded to experimental treatments. Mast cell counts were derived by averaging the number of mast cells observed in 10 high power fields per tissue slide and were expressed as number of mast cells per high power field. The animal served as the experimental unit for statistical analysis.
Gel electrophoresis and Western blotting
Supernatants from colonic explants were stored at −70°C before SDS-PAGE analysis. Aliquots were thawed at 4°C, and protein analysis was performed (Pierce BCA protein assay kit, Thermo Scientific, Rockford, IL). Aliquots (amounts equalized by protein concentration) were mixed with an equal volume of 2× SDS-PAGE sample buffer and boiled for 10 minutes. Western immunoblots for tryptase were performed using a mouse mast cell tryptase primary antibody (Chemicon International, Temecula, CA) as previously described.27 Quantitative results were obtained by scanning the resulting images and densitometric analysis using QuantityOne software (Bio-Rad Laboratories, Hercules, CA).
Statistics
Data were reported as mean ± SE based on experimental number (n). Data were analyzed using a standard 2-way ANOVA (SigmaStat, Jandel Scientific, San Rafael, CA) with stress and genotype as the main effects. A post-hoc Tukey's test was used to determine differences between experimental groups. Histology scores were compared using a Mann-Whitney U test. p<0.05 was considered significant and p values ≥ 0.05 and ≤0.1 were reported as trends. Cytokine data were analyzed with a Mann-Whitney U test using GraphPad Prism (Graphpad software, San Diego, CA).
Results
Neonatal maternal separation exacerbates histologic colitis in IL-10 deficient mice
In IL-10-/- mice, NMS resulted in more severe colitis, characterized by marked colonic epithelial hyperplasia as well as increased frequency and severity of inflammatory aggregates within the lamina propria and submucosa compared to controls (Figure 2). The inflammatory infiltrate consisted primarily of lymphocytes, with macrophages and neutrophils representing less than 3% and 1% of the inflammatory cell infiltrate, respectively (Table 1). Mice subjected to NMS had higher histological scores and decreased colon length compared to controls (Figure 3). Crypt abscesses were not a prominent feature of the colitis in these mice. Mice exposed to NMS displayed a trend towards higher percentage of T-lymphocyte infiltration (p=0.05) than control mice. Mucosal hypertrophy was evident in control and NMS mice, but was similar between the two groups. There was no evidence of histological inflammation in the NMS group in wild type mice.
Figure 2.
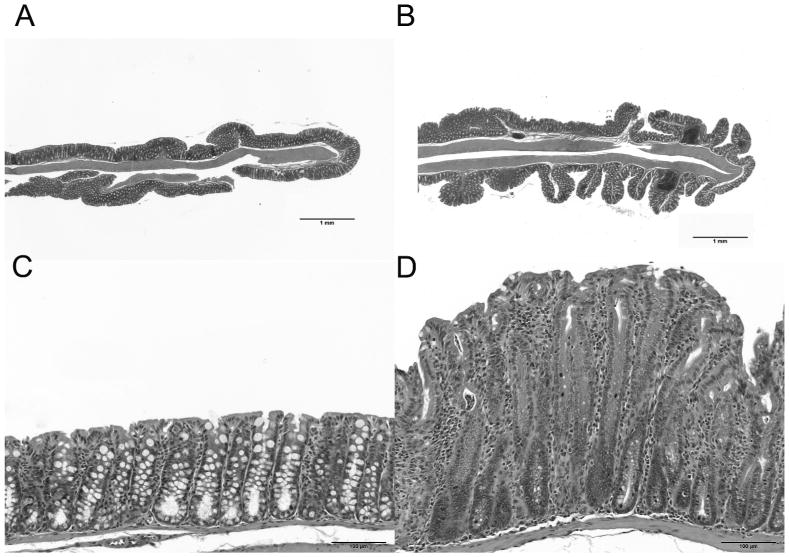
Colon, H&E staining: Top row: 2× magnification; Bottom row: 60× magnification. NMS IL-10-/- mice display marked epithelial hyperplasia compared to control. Increased frequency and severity of inflammatory aggregates within the lamina propria and submucosa of the NMS mouse (B and D) compared to the control mouse (A and C).
Table 1. Histological scores and inflammatory cell count.
| % of lamina propria inflammatory cells | |||||
|---|---|---|---|---|---|
| Colitis Score (SE) | Hypertrophy score (SE) | T-lymphocyte (SE) | Macrophage (SE) | Neutrophil (SE) | |
| Control | 2 (0.5) | 8.67 (1.9) | 31.8 (1.8) | 2.9 (0.05) | 0.1 (0.05) |
| NMS | 4.5 (0.5) | 6.9 (1.2) | 46.7 (1.7) | 2.8 (0.03) | 1.8 (0.05) |
| p | 0.012 | 0.79 | 0.05 | 0.975 | 0.326 |
Key: SE= Standard Error; NMS=Neonatal Maternal Separation group
Figure 3.
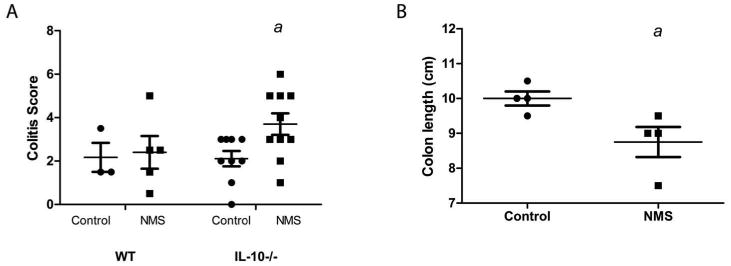
Neonatal maternal separation stress exacerbates colitis. IL-10-/- mice (n=3-10/group) were either exposed (NMS group) or not exposed (control group) to neonatal maternal separation. (A) Colitis was more severe in NMS group as assessed by histologic colitis scores (p=0.01) (B) Colon length was significantly decreased in IL-10-/- mice exposed to NMS (p<0.04)
Neonatal maternal separation causes increased colonic proinflammatory cytokine production in IL-10 deficient mice
NMS resulted in markedly elevated colonic IL-12 p40 levels in colonic explant cultures compared to controls, as assessed by ELISA (Figure 4A). This elevation was also present at the level of mRNA (Figure 4B). Colonic Ifng mRNA was also significantly elevated in IL10-/- mice subjected to NMS compared to control mice (Figure 4C). Colonic levels of IL-1β, IL-6, IL-17, and TNFα were not significantly different between the two groups, although these cytokines trended higher in the NMS group (Figure 4, D-G).
Figure 4.
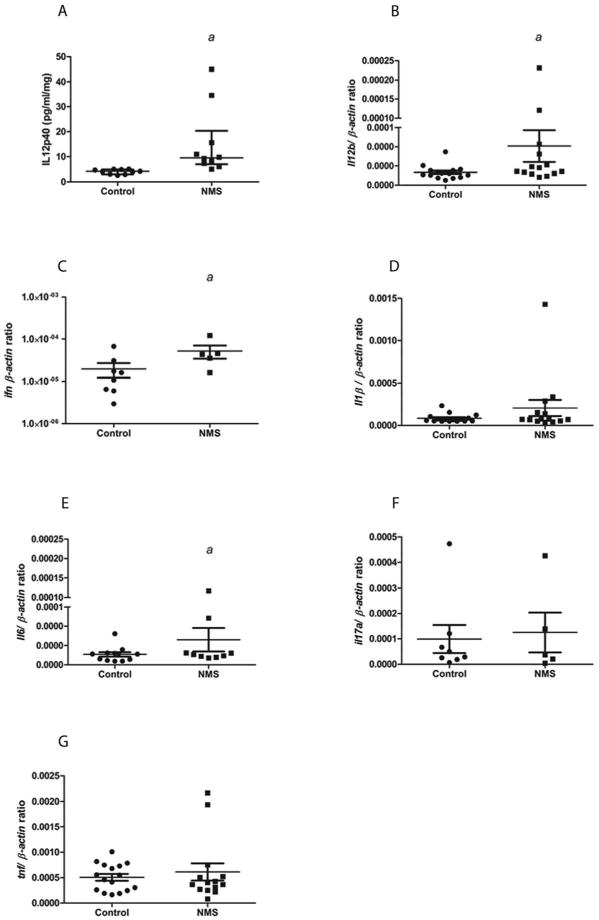
Colonic cytokine levels are increased in IL-10-/- mice subjected to NMS (n=5-10/group). IL-12p40 (A; p<0.003), IL-12b mRNA (B; p<0.001), and IFNγ (C; p<0.05). IL-1β, IL-6, IL-17, and TNFα were not significantly different from control mice (D-G).
IL-10 deficiency and neonatal maternal separation stress compromise intestinal barrier function
IL-10 deficiency and NMS had deleterious influences on colonic epithelial barrier function in the present study. In colonic mucosa from control (no NMS), IL-10-/- mice exhibited elevated colonic permeability (p<0.05; Figure 5) compared with wild type mice. NMS induced elevations in colonic permeability to FD4 in both wild type and IL-10-/- mice. IL-10-/- NMS mice exhibited the highest colonic permeability of all the experimental groups.
Figure 5.

Neonatal maternal separation and IL-10 deficiency cause long-term alterations in colonic barrier function. NMS caused significant increases in mucosal-to-serosal FITC-dextran flux in wild type and IL-10-/- mice (b,c: p<0.05). Effects of NMS and IL-10 deficiency were additive in causing increased permeability (c) (n=8/group).
Transepithelial short circuit current (Isc)
IL-10-/- mice displayed a trend for lower baseline colonic Isc compared with wild type controls (p<0.01). Wild type mice subjected to NMS exhibited elevated Isc (p<0.05) values compared with respective, normally-reared controls (Figure 6). Colonic mucosa from IL-10-/- mice previously subjected to NMS exhibited a trend towards elevated Isc values compared to normally reared IL-10-/- controls (p=0.058).
Mast cell activity
Mast cells have been previously demonstrated to mediate intestinal barrier dysfunction and contribute to chemical-induced colitis;27-30 therefore, we evaluated the effects of NMS on colonic mast cell activity in IL-10-/- mice. Colonic lamina propria mast cell counts, as assessed by toluidine blue staining, displayed a trend towards a decrease in mice exposed to NMS (p=0.10), indicative of increased mast cell activation and granule release. Consistent with these results, tryptase concentrations in colonic explant supernatants were elevated (p<0.05) in IL-10-/- mice exposed to NMS compared with normally reared mice.
Discussion
The results of this study demonstrate that NMS stress in IL-10-/- mice triggered the development of severe colitis which was sustained later in life. NMS exacerbated colitis in IL-10-/- mice, which was associated with marked defects in colonic epithelial function, elevated proinflammatory cytokines, and evidence for enhanced mast cell activation. Overall, these findings indicate that NMS in the IL-10-/- mouse represents a novel model for studying the effect of stress on the pathogenesis of IBD.
In this study, normal reared IL-10-/- mice exhibited elevations in colonic permeability compared with wild type controls demonstrating that IL-10 deficiency plays a role in baseline regulation of intestinal barrier function. These findings are supported by Madsen et al (1999) who showed that Sv/Ev 129, IL-10-/- mice exhibited a higher baseline intestinal permeability compared with controls and this preceded colitis development. 31 A subsequent study by Arrieta et al (2009) showed that administration of the zonulin antagonist (AT-1001) significantly reduced small intestinal permeability and colitis indicating that intestinal barrier dysfunction is a primary defect associated with colitis in IL-10-/- mice.32 In this study, we showed that NMS induced long-term increases in colonic permeability in wild type and IL-10-/- mice; however, colitis was observed only in IL-10-/- mice subjected to NMS. These findings support the “two-hit hypothesis” implicated in inflammatory bowel disease, in which a single factor, such as genetic predisposition, barrier dysfunction, or dysbiosis, is not sufficient to induce significant IBD.33
The precise mechanisms by which NMS resulted in long-lasting exacerbations in colitis in IL-10-/- mice are unclear. In addition to causing increased intestinal permeability and more severe colitis in IL-10-/- mice, NMS also caused an increase in intestinal permeability without inducing colitis in wild type mice. Therefore, it is likely that the enhanced colonic permeability observed in NMS-exposed mice results in increased luminal antigen and adjuvant translocation across the intestinal epithelium, thus aggravating colitis in IL-10-/- mice. Intestinal barrier dysfunction is a critical event that precedes inflammation in IBD patients and animal models.32 In patients with Crohn's disease, increased intestinal permeability is present and precedes inflammation and can predict relapse.34, 35 The presence of intestinal barrier dysfunction in relatives of Crohn's disease patients suggests that increased permeability may be a single early defect that may precede the development of overt disease or may be a contributing factor to disease.36-39 In the current model, increased intestinal permeability could be a cause or effect of the increase in colitis scores in IL-10-/- mice. Further studies are necessary to determine if the increased colitis is preceded by an increase in intestinal permeability.
Alternatively, enhanced colitis in stressed IL-10-/- mice could be due to the direct influence of stress mediators on intestinal immune system. For example, peripheral stress mediators, such as corticotrophin releasing factor (CRF) have been implicated as contributing to intestinal inflammation independent of intestinal barrier dysfunction.40 For example, CRF receptors are expressed on a variety of cell types in the intestine, including mast cells and macrophages.41-43 CRF signaling on these cells may contribute to IBD either by triggering intestinal barrier dysfunction or by initiating direct release of pro-inflammatory mediators that in turn result in barrier dysfunction.41, 44-46 Although there were only modest effects of NMS on measures of mast cell activation as evidenced by (1) a trend for decreased toluidine blue-stained mast cells, an indicator or intracellular loss (exocytosis) of mast cell granule47 and (2) elevated colonic tryptase release, these data are in line with a well-established of the mast cell in mediating intestinal barrier dysfunction30 and colitis.48-50 Mast cell tryptase has been shown to induce multiple effects that can contribute to colitis including breakdown of intestinal epithelial barrier function via tight junction protein disruption and neutrophil recruitment. 50, 51 Given that we only assessed mast cell activation, the functional role of the mast cell in this model remains to be fully understood.
In the present study, IL-10 deficiency and NMS induced alterations in Isc, an index of net electrogenic ion transport. Baseline Isc was elevated by NMS in wild type mice and displayed a trend towards increase in IL-10-/- mice, which is consistent with previous studies utilizing NMS in rats.30, 52 Previous studies demonstrated that elevated Isc induced by NMS was not observed in mast cell deficient rats suggesting that NMS induced mast cell activation was responsible for the chronically elevated Isc.30 Although others have demonstrated altered Isc in several models of colitis, the direct role of altered Isc remains unclear.53
In summary, stress is a major factor triggering the onset of IBD but the exact mechanisms remain unclear. Here we present a novel animal model to investigate the mechanisms by which early life stress influences development of IBD in adulthood. Future research into the mechanisms through which stress influences IBD will provide insight into the mechanism of stress-induced IBD onset or IBD flares and potentially reveal therapeutic targets that control IBD development and relapse; therefore, this novel NMS/ IL-10-/- model may serve as a useful model to study the influence of early life stress on the development of IBD in human patients.
Acknowledgments
This work was supported by the NIH K08 DK084313 (AJM), R01 DK54452 (SEP), and P30 DK34987 (AJM and SEP, Immunotechnologies Core and Histology Core). Nitsan Maharshak is supported by the Crohn's and Colitis Foundation of America Research Fellowship Award and the American Physicians Fellowship for Medicine in Israel. Elizabeth Lennon is supported by the Ruth L. Kirschstein National Research Service Award T32 RR024394 as part of North Carolina State University's Comparative Medicine and Translational Research Training Program.
References
- 1.Tocchi A, Lepre L, Liotta G, et al. Familial and psychological risk factors of ulcerative colitis. Ital J Gastroenterol Hepatol. 1997 Oct;29(5):395–398. [PubMed] [Google Scholar]
- 2.Lerebours E, Gower-Rousseau C, Merle V, et al. Stressful life events as a risk factor for inflammatory bowel disease onset: A population-based case-control study. Am J Gastroenterol. 2007 Jan;102(1):122–131. doi: 10.1111/j.1572-0241.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 3.Levenstein S, Prantera C, Varvo V, et al. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am J Gastroenterol. 1994 Aug;89(8):1219–1225. [PubMed] [Google Scholar]
- 4.Levenstein S, Prantera C, Varvo V, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000 May;95(5):1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- 5.Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn's disease: a biopsychosocial model. Gut. 2008 Oct;57(10):1386–1392. doi: 10.1136/gut.2007.134817. [DOI] [PubMed] [Google Scholar]
- 6.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002 Nov;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 7.Agostini A, Rizzello F, Ravegnani G, et al. Parental bonding and inflammatory bowel disease. Psychosomatics. Jan;51(1):14–21. doi: 10.1176/appi.psy.51.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Agostini A, Rizzello F, Ravegnani G, et al. Adult attachment and early parental experiences in patients with Crohn's disease. Psychosomatics. May;51(3):208–215. doi: 10.1176/appi.psy.51.3.208. [DOI] [PubMed] [Google Scholar]
- 9.Ercolani M, Farinelli M, Agostini A, et al. Gastroesophageal reflux disease (GERD) and inflammatory bowel disease (IBD): attachment styles and parental bonding. Percept Mot Skills. Oct;111(2):625–630. doi: 10.2466/02.09.15.21.PMS.111.5.625-630. [DOI] [PubMed] [Google Scholar]
- 10.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricius K, Wortwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 2008 Feb;212(5):403–416. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin J, Makharia GK, Ahuja V, Kalaivani M, Joshi YK. Intestinal permeability and its association with the patient and disease characteristics in Crohn's disease. World J Gastroenterol. 2008 Mar 7;14(9):1399–1405. doi: 10.3748/wjg.14.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006 Aug;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 14.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008 Jun;8(4):274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- 15.Kiliaan AJ, Saunders PR, Bijlsma PB, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998 Nov;275(5 Pt 1):G1037–1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- 16.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000 Oct;119(4):1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 17.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005 Dec;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 18.Moeser AJ, T S, Blikslager AT, Keku TO. The Role of the Intestinal Microbiota in Colonic Barrier Dysfunction Induced by Neonatal Stress. Gastroenterology. 2009;136(5):A102. [Google Scholar]
- 19.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010 Mar;298(3):G352–363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudry G, Jury J, Yang PC, Perdue MH. Chronic psychological stress alters epithelial cell turn-over in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1228–1232. doi: 10.1152/ajpgi.00358.2006. [DOI] [PubMed] [Google Scholar]
- 21.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996 Aug 15;98(4):1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007 Jun 11;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 23.de Jong D, Vyth-Dreese F, Dellemijn T, et al. Histological and immunological parameters to predict treatment outcome of Helicobacter pylori eradication in low-grade gastric MALT lymphoma. J Pathol. 2001 Mar;193(3):318–324. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH811>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005 Dec 19;202(12):1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh SZ, Hegazi RA, Kobayashi T, et al. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. May 1;186(9):5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argenzio RA, Henrikson CK, Liacos JA. Restitution of barrier and transport function of porcine colon after acute mucosal injury. Am J Physiol. 1988 Jul;255(1 Pt 1):G62–71. doi: 10.1152/ajpgi.1988.255.1.G62. [DOI] [PubMed] [Google Scholar]
- 27.Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007 Aug;293(2):G413–421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 28.Snoek SA, Dhawan S, van Bree SH, et al. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterol Motil. Nov 29; doi: 10.1111/j.1365-2982.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 29.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001 May;48(5):630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999 Nov;5(4):262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009 Jan;58(1):41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaser A, Zeissig S, Blumberg RS. Genes and environment: how will our concepts on the pathophysiology of IBD develop in the future? Dig Dis. 28(3):395–405. doi: 10.1159/000320393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 35.Meddings JB. Review article: Intestinal permeability in Crohn's disease. Aliment Pharmacol Ther. 1997 Dec;11(Suppl 3):47–53. doi: 10.1111/j.1365-2036.1997.tb00808.x. discussion 53-46. [DOI] [PubMed] [Google Scholar]
- 36.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 37.D'Inca R, Annese V, di Leo V, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment Pharmacol Ther. 2006 May 15;23(10):1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 38.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology. 1996 May;110(5):1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- 39.Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997 Sep;113(3):802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 40.la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci U S A. 2005 May 24;102(21):7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008 Jan;57(1):50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 42.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998 Apr;114(4):640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 43.Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004 Jul;90(2):309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 44.Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998 Jan;139(1):403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 45.Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004 Nov;89(11):5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- 46.Kokkotou E, Torres D, Moss AC, et al. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006 Sep 1;177(5):3355–3361. doi: 10.4049/jimmunol.177.5.3355. [DOI] [PubMed] [Google Scholar]
- 47.Augusto C, Lunardi LO, Vugman I. Reduction in rat mesentery mast cell staining after degranulation by protamine. A competitive antagonism. Cell Mol Biol. 1990;36(4):357–361. [PubMed] [Google Scholar]
- 48.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008 May;57(5):582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 49.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004 Apr;53(4):501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton MJ, Sinnamon MJ, Lyng GD, et al. Essential role for mast cell tryptase in acute experimental colitis. Proc Natl Acad Sci U S A. Jan 4;108(1):290–295. doi: 10.1073/pnas.1005758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005 Sep 9;280(36):31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 52.Zareie M, Johnson-Henry K, Jury J, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006 Nov;55(11):1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Navarro R, Ballester I, Zarzuelo A, Sanchez de Medina F. Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci. 2005 Feb 11;76(13):1489–1501. doi: 10.1016/j.lfs.2004.09.019. [DOI] [PubMed] [Google Scholar]


