Abstract
Background
Dopamine is a principal natriuretic hormone in mammalian Na+ homeostasis. Dopamine acutely alters glomerular filtration rate (GFR) and decreases Na+ absorption in both the proximal and distal nephron. Proximal tubule natriuresis is effected through inhibition of the apical membrane Na/H exchanger NHE3.
Methods
We examined whether dopamine directly and acutely decreases apical membrane NHE3 protein using renal tissue in two in vitro systems: renal cortical slices and in vitro perfused single tubules. After incubation with dopamine, NHE3 activitry was measured by 22Na flux and NHE3 antigen was measured by immunoblot in apical membrane and total cellular membranes.
Results
Direct application of dopamine to either cortical slices or microperfused tubules acutely decreases NHE3 activity and antigen at the apical membrane of the proximal tubule. No change in total cellular NHE3 was detected.
Conclusion
One mechanism by which dopamine causes natriuresis is via direct and acute reduction of NHE3 protein at the apical membrane via changes in NHE3 protein trafficking.
Keywords: dopamine, Na/H exchange, trafficking, proximal tubute, natriuresis
Dopamine is an intrarenal autocrine/paracrine hormone with diverse actions in the kidney. With respect to regulation of Na+ balance, dopamine increases renal plasma flow and to a much lesser extent glomerular filtration rate (GFR) with a resultant decrease in filtration fraction [1, 2]. Independent of its hemodynamic effects, dopamine also acutely inhibits volume absorption in the proximal tubule [3, 4] and thick ascending limb [5] and it decreases Na+/K+-ATPase activity in the cortical collecting duct [6] via direct actions on renal epithelia. These effects of dopamine on the renal vasculature and epithelia operate in concert to produce natriuresis. The natriuresis in response to acute volume expansion is associated with increased urinary dopamine excretion [7] and infusion of the renal precursor of dopamine, gludopa, which selectively increases renal dopamine synthesis, has been shown to cause Na+ excretion [8]. Finally, the natriuretic response to acute saline infusion can be partially negated by blockade of the DR1 dopamine receptor [9, 10].
The mammalian proximal tubule absorbs about half of the isotonic glomerular filtrate. Dopamine acutely inhibits three Na+ transporters in the proximal tubule: the apical membrane Na+/H+ exchanger [11–17], Na+ inorganic phosphate cotransporter [18–21], and the basolateral Na+/K+-ATPase [22–26]. Apical membrane Na+/H+ exchange activity is responsible for all of the transcellular NaCl absorption in the proximal tubule via coupling with parallel Cl−/base− exchange and apical Na+/H+ exchange lowers luminal [Cl−] and enhances paracellular Cl− absorption [27, 28]. Proximal tubule apical membrane Na+/H+ exchange activity is encoded primarily by NHE3 based on antigenic [29, 30] and functional evidence [31–33]. The importance of NHE3 in maintaining extra-cellular fluid volume is underscored by the hypovolemia and hypotension seen in NHE3-deficient rodents [34, 35].
NHE3 is regulated via a panoply of mechanisms over an extended time course. Regulation can be effected at the levels of alteration in transporter activity, changes in NHE3 trafficking, changes in NHE3 protein synthesis and half-life and changes in NHE3 transcription [36, 37]. Although these regulatory mechanisms are well demonstrated in cultured cells, only few of them have actually been confirmed in native renal tissue. Acute inhibition of Na+/H+ exchanger activity by dopamine has been described in suspended tubules [11], apical membrane vesicles [13, 14], and cultured renal cells [16]. The only study that measured NHE3 antigen was in cultured cells, where dopamine acutely activates NHE3 endocytosis [17] but evidence for this mode of regulation is lacking in intact renal tissue. In the present study, we seek to provide evidence in renal tissue that dopamine directly and acutely decreases apical membrane NHE3 activity and antigen.
Methods
Preparation of cortical slices and membrane vesicles
Balb/c mice with free access to food and water (20 to 25 g) were anesthetized with KXA cocktail (vol:vol 1.5/5/2.5/2 H2O/ketamine/xylazine/Apr; 0.3 mL per animal intraperitoneally) and kidneys were removed, decapsulated, and rinsed in ice-cold phosphate buffered saline (PBS). Cortices were dissected out on inverted ice-cold Petri dish, and sliced with a Staedie-Riggs tissue microtome. Cortical slices were rinsed in Ham's F12/Dulbecco's modified Eagle's medium (DMEM) (1:1) supplemented with 7 mmol/L heptanoic acid and resuspended in dopamine-containing incubation media [F12/DMEM (1:1) + 7 mmol/L heptanoic acid, 1 mmol/L sodium ascorbate, 10−8 mol/L benserazide, dopamine 10−8 to 10−4 mol/L, 5% CO2, and 21% O2 at 37°C]. Vehicle media was identical with omission of dopamine. Slices were resuspended in buffer (in mmol/L) [300 mannitol, 6 ethyleneglycol tetraacetate (EGTA), 12 Tris-HCl, pH 7.5, and 0.1 phenylmethylsulfonyl fluoride (PMSF)] and mechanically homogenized (hand-held Dounce ×15 strokes then Potter- Elvehjelm Teflon-pestle ×15 strokes). Residual unbroken cortical slices were pelleted (200 × g @ rmax, 4°C, 1 minute) (Beckman Acuspin, Beckman, Fullerton, CA, USA) and subjected to a second round of homogenization. The two homogenates were combined and an aliquot was saved and total cortical membranes were pelleted (55,000 × g @ rmax, 20,000 rpm, 40 minutes, 4°C) (Beckman J2-21M, JA-20 rotor) for immunoblotting. The remaining cortical homogenate were exposed to 20 mmol/L MgCl2 (rocking 4°C, 1 hour) and the Mg2+-induced aggregate was removed by centrifugation (200 × g @ rmax, 4°C, 1 minute) (Beckman Acuspin). The apical membrane in the supernatant was pelleted (55,000 × g @ rmax, 20,000 rpm, 40 minutes, 4°C) (Beckman J2–21M, JA-20 rotor) and resuspended in 1 mL of wash buffer (in mmol/L) (300 mannitol, 12 Tris-HCl, pH 7.5, and 0.1 PMSF). Apical membranes were again pelleted (109,000 × g @ rmax, 50,000 rpm, 25 minutes, 2°C) (Beckman TLX ultracentrifuge, TLA 100.3 rotor) and resuspended in the appropriate buffer for the subsequent assay.
Measurement of NHE3 activity
NHE3 activity in apical membrane vesicles was measured by rapid filtration technique as previously described [38]. Two hundred micrograms (protein) of vesicles were acid loaded (in mmol/L) [300 mannitol, 20 methyl ethane sulphonate (MES), pH 5.5, 4°C, 2 hours] and exposed to 10× volume uptake solution (in mmol/L) (300 mannitol, 20 Tris, pH 7.5, 0.1 22NaCl) to initiate transport at 20°C for 10 seconds. Uptake was stopped by dilution with stop solution (in mmol/L) (150 NaCl, 20 Tris, pH 7.5) at 4°C and 22Na uptake was quantified by rapid filtration on 0.65 μmol/L Millipore filters (Millipore, Bedford, MA, USA) and scintillation counting. Specific activity was expressed per milligram membrane protein as well as normalized to the relative amounts of NHE3 antigen.
Measurement of NHE3 antigen
For immunoblots, either total cortical membranes or apical membranes were resuspended in dye-free sodium dodecyl sulfate (SDS) loading buffer [5 mmol/L Tris-HCl, pH 6.8, 10% (vol:vol) glycerol, 1% (wt:vol) mercaptoethanol, 0.1% (wt:vol) SDS, and 0.01% (wt:vol)]. Protein concentrations were determined by the Bradford colorimetric assay. Thirty micrograms of total cortical membrane and 10 μg of apical membrane were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nylon membranes, and blotted with anti-NHE3 (antiserum #1568) exactly as previously described [30]. Some experiments were repeated with the monoclonal antibody used for the single tubule immunohistochemistry (see below) and showed basically identical results. After incubation with horseradish peroxidase-conjugated secondary antibody, signals were detected by enhanced chemiluminescence and quantified by densitometry.
For immunohistochemistry of kidneys, five NMRI mice (BRL, Basel, Switzerland) weighing 25 to 30 g were used. Animals were kept on a standard laboratory diet and had free access to food and water. The mice were anesthetized with intraperitoneal ketamine (Narketan 10) (Chassot AG, Belp, Switzerland) (80 mg/kg body weight) and xylazine (Rompun) (Bayer, Leverkusen, Germany) (33 mg/kg body weight) and the animals were fixed by intravascular perfusion via the abdominal aorta as described previously [39]. The fixative consisted of 3% paraformaldehyde and 0.05% picric acid dissolved in a 3:2 mixture of 0.1 mol/L cacodylate buffer (pH 7.4, adjusted to 300 mOsm with sucrose) and 10% hydroxyethyl starch in saline (HAES-steril) (Fresenius, Stans, Switzerland). After 5 minutes of fixation, the kidneys were rinsed by perfusion for 5 minutes with the 0.1 mol/L cacodylate buffer. Slices of fixed mice kidneys were frozen in liquid propane and mounted onto thin cork slices. Cryosections (2 to 3 μm) were mounted on chromalum/gelatine-coated glass slides, thawed, and stored in PBS until use. Antigen retrieval was performed with microwave in 0.01 mol/L citrate buffer, pH 6.0, at 20% power for 10 minutes. After rising with PBS, sections were incubated for 10 minutes in PBS containing 3% (wt:vol) nonfat milk powder and 0.02% (vol:vol) Triton X-100 to block nonspecific staining. Primary anti-NHE3 monoclonal antibody (clone 53 mouse IgG raised against rat NHE3 aa 725–831) (formerly Transduction Laboratories now Pharmingen, Lexington, KY, USA) diluted 1:100 in blocking solution was applied overnight at 4°C. Sections were then rinsed three times with PBS and overlayed with the secondary antibody (Cy3-conjugated goat-antimouse IgG at 1:1000 dilution) (Jackson Immunoresearch Laboratories, West Grove, PA, USA) for 45 minutes at room temperature. Finally, the sections were rinsed with PBS and coverslips were applied with Dako Glycergel (Dakopatts, Glostrup, Denmark) containing 2.5% 1,4-diazabicyclo-(2.2.2)-octane (DABCO) (Sigma Chemical Co., St. Louis, MO, USA) as a fading retardant and studied using an epifluorescense microscope (Polyvar, Reichert-Jung, Austria).
In vitro microperfusion and single-tubule immunohistochemistry
Mouse proximal straight and convoluted tubules were dissected free hand in cooled (4°C) modified Hank's solution (in mmol/L) (137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 Tris-HCl, 0.25 CaCl2, 2 glutamine, and 2 l-lactate bubbled with 100% O2, pH 7.4). Mouse tubules were perfused in vitro as previously described [33]. Briefly, dissected tubules were transferred to a 1.2 mL thermostatically controlled bathing chamber and perfused with concentric glass pipettes at 38°C. The perfusion and solutions simulated an ultrafiltrate solution (in mmol/L) (115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 mmol/L CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, 2 glutamine, and 2 l-lactate). This solution was gassed with 95% O2 and 5% CO2 and had a pH of 7.4 and the osmolality was adjusted to 295 mOs mol/kg H2O.
After the tubules were perfused or bathed with either 10−5 mol/L dopamine or vehicle, they were fixed for 5 to 10 minutes in the bathing chamber with fixative (see above for content) as described by Traebert et al [40]. The tubule was then released near the bottom of the bathing chamber and the bathing chamber removed to a dissection microscope for better visualization of the tubule. Immediately before transferring the tubule, a drop of warm (37°C) 10% gelatin (Gelatin Type A 175 Bloom) (Sigma Chemical Co.) in PBS was placed on a microscope slide cooled to 4°C. After the gelatin hardened slightly, the tubule was transferred with an Eppendorf pipette to the gelatin. The excess fixative was removed from the drop of gelatin and a second drop of cooled but still molten gelatin was placed on top of the tubule. The slide was placed at 4°C for 1 hour and then a cube of gelatin containing the tubule oriented in the long axis was cut and stored in fixative at 4°C.
For immunodetection of NHE3 in single tubules, the gelatin droplets containing the tubules were washed for 2 hours in PBS and cut into cubes before freezing in liquid propane. Cryosections of 2 to 3 μm thickness were mounted on chromalum/gelatine-coated glass slides, thawed, and stored in PBS until labeling use. For immunofluorescence, sections were pretreated with 3% milk powder in PBS for 10 minutes and incubated overnight at 4°C with antiserum against the NHE3 (clone 53) (Transduction Laboratories) diluted 1:500 in 3% milk powder in PBS containing 0.3% Triton X-100. Sections were then rinsed three times with PBS and incubated for 45 minutes at 4°C with the secondary antibody (swine antirabbit IgG conjugated to fluorescein isothiocyanate) (Dakopatts) diluted 1:50 in PBS/milk powder. Finally, the sections were rinsed three times with distilled water, plated on coverslips by using Dako-Glycergel (Dakopatts) containing 2.5% DABCO (Sigma Chemical Co.) as a fading retardant and studied by an epifluorescense microscope (Polyvar, Reichert-Jung). Controls performed with omission of the primary antiserum or with nonimmune sera were all negative.
Results
Effect of dopamine on NHE3 activity and antigen in cortical slices
Since dopamine has significant effects on the systemic circulation as well as renal hemodynamics, we studied the epithelial effects of dopamine by applying it directly to cortical slices. Figure 1A shows that dopamine acutely decreased NHE3 antigen in apical membrane fraction without affecting the abundance of NHE3 antigen in the total cortex. A summary of the time-dependence of the dopamine effect on apical NHE3 antigen is shown in Figure 1B. The effect on apical membrane NHE3 antigen is evident within 10 minutes of dopamine application and persisted for at least 1 hour. No changes in total cortical NHE3 antigen were detected throughout 1 hour of dopamine treatment (Fig. 1A). Changes in NHE3 activity without changes in apical NHE3 protein has been shown [37, 41]. We next examined NHE3 activity and antigen in the same apical membranes treated with either dopamine or vehicle and normalized the effect of dopamine on transport activity of NHE3 to NHE3 antigen as described by Yang et al [42]. With this system, there were no discernible changes in NHE3 activity without changes in NHE3 antigen (Fig. 2). Figure 3 shows the dose-dependence of the dopamine effect and the summary is shown in Figure 3B. The half maximal effect was seen between 10−6 and 10−5 mol/L dopamine. This dose response is similar to that previously described for the effect of dopamine on NHE3 activity in brush border vesicles and cultured cells [13, 16] as well as on surface NHE3 antigen in cultured cells [17]. The effect of dopamine was more variable and somewhat blunted in the absence the aromatic amino acid decarboxylase inhibitor benserazide (data not shown), suggesting that there is some endogenous production of dopamine by the cortical slices.
Fig. 1. Effect of dopamine on NHE3 antigen: Time response.

(A) Representative experiments. Mouse renal cortical slices were incubated with 10−5 mol/L dopamine (DA) and apical and total cortical membranes were prepared and NHE3 and β-actin antigen were measured by immunoblot. Con is control. (B) Summary of the effect of dopamine on NHE3 antigen. Y-axis shows the abundance of NHE3 antigen in the apical membrane vesicles as a percentage of the vehicle-treated controls. Symbols denote means ± SE. The number of independent experiments for each time point is N = 8, 5 minutes; N = 4, 10 minutes; N = 4, 15 minutes; N = 8, 30 minutes; N = 3, 60 minutes. *P < 0.05 (unpaired t test); #P = 0.08.
Fig. 2. Effect of dopamine on NHE3 activity and NHE3 antigen on the same apical membrane vesicles.
Mouse renal cortical slices were incubated with 10−5 mol/L dopamine (DA) for either 5 or 30 minutes and apical and cortical membranes were prepared for NHE3 activity (pH gradient driven 22Na flux) and NHE3 antigen (immunoblot) assays. The left panel shows NHE3 activity normalized to membrane protein and NHE3 activity from dopamine-treated tissue is expressed as percentage of the vehicle-treated ones. The middle panel shows the quantity of NHE3 antigen expressed also as a percentage of vehicle. The right panel shows NHE3 activity divided by NHE3 antigen and expressed as dopamine as percentage of control. The number of independent experiments = 4 pairs for either 5 or 30 minutes. *P < 0.05 (unpaired t test).
Fig. 3. Effect of dopamine on NHE3 antigen: Dose response.
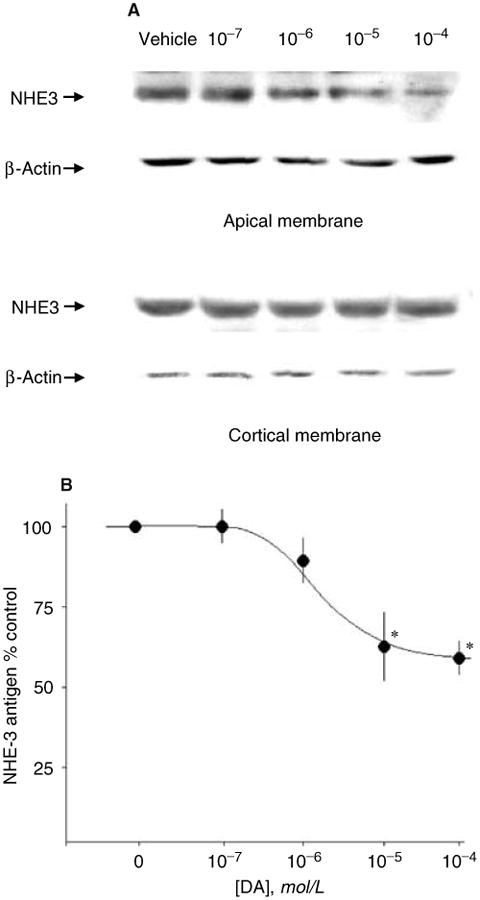
(A) Representative experiment. Mouse renal cortical slices were incubated with various concentration of dopamine (DA) for 30 minutes and apical and cortical membranes were prepared and NHE3 and β-actin antigen were measured by immunoblot. (B) Summary of all experiments. Y-axis shows the abundance of NHE3 antigen in the apical membrane vesicles as a percentage of the vehicle-treated controls. Each symbol represents the mean ± SE of three to four independent experiments. The number of independent experiments for each dose were N = 3, 10−7 mol/L; N = 4, 10−6 mol/L; N = 8, 10−5 mol/L; N = 4, 10−4 mol/L. *P < 0.05 (unpaired t test).
Effect of dopamine on NHE3 antigen in isolated proximal tubules
Expression of NHE3 is different in the rabbit (proximal tubule), rat (proximal tubule and thick ascending limb), and pig (distal tubule) kidney [29, 30, 43] but no published data are available on mouse kidney. We first examined NHE3 distribution in the mouse kidney by immunohistochemistry. NHE3 staining was observed in the apical membrane of the proximal tubule and the thick ascending limb (Fig. 4). In the proximal tubule, there is an axial decrease in apical membrane NHE3 protein with highest expression in S1 and lowest in S3 (Fig. 4). We next corroborated the dopamine effect seen with immunoblots with actual immunohistochemical visualization of NHE3 antigen in intact proximal tubules. To avoid confounding systemic and/or renal hemodynamic effects of dopamine, we examined the effect of luminal or bath dopamine on single microdissected tubules perfused in vitro on NHE3 antigen by immunofluorescence. NHE3 was seen as a brush border protein under control conditions in both the proximal convoluted and proximal straight tubule (Figs. 5 and 6 vehicle). Addition of dopamine to the lumen or the bath for 30 minutes both decreased NHE3 antigen on the brush border by comparable extent (Figs. 5 and 6). This finding is compatible with the biochemical data obtained from homogenized cortical slices presented above, as well as findings described in cultured cells [17]. Subapical staining of NHE3 in tubules was not strong and consistent enough to enable us to conclude whether there is increase subapical NHE3 in response to dopamine.
Fig. 4. Immunohistochemical staining of NHE3 antigen in mouse kidney.


Mouse kidneys were perfusion fixed and stained with anti-NHE3 as described in the Methods section. (A) Whole kidney overview. (B and C) Proximal tubule S1, S2, and S3 segment and thick ascending limb (TAL) are labeled.
Fig. 5. Single-tubule immunohistochemistry: Effect of dopamine (DA) on proximal convoluted tubule.

Microdissected proximal convoluted tubules were perfused with 10−5 mol/L dopamine in the lumen or the bath for 30 minutes and fixed and stained for NHE3 immunohistochemistry as described in the Methods section. Three independent experiments showed similar results.
Fig. 6. Single-tubule immunohistochemistry: Effect of dopamine (DA) on proximal straight tubule.

Microdissected proximal convoluted tubules were perfused with 10−5 mol/L dopamine in the lumen or the bath for 30 minutes and fixed and stained for NHE3 immunohistochemistry as described in the Methods section. Three independent experiments showed similar results.
Discussion
The renal proximal tubule is an important segment in the regulation extracellular fluid volume. Several features regarding proximal tubule Na+ absorption are relevant to the study of dopamine as a natriuretic hormone. Traditional views of control of proximal tubule Na+ and water transport have focused on peritubular hydrostatic and osmotic driving forces [27] that permits changes in glomerular hemodynamics to be coupled instantaneously and reversibly to changes in proximal tubule Na+ and water absorption. Besides peritubular physical factors, active transcellular Na+ transport is also directly modulated by hormones [37]. The relative importance of the two parallel regulatory modes is not known but dopamine reduces filtration fraction as well as directly inhibits proximal tubule Na+ transporters thereby favoring natriuresis by both paracellular and transcellular pathways [1–4]. Inhibition of proximal tubule Na+ absorption per se may have variable impact on whole organ Na+ balance because increased distal Na+ delivery can reduce the filtered Na+ load via activation of tubuloglomerular feedback and the Na+ exiting the proximal tubule is subjected to reabsorption by the distal nephron. Both of these counter measures have to be dampened in order for proximal natriuresis to effect negative Na+ balance. The dopamine-induced proximal natriuresis is accompanied by inhibition of tubuloglomerular feedback [44] and distal tubule transport [5, 6], rendering dopamine an effective natriuretic hormone.
The importance of NHE3 in mediating proximal tubule Na+ and volume absorption has been shown at the level of the tubule [32] and the whole organism [34, 35]. These conditions include acute regulation by parathyroid hormone [41, 43] and hypertension [46, 47] and chronic regulation by hypertension [47, 48], steroid hormones [49–52], and metabolic acidosis [31, 53]. The acute regulation of NHE3 by parathyroid hormone appears to proceed via a biphasic mechanism in kidney as well as in cultured cells [41, 54], whereas the acute regulation by hypertension is mediated solely by NHE3 trafficking [42]. Changes in surface NHE3 without changes in total cellular NHE3 have been shown in response parathyroid hormone [54], dopamine [17], endothelin [55], and acidic pH [56] in cell culture models. The effects of parathyroid hormone and acidosis have also been shown in intact animals [41, 45]. In the present study, we cannot detect changes in NHE3 activity that was not accompanied by changes in apical NHE3 antigen. It is presently unknown whether dopamine can change NHE3 activity without changes in NHE3 antigen in the kidney.
We have previously demonstrated in a renal cell line that dopamine acutely activates NHE3 endocytosis via the clathrin-coated pathway by a process dependent on phosphorylation of NHE3 by protein kinase A [17]. This mode of regulation allows rapid and reversible changes in proximal tubule Na+ transport. Reversible decreases in apical membrane NHE3 have been shown with the hyper-tensive model [46] and recycling of endocytosed NHE3 has been shown in transfected cells [57, 58]. We cannot appreciate increased subapical NHE3 staining in response to dopamine in every section of tubule, although it is visible in some sections (Fig. 6, middle panel). This may reflect the pervasive difficulty encountered in visualizing intracellular NHE3 on conventional light immunohistochemistry [29, 30]. However, by high-resolution immunohistochemistry on semithin sections and electro-microscopy, subapical NHE3 can be detected [59]. In total cortical membranes, it was unequivocal that dopamine did not decrease total cellular NHE3 over 1 hour. These results are supportive of a trafficking event similar to that described in cultured cells [17].
In cortical slices, the effect of exogenous dopamine was more variable and smaller in magnitude in the absence of inhibition of aromatic amino acid decarboxylase. This finding is in congruence with endogenous dopamine production by cortical tissue in vitro [60–62]. Since no exogenous l-dopa was supplemented, one assumes that there exists an endogenous stored pool of l-dopa in the proximal tubule that is available for in vitro dopamine synthesis. In our studies with isolated tubules, the dopamine effect was evident even in the absence of decarboxylase inhibition. This is likely due to the smaller mass of proximal tubule cells in a single tubule and more importantly the dilution of endogenous dopamine by the bath and the luminal perfusate. There are notable differences between this study and previous ones in isolated tubules and cultured cells. Both apical and basolateral application of dopamine reduced apical NHE3 protein abundance in the isolated mouse tubules. This finding is compatible with the presence of dopamine receptors on both the apical and basolateral surface [63] and studies of dopamine on NHE3 activity in cultured cells [16]. However, our previous study measuring volume flux in rabbit proximal convoluted tubules found only a luminal dopamine effect [4]. The basis for this difference is not clear. Although the rodent proximal tubule expresses dopamine receptors in both the apical and basolateral membrane, similar data in rabbit kidney are lacking. One in vitro microperfusion study in rabbits showed that dopamine inhibited transepithelial fluid flux only in the proximal straight tubule [3]. We have shown that in the rabbit proximal convoluted tubule, the dopamine-induced inhibition of volume absorption can be unmasked if transport was first stimulated by norepinephrine [4]. The present study shows that dopamine decreases apical NHE3 protein in both the proximal convoluted and straight tubules. The baseline status of proximal Na+ and water transport of the animal will likely affect the ability to observe the dopamine effect.
Conclusion
We have shown that in the absence of hemodynamic changes, dopamine can directly reduce the abundance of NHE3 protein on the proximal tubule apical membrane without changing total cellular NHE3. This action of dopamine on NHE3 trafficking is likely an important mechanism by which dopamine effects natriuresis.
Acknowledgments
This work was supported by the National Institutes of Health (DK-48482 and DK-54392 to O.W.M. and DK-41612 to M.B.), the Swiss National Science Foundation (31-47742.96 to B.K.), the American Heart Association Texas Affiliate (98G-052 to O.W.M.), and the Department of Veteran Affairs Research Service (O.W.M.).
References
- 1.Mcdonald RH, Goldberg LI, Mcnay JL, Tuttle EP. Effect of dopamine in man, augmentation of sodium excretion, glomerular filtration rate and renal blood flow. J Clin Invest. 1964;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenberg NK, Adams DF, Mendell P, et al. Renal vascular responses to dopamine: Hemodynamic and angiographic observations in normal man. Clin Sci Mol Med. 1973;45:733–742. doi: 10.1042/cs0450733. [DOI] [PubMed] [Google Scholar]
- 3.Bello-Reuss E, Pastoraiza-Munoz E, Colindres RE. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol. 1977;232:F26–F32. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int. 1998;54:1593–1600. doi: 10.1046/j.1523-1755.1998.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grider J, Kilpatrick E, Ott C, Jackson B. Effect of dopamine on NaCl transport in the medullary thick ascending limb of the rat. Eur J Pharmacol. 1998;342:281–284. doi: 10.1016/s0014-2999(97)01564-1. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto F, Cohen HT, Satoh T, Katz AI. Dopamine inhibits Na/K-ATPase in single tubules and cultured cells from distal nephron. Pflugers Arch. 1992;421:302–306. doi: 10.1007/BF00374216. [DOI] [PubMed] [Google Scholar]
- 7.Sowers JR, Crane PD, Beck FWJ, et al. Relationship between urinary dopamine production and natriuresis after acute intravascular volume expansion with NaCl in dogs. Endocrinology. 1984;115:2085–2090. doi: 10.1210/endo-115-6-2085. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZQ, Way D, Trigg L, Mcgrath BP. DA-2 receptors mediate renal effects of the dopamine prodrug gludopa, in conscious rabbits. Clin Exp Pharmacol Physiol. 1992;19:369–372. doi: 10.1111/j.1440-1681.1992.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 9.Hedge SS, Jadhav AL, Lokhandwala MF. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension. 1989;13:828–834. doi: 10.1161/01.hyp.13.6.828. [DOI] [PubMed] [Google Scholar]
- 10.Hansel LP, Fasching A. The effect of dopamine receptor blockade on natriuresis is dependent on the degree of hypervolemia. Kidney Int. 1991;39:253–258. doi: 10.1038/ki.1991.30. [DOI] [PubMed] [Google Scholar]
- 11.Gesek F, Schoolworth A. Hormonal interactions with the proximal Na/H exchanger. Am J Physiol. 1990;258:F514–F521. doi: 10.1152/ajprenal.1990.258.3.F514. [DOI] [PubMed] [Google Scholar]
- 12.Winaver J, Burnett JC, Tyce GM, Dousa TP. ANP inhibits Na/H antiport in proximal tubule brush border membrane: Role of dopamine. Kidney Int. 1990;38:1133–1140. doi: 10.1038/ki.1990.323. [DOI] [PubMed] [Google Scholar]
- 13.Felder CC, Albrecht FE, Campbell T, et al. cAMP-independent G-protein linked inhibition of Na/H exchange in renal brush border by D1 dopamine agonist. Am J Physiol. 1993;263:F1031–1037. doi: 10.1152/ajprenal.1993.264.6.F1032. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh-Hamad D, Wang YP, Jo OD, Yanagawa N. Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol. 1993;264:F737–F743. doi: 10.1152/ajprenal.1993.264.4.F737. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht FE, Xu J, Moe OW, et al. Regulation of NHE-3 activity by G-protein subunits in renal brush border membranes. Am J Physiol. 2000;278:R1064–R1073. doi: 10.1152/ajpregu.2000.278.4.R1064. [DOI] [PubMed] [Google Scholar]
- 16.Wiederkehr MR, Di Sole F, Fan L, et al. Characterization of acute regulation of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney Int. 2001;59:197–209. doi: 10.1046/j.1523-1755.2001.00480.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu MC, Fan L, Quiñones H, et al. Dopamine stimulates dynamin-dependent endocytosis of NHE3 via PKA-mediated phosphorylation of NHE3. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 18.Glahn RP, Onsgaad MJ, Tyce GM, et al. Autocrine/paracrine regulation of renal Na-phosphate co-transport by dopamine. Am J Physiol. 1993;264:F614–F622. [Google Scholar]
- 19.Perrichot R, Garcia-Ocaña A, Couette S, et al. Locally formed dopamine modulates renal Na-Pi cotransport through DA1 and DA2 receptors. Biochem J. 1995;312:433–437. doi: 10.1042/bj3120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells. J Am Soc Nephrol. 1998;9:1604–1612. doi: 10.1681/ASN.V991604. [DOI] [PubMed] [Google Scholar]
- 21.Lederer ED, Sohi SS, Mcleish KR. Dopamine regulates phosphate uptake by opossum kidney cells through multiple counter-regulatory receptors. J Am Soc Nephrol. 1998;9:975–985. doi: 10.1681/ASN.V96975. [DOI] [PubMed] [Google Scholar]
- 22.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na-K-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987;252:F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- 23.Bertorello AM, Hopfield JF, Aperia A, Greengard P. Proximal tubule Na-K-ATPase activity is inhibited during high salt diet: Evidence for DA-mediated effect. Am J Physiol. 1988;254:F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- 24.Bertorello A, Aperia A. Both DA1 and DA2 receptor agonists are necessary to inhibit NaK-ATPase activity in proximal tubules from the rat kidney. Acta Physiol Scand. 1988;132:441–443. doi: 10.1111/j.1748-1716.1988.tb08350.x. [DOI] [PubMed] [Google Scholar]
- 25.Bertorello A, Aperia A. Inhibition of proximal tubule Na-K-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990;259:F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- 26.Bertorello A, Aperia A. Short term regulation of Na-K-ATPase activity by dopamine. Am J Hyperten. 1990;3:51S–54S. doi: 10.1093/ajh/3.6.51s. [DOI] [PubMed] [Google Scholar]
- 27.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 28.Aronson PS. Role of ion exchangers in mediating NaCl transport in the proximal tubule. Kidney Int. 1996;49:1665–1670. doi: 10.1038/ki.1996.243. [DOI] [PubMed] [Google Scholar]
- 29.Biemesderfer D, Pizzonia J, Abu-Alfa A, et al. NHE-3: A Na/H exchanger isoform of renal brush border. Am J Physiol. 1993;265:F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- 30.Amemiya M, Loffing J, Lötscher M, et al. Expression of NHE-3 in the apical membrane of rat proximal convoluted tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 31.Wu MS, Biemesderfer D, Giebisch G, Aronson PS. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem. 1996;271:32749–32752. doi: 10.1074/jbc.271.51.32749. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Yang CL, Abbiati T, et al. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol. 1999;277:F298–F302. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 33.Choi JY, Shah M, Lee MG, et al. Novel amiloride sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 35.Ledoussal C, Lorenz JN, Nieman ML, et al. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol. 2001;281:F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 36.Yun CH, Tse CM, Nath SK, et al. Mammalian Na+/H+ exchanger gene family: Structure and function studies. Am J Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 37.Moe OW. Acute regulation of proximal tubule Na/H exchanger NHE-3: Role of NHE-3 phosphorylation, trafficking and regulatory cofactors. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 38.Moe OW, Tejedor A, Levi M, et al. Dietary NaCl modulates Na(+)H+ antiporter activity in renal cortical apical membrane vesicles. Am J Physiol. 1991;260:F130–F137. doi: 10.1152/ajprenal.1991.260.1.F130. [DOI] [PubMed] [Google Scholar]
- 39.Dawson TP, Gandhi R, Le Hir M, Kaissling B. Ecto-5′-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem. 1989;37:39–47. doi: 10.1177/37.1.2535703. [DOI] [PubMed] [Google Scholar]
- 40.Traebert M, Volkl H, Biber J, et al. Luminal and contraluminal action of 1-34 and 3-34 PTH peptides on renal type Iia Na-P(i) cotransporter. Am J Physiol. 2000;278:F792–F798. doi: 10.1152/ajprenal.2000.278.5.F792. [DOI] [PubMed] [Google Scholar]
- 41.Fan L, Wiederkehr MR, Collazo R, et al. Dual mechanism of acute regulation of Na/H exchanger NHE-3 by parathyroid hormone in rat kidney. J Biol Chem. 1999;274:11289–11295. doi: 10.1074/jbc.274.16.11289. [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Leong PK, Chen JO, et al. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol. 2002;282:F730–F740. doi: 10.1152/ajprenal.00298.2001. [DOI] [PubMed] [Google Scholar]
- 43.Shugrue CA, Obermuller N, Bachmann S, et al. Molecular cloning of NHE3 from LLC-PK1 cells and localization in pig kidney. J Am Soc Nephrol. 1999;10:1649–1657. doi: 10.1681/ASN.V1081649. [DOI] [PubMed] [Google Scholar]
- 44.Schnermann J, Todd KM, Briggs JP. Effect of dopamine on the tubuloglomerular feedback mechanism. Am J Physiol. 1990;258:F790–F798. doi: 10.1152/ajprenal.1990.258.4.F790. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Norian JM, Magyar CE, et al. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol. 1999;276:F711–F719. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Magyar CE, Norian JM, et al. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol. 1998;274:C1090–C100. doi: 10.1152/ajpcell.1998.274.4.C1090. [DOI] [PubMed] [Google Scholar]
- 47.Yip KP, Tse CM, Mcdonough AA, Marsh DJ. Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol. 1998;275:F565–F575. doi: 10.1152/ajprenal.1998.275.4.F565. [DOI] [PubMed] [Google Scholar]
- 48.Magyar CE, Zhang Y, Holstein-Rathlou NH, Mcdonough AA. Proximal tubule Na transporter responses are the same during acute and chronic hypertension. Am J Physiol. 2000;279:F358–F369. doi: 10.1152/ajprenal.2000.279.2.F358. [DOI] [PubMed] [Google Scholar]
- 49.Baum M, Moe OW, Gentry DL, Alpern RJ. Effect of glucocorticoids on renal cortical NHE-3 and NHE-1 mRNA. Am J Physiol. 1994;267:F437–F442. doi: 10.1152/ajprenal.1994.267.3.F437. [DOI] [PubMed] [Google Scholar]
- 50.Loffing J, Lotscher M, Kaissling B, et al. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol. 1998;9:1560–1567. doi: 10.1681/asn.v991560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on the neonatal renal cortical Na+/H+ antiporter. Kidney Int. 1998;53:1254–1258. doi: 10.1046/j.1523-1755.1998.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cano A, Baum M, Moe OW. Thyroid hormone stimulates the renal Na/H exchanger NHE3 by transcriptional activation. Am J Physiol. 1999;276:C102–C108. doi: 10.1152/ajpcell.1999.276.1.C102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ambühl PM, Amemiya M, Danczkay M, et al. Chronic metabolic acidosis increases NHE-3 protein abundance in rat kidney. Am J Physiol (Renal) 1996;271:F917–F925. doi: 10.1152/ajprenal.1996.271.4.F917. [DOI] [PubMed] [Google Scholar]
- 54.Collazo R, Fan L, Zhao H, et al. Acute regulation of Na/H exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;276:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 55.Peng Y, Amemiya M, Yang X, et al. ET(B) receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol. 2001;280:F34–F42. doi: 10.1152/ajprenal.2001.280.1.F34. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Amemiya M, Peng Y, et al. Acid incubation causes exocytotic insertion of NHE3 into the apical membrane of OK cells. Am J Physiol. 2000;279:C410–C419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- 57.Chow CW, Khurana S, Woodside M, et al. The epithelial Na+/H+ exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem. 1999;274:37551–37558. doi: 10.1074/jbc.274.53.37551. [DOI] [PubMed] [Google Scholar]
- 58.D'souza S, Garcia-Cabado A, Yu F, et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 59.Biemesderfer D, Rutherford PA, Nagy T, et al. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 60.Soares-da-Silva P, Fernandes MH. Sodium-dependence and ouabain-sensitivity of the synthesis of dopamine in renal tissues of the rat. Br J Pharmacol. 1992;105:811–816. doi: 10.1111/j.1476-5381.1992.tb09062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soares-da-Silva P, Fernandes MH, Pestana M. A comparative study on the synthesis of dopamine in the human, dog and rat kidney. Acta Physiol Scand. 1993;148:347–351. doi: 10.1111/j.1748-1716.1993.tb09566.x. [DOI] [PubMed] [Google Scholar]
- 62.Pestana M, Soares-da-Silva P. The renal handling of dopamine originating from l-dopa and gamma-glutamyl-l-dopa. Br J Pharmacol. 1994;112:417–422. doi: 10.1111/j.1476-5381.1994.tb13088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jose PA, Raymond JR, Bates MD, et al. The renal dopamine receptors. J Am Soc Nephrol. 1992;2:1265–1278. doi: 10.1681/ASN.V281265. [DOI] [PubMed] [Google Scholar]



