Abstract
Background
New WHO guidelines recommend ART initiation for HIV-positive persons with CD4 cell counts ≤500 cells/µL, a higher threshold than was previously recommended. Country decision makers must consider whether to further expand ART eligibility accordingly.
Methods
We used multiple independent mathematical models in four settings—South Africa, Zambia, India, and Vietnam—to evaluate the potential health impact, costs, and cost-effectiveness of different adult ART eligibility criteria under scenarios of current and expanded treatment coverage, with results projected over 20 years. Analyses considered extending eligibility to include individuals with CD4 ≤500 cells/µL or all HIV-positive adults, compared to the previous recommendation of initiation with CD4 ≤350 cells/µL. We assessed costs from a health system perspective, and calculated the incremental cost per DALY averted ($/DALY) to compare competing strategies. Strategies were considered ‘very cost-effective’ if the $/DALY was less than the country’s per capita gross domestic product (GDP; South Africa: $8040, Zambia: $1425, India: $1489, Vietnam: $1407) and ‘cost-effective’ if $/DALY was less than three times per capita GDP.
Findings
In South Africa, the cost per DALY averted of extending ART eligibility to CD4 ≤500 cells/µL ranged from $237 to $1691/DALY compared to 2010 guidelines; in Zambia, expanded eligibility ranged from improving health outcomes while reducing costs (i.e. dominating current guidelines) to $749/DALY. Results were similar in scenarios with substantially expanded treatment access and for expanding eligibility to all HIV-positive adults. Expanding treatment coverage in the general population was therefore found to be cost-effective. In India, eligibility for all HIV-positive persons ranged from $131 to $241/DALY and in Vietnam eligibility for CD4 ≤500 cells/µL cost $290/DALY. In concentrated epidemics, expanded access among key populations was also cost-effective.
Interpretation
Earlier ART eligibility is estimated to be very cost-effective in low- and middle-income settings, although these questions should be revisited as further information becomes available. Scaling-up ART should be considered among other high-priority health interventions competing for health budgets.
Funding
The Bill and Melinda Gates Foundation and World Health Organization
Introduction
In July 2013, the World Health Organization (WHO) issued consolidated guidelines for the use of antiretroviral drugs (ARVs) for treating and preventing HIV infection.1 These guidelines recommended antiretroviral therapy (ART) for all HIV-positive adults when their CD4 cell count falls to 500 cells/µL or below, with treatment irrespective of CD4 cell count for pregnant women, HIV serodiscordant couples, and persons with active tuberculosis or hepatitis B associated severe chronic liver disease. The decision to increase this threshold from the 350 cells/µL recommended in 2010 was determined through a structured GRADE review process that evaluated evidence for clinical and epidemiological benefits of earlier HIV initiation.2
Evidence for ART reducing HIV infectiousness3,4 suggests that increasing the number of HIV-positive adults who are on ART may have the potential to change the course of the epidemic in highly-affected regions.5,6 However, the resources required to implement these changes could be substantial.1 The recommendation for earlier ART initiation comes at a time when progress towards implementing ART is varied: currently only an estimated 58% of those with CD4 ≤350 cells/µL in low- and middle-income countries (LMIC) are receiving treatment.7 Even in settings that have achieved high coverage, many patients still initiate treatment late due to lack of early HIV diagnosis and poor linkage and retention in pre-ART care.8–10 In this context, decision makers must consider whether resources should be devoted to implementing earlier eligibility, achieving high coverage and timely ART initiation for those with the greatest clinical need, or expanding other health programmes that might generate greater health gains. This decision requires assessment of the population-level impact and costs of prospective strategies to expand ART eligibility or increase access, accounting for the additional resources that would be required. While clinical trials assess the individual-level consequences of expanded eligibility criteria, mathematical models are used to project these long-term policy consequences.11 Over the past decade mathematical models have been useful in understanding the potential epidemiologic consequences, public health benefits, and costs of ART in many populations.5,11–14
To better inform current ART policy, we assembled twelve independently developed HIV epidemic models to generate estimates for the health benefits, costs, and cost-effectiveness of earlier ART eligibility using the latest available evidence. We also assessed the cost-effectiveness of increasing HIV testing and linkage to care to achieve higher levels of ART coverage. The use of several models allows us to identify conclusions that are robustly reproduced across the models, which is critical given the wide range of results demonstrated in previous analyses.6 As optimal strategies might be expected to differ in settings with different epidemic types, current ART coverage, and income levels, we selected four countries with existing models of the impact of ART as case studies in an effort to produce guidance applicable to a broad set of epidemic settings: South Africa (generalized epidemic, medium ART coverage), Zambia (generalized epidemic, high ART coverage), India (concentrated epidemic, medium ART coverage), and Vietnam (concentrated epidemic, low ART coverage).
Methods
Overview
We assessed the potential impact of changes to adult ART eligibility guidelines and improvements in HIV testing and linkage to care in four LMIC settings. Existing, independently developed mathematical models were calibrated to epidemic settings in South Africa (7 models), Zambia (4 models), India (3 models), or Vietnam (1 model). All models were dynamic HIV transmission models that simulate HIV transmission at the population level, HIV disease progression, and incorporate both the therapeutic benefits of ART for reducing HIV morbidity and mortality and the preventive benefits associated with reduced HIV infectiousness (Table 1). Model outputs describing changes in healthcare utilisation were used to estimate changes in costs borne by the HIV program and broader health system. We estimated the impact of intervention strategies on HIV incidence, ART and non-ART health costs, and disability-adjusted life-years (DALYs) averted by comparing model projections of different ART eligibility and access strategies over 20 years. Incremental cost-effectiveness ratios (ICERs) were calculated to compare alternative strategies.
Table 1.
Epidemiological models included in analysis and strategies simulated
| Model name [Key reference(s)] |
Setting | Model Typea | Age- structured |
Heterogeneous sexual risk in general pop.b |
Key populations included in modelc |
Notesd |
|---|---|---|---|---|---|---|
| Goals41 | South Africa; Zambia | Determ. | Yes | Yes | FSW, MSM | includes TB disease |
| STDSIM42 | South Africa | Microsim. | Yes | Yes | FSW | |
| EMOD43 | South Africa; Zambia | Microsim. | Yes | Yes | ||
| BBH37 | South Africa | Determ. | No | No | FSW, MSM | |
| PopARTe | South Africa; Zambia | Determ. | No | Yes | includes TB disease | |
| Synthesis44 | South Africa | Microsim. | Yes | Yes | incl. WHO stage IV | |
| Menzies45 | South Africa | Determ. | No | No | includes TB disease, no CD4 ≤500 | |
| Macha46 | Zambia | Determ. | No | No | ||
| Pruddelle | Bangalore, India | Determ. | No | N/A | FSW, MSM | no CD4 ≤500 |
| Mishra47 | Belgaum, Indiaf | Determ. | No | No | FSW | no CD4 ≤500 |
| IDU-Manipur48 | Churachandpur, India | Determ. | No | N/A | PWID, former-PWID | includes HCV transmission, no CD4 ≤500 |
| Prevtool49 | Vietnam | Determ. | No | No | FSW, MSM, PWID |
Determ. = deterministic compartment model structure; Microsim. = Individual-based microsimulation model.
All models for South Africa and Zambia simulate entire adult population (age 15+ years). Mishra model for Belgaum simulates the general adult population (age 15+ years) of Belgaum municipality. Pruddell simulates only subpopulations consisting of current and former FSW, MSM, and clients of FSW, and IDU-Manipur simulates current and former male PWID and their heterosexual partners. Prevtool model for Vietnam simulates the general adult population aged 15–49 years.
Abbreviations: FSW = female sex workers; MSM = men who have sex with men; PWID = people who inject drugs. Concentrated epidemic models (India, Vietnam) consider expanded access to ART among these populations.
All models simulate ART eligibility for CD4 ≤350 cells/µL, and eligibility for all HIV-positive adults. Most models simulate ART eligibility for CD4 ≤500 cells/µL. Menzies, Pruddell, Mishra, and IDU-Manipur do not consider eligibility for CD4 ≤500 cells/µL. Synthesis model also simulates ART eligibility for patients with a WHO Stage IV diagnosis.
All models except for Pruddell and IDU-Manipur simulate the entire adult (age 15+) general population in the defined setting.
Description of model available online at http://www.hivmodelling.org.
Mishra contributes a second baseline simulation assuming that increases in condom usage and ART access for FSW resulting from the successful Avahan intervention program18 had not occurred and ART access for HIV-positive individuals (including FSW) remained poor, resulting in higher HIV incidence. This is referred to as ‘Mishra – no FSW intervention’.
Modelling the epidemiological impact of expanding ART eligibility and expanding access to care
The models represented three eligibility criteria by which ART could be initiated for those in care: (1) HIV-positive adults with CD4 ≤350 cells/µL (assumed to be the existing, baseline strategy); (2) HIV-positive adults with CD4 ≤500 cells/µL and (3) all HIV-positive adults. Each model simulated a baseline projection representing current patterns of HIV testing, linkage to and retention in pre-ART care, and ART uptake, which we refer to as the ‘status quo’ healthcare access strategy. All three ART eligibility criteria were simulated assuming a continuation of status quo access to HIV care—i.e. patients initiated on ART are those already being linked to HIV care programmes according to current access patterns. Models also simulated each ART eligibility strategy assuming substantial increases in routine HIV testing and linkage to care across the adult population such that 80% of persons infected with HIV would be in care when they became eligible for ART. For concentrated epidemic settings (India, Vietnam), models examined increased HIV testing and linkage-to-care in specific key populations (female sex workers – FSW, men who have sex with men – MSM, and people who inject drugs – PWID) to achieve 80% ART access in this population, while access for the general population remained at status quo levels.
Alternative ART eligibility and healthcare access strategies were simulated for a 20-year period from 2014 through 2033. Changes in ART eligibility were assumed to occur at the beginning of 2014. For strategies involving expanded access to HIV care, the change in access was implemented progressively over two years from the beginning of 2014.
Estimation of costs and cost-effectiveness
Incremental costs of each strategy were assessed from a health system perspective, using a common costing framework across all models. Costs included service delivery costs required to identify and link HIV-positive individuals to care, service delivery costs for patients receiving ART or pre-ART care, potential cost savings due to reduced healthcare utilisation in the wider health system as HIV positive individuals begin receiving care through the HIV programme, and the costs associated with programmatic support and supply-chain management (Table 2). All costs are additional to the basic level of spending required to support the program. Country-specific unit cost accounted for differences in price levels between countries, and all costs are reported in 2012 US dollars. The upfront costs of infrastructure investments are spread over their useful life. The costing framework and sources of cost estimates are described in the Supplementary Information, Section 1.3.
Table 2.
Unit costs and disability weights (all costs in 2012 US dollars).e
| Costs | Disability weights | |||||
|---|---|---|---|---|---|---|
| South Africa |
Zambia | India | Vietnam | Health state | Disability weighta |
|
| On ART, ARV drug cost (ppy) | $143 | $141 | $91 | $105 | HIV+, CD4 >350 (untreated)b | 0.053 |
| On ART, non-ARV cost (ppy) | $422 | $217 | $128 | $198 | HIV+, CD4 200–350 (untreated) | 0.221 |
| ART initiation, from pre-ART care (per initiation) | $95 | $49 | $29 | $45 | HIV+, CD4 ≤200 (untreated) | 0.547 |
| ART initiation, not in pre-ART care (per initiation) | $126 | $65 | $38 | $59 | HIV+, on ART | 0.053 |
| Pre-ART care, CD4 > 350 (ppy) | $205 | $127 | $73 | $145 | TB disease | 0.331 |
| Pre-ART care, CD4 200–350 (ppy) | $238 | $139 | $81 | $150 | ||
| Pre-ART care, CD4 ≤200 (ppy) | $359 | $185 | $109 | $169 | ||
| HIV testing & linkage: general pop. (per client) | $20 | $10 | $6 | $9 | ||
| HIV testing & linkage: FSW, MSM, PWID (per client) | $67 | $34 | $20 | $31 | ||
| Healthcare utiliz., CD4 >350, not in care (ppy) | $13 | $5 | $3 | $2 | ||
| Healthcare utiliz., CD4 200–350, not in care (ppy) | $46 | $17 | $11 | $7 | ||
| Healthcare utiliz., CD4 ≤200, not in care (ppy) | $167 | $63 | $39 | $26 | ||
| End of life cost (per death) | $160 | $50 | $34 | $32 | ||
| TB treatment (per case treated) | $364 | $188 | $110 | $172 | ||
| Supply chain management (percentage mark-up)c | 20% | 20% | 20% | 20% | ||
| Programmatic support (percentage mark-up)d | 50% | 50% | 50% | 50% | ||
ppy = per person-year
Disability weights based on Salomon et al (2012).15 For individuals with co-morbidity (e.g. concurrent HIV and TB disease), disability weights were compounded multiplicatively.
It was assumed that HIV-infection with CD4 ≥350 incurs the same disability, 0.053, as individuals receiving ART.
Mark-up assessed on ARV drug cost.
Mark-up assessed on all costs except for ARV drugs.
Full description of costing framework and sources of cost data described in the Supplementary Information, Section 1.3.
Health benefits were summarized as DALYs averted, which capture improvements in both survival and quality of life resulting from the direct benefits of ART in extending life for HIV-positive persons and through reduced numbers of HIV infections. Disability weights were drawn from the Global Burden of Disease Study 2012, which assessed the value of life years lived with defined health conditions, in comparison to full health, through sample surveys conducted in different world regions.15
Incremental cost effectiveness ratios (ICERs) were calculated as the incremental cost per DALY averted over 20 years by an intervention compared to a less effective, less costly alternative. Costs and health benefits were discounted by 3% per annum.16 Following WHO recommended benchmarks, an intervention was categorised as ‘very cost-effective’ if its ICER was less than the country’s per capita GDP (South Africa: $8040, Zambia: $1425, India: $1489, Vietnam: $1407 in 2012),17 and ‘cost-effective’ if it was less than three times per capita GDP.16
Role of the funding source
WHO authors contributed to the design of the study, the selection of settings considered and strategies evaluated, but had no role in the development or selection of epidemiological models, conduct of the analyses or interpretation of results. The Bill and Melinda Gates Foundation had no role in the design of the analysis, interpretation of the results, or the decision to submit the manuscript for publication. The corresponding author had final responsibility for the decision to submit for publication.
Results
We present the results of the analysis in four parts: (i) overall estimates of the cost-effectiveness of earlier ART eligibility in generalised epidemic settings; (ii) overall estimates of the cost-effectiveness of earlier ART eligibility in concentrated epidemic settings; (iii) the costs and benefits of expanding access to HIV care in generalised epidemic settings; and (iv) the costs and benefits of expanding access to HIV care in concentrated epidemic settings.
(i) The cost-effectiveness of earlier ART eligibility in generalised epidemics
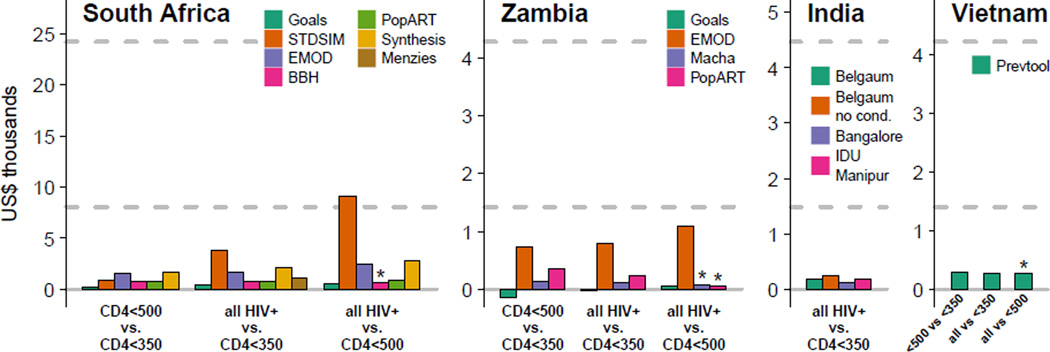
We first examine whether it would be cost-effective to change ART eligibility in adults. In all four settings, expanding ART eligibility to CD4 ≤500 cells/µL or all HIV-positive adults was estimated to be ‘very cost effective’ over 20 years (Figure 1).
Figure 1.
The incremental cost per DALY averted for expanding ART eligibility criteria to include HIV-positive adults with CD4 ≤500 cells/µL or all HIV-positive adults, assuming continuation of status quo patterns of healthcare access. Results calculated over a 20 year time horizon, with all costs and health benefits discounted at 3% per annum. All costs reported in 2012 US dollars. Horizontal dashed lines represent cost-effectiveness benchmarks of one times and three times per capita GDP. Menzies (South Africa) and models for India only simulated eligibility for all HIV-positive adults, not restricted to those with CD4 ≤500 cells/µL. ‘*’ indicates that eligibility for CD4 ≤500 cells/µL is dominated by eligibility for all HIV-positive adults. For the Goals model in Zambia, the estimated ICER is negative because over 20 years the strategy produces health benefits and is estimated to be cost saving over 20 years due to the reduced treatment and care burden, including savings due to averted TB treatment costs.
In South Africa, the cost-effectiveness of changing ART eligibility from CD4 ≤350 cells/µL to CD4 ≤500 cells/µL ranged from $273 to $1,691 per DALY averted over 20 years (results from 6 models). The cost per DALY averted for changing eligibility to all HIV-positive adults compared to eligibility for CD4 ≤350 cells/µL ranged from $438 to $3,790 (7 models). In Zambia, the cost-effectiveness of expanding eligibility to CD4 ≤500 cells/µL from CD4 ≤350 cells/µL ranged from improving health outcomes while reducing costs (i.e. dominating current guidelines) to $749/DALY. For expanding eligibility to all HIV-positive patients compared to CD4 ≤350 cells/µL, results ranged from dominating current guidelines to $790/DALY (4 models). The lower cost-effectiveness ratios in Zambia compared to South Africa are in part due to lower non-ARV costs in Zambia (Table 2). Most models found slightly higher costs per DALY averted for expanding ART eligibility to all HIV-positive adults compared to expansion to CD4 ≤500 cells/µL (5 of 6 for South Africa and 2 of 4 for Zambia). But, these models also found that the expansion of ART eligibility from 500 cells/µL to all HIV-positive adults was still either ‘cost effective’ or ‘very cost-effective’ (Figure 1).
The analysis was repeated assuming greatly expanded HIV testing and linkage. Similar cost-effectiveness ratios were obtained (Tables S7 and S8). ICERs that compare costs and benefits over a shorter time-period were much greater (Tables S7 and S8): for example, in South Africa, over 5 years the highest ICER for changing eligibility to CD4 ≤500 cells/µL from CD4 ≤350 cells/µL is $11,646 compared with the $1,691 when considering a twenty year period (above). This is because the impact of expanded ART in reducing HIV transmission tends to increase over time in the models (Figure S6).
(ii) The cost-effectiveness of earlier ART eligibility in concentrated epidemics
In Vietnam, where the epidemic is driven by FSW, MSM, and PWID, the ICER was $290 and $289 respectively for extending eligibility to CD4 ≤500 cells/µL and all HIV-positive compared to eligibility for CD4 ≤350/µL. In Bangalore, where the epidemic is driven by both FSW and MSM, the ICER associated with eligibility for all HIV-positive adults compared to eligibility for CD4 ≤350 cells/µL was $131/DALY. In Manipur, where HIV is primarily spread by unsafe injections, the ICER for immediate ART eligibility was $197 compared to CD4 ≤350 cells/µL eligibility. Each of these policy changes would be very cost-effective.
In Belgaum district, in western India, where the epidemic is primarily associated with sex work, the ICER for eligibility for all HIV-positive adults compared to CD4 ≤350 cells/µL eligibility criteria was $198/DALY. Belgaum has experienced significant reductions in HIV incidence in recent years, which are thought to be the result of targeted interventions that increased use of condoms and access to HIV care and treatment among sex workers.18 In a simulated scenario in which this intervention program did not occur, the ICER would be $241/DALY. Thus, earlier ART eligibility would be very cost-effective in epidemic settings similar to Belgaum which have had such programmes and those that have not.
(iii) The costs and benefits of expanding access to HIV care in generalised epidemic settings
Changes in eligibility for ART initiation is only one way in which decision-makers could respond to the new guidelines. They could instead invest in expanding HIV testing and linkage to treatment to improve treatment coverage among those in greatest need with CD4 ≤350 cells/µL, or they could simultaneously adopt earlier eligibility criteria and expand testing and linkage to treatment. In this section, we use the model results to compare these alternatives.
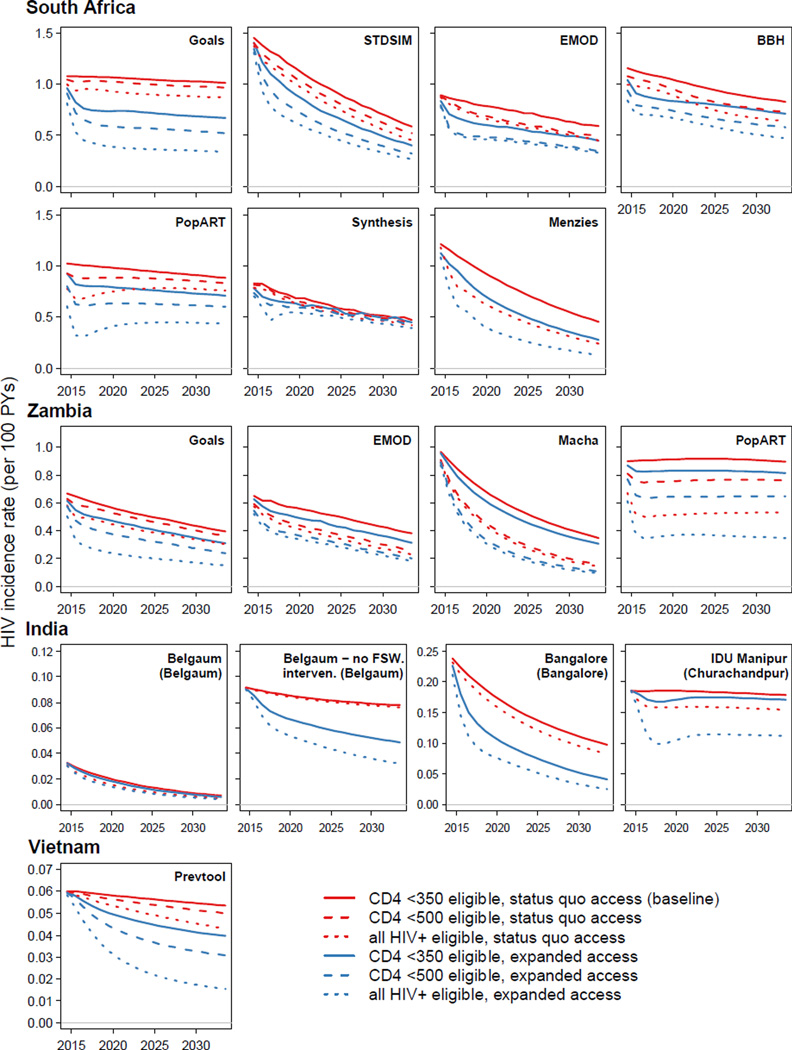
The relative impact of these competing approaches on incidence reduction differs between settings (Figure 2). In South Africa, where current ART coverage is moderate, eligibility expansion would avert only 5–12% (for CD4 ≤500 cells/µL) of new infections over 20 years. In contrast, expanding access to ART whilst keeping the existing CD4 ≤350 cells/µL eligibility criteria would have a larger impact in each model (6–28% of infections averted). Changing eligibility to all HIV-positive adults would avert 9–32% without expansions in access, or 19–60% with both. This relationship is reversed in Zambia, which has higher ART coverage with current guidelines: in each model, expanding eligibility to all HIV-positive adults (21–40% infections averted) averted more infections than expanded access with CD4 ≤350 cells/µL (8–17%).
Figure 2.
The projected annual HIV incidence rate per 100 person-years for ART eligibility (CD4 ≤350 cells/µL: solid; CD4 ≤500 cells/µL: dashed; all HIV-positive: dotted) and health access strategies (status quo: red; expanded access: blue). In the generalized epidemic settings (South Africa, Zambia), ‘expanded access’ refers to expanded access for the general population. In concentrated epidemic settings (India, Vietnam), ‘expanded access’ refers to expanded access for all high-risk groups (FSW, MSM, PWID; see Table 1).
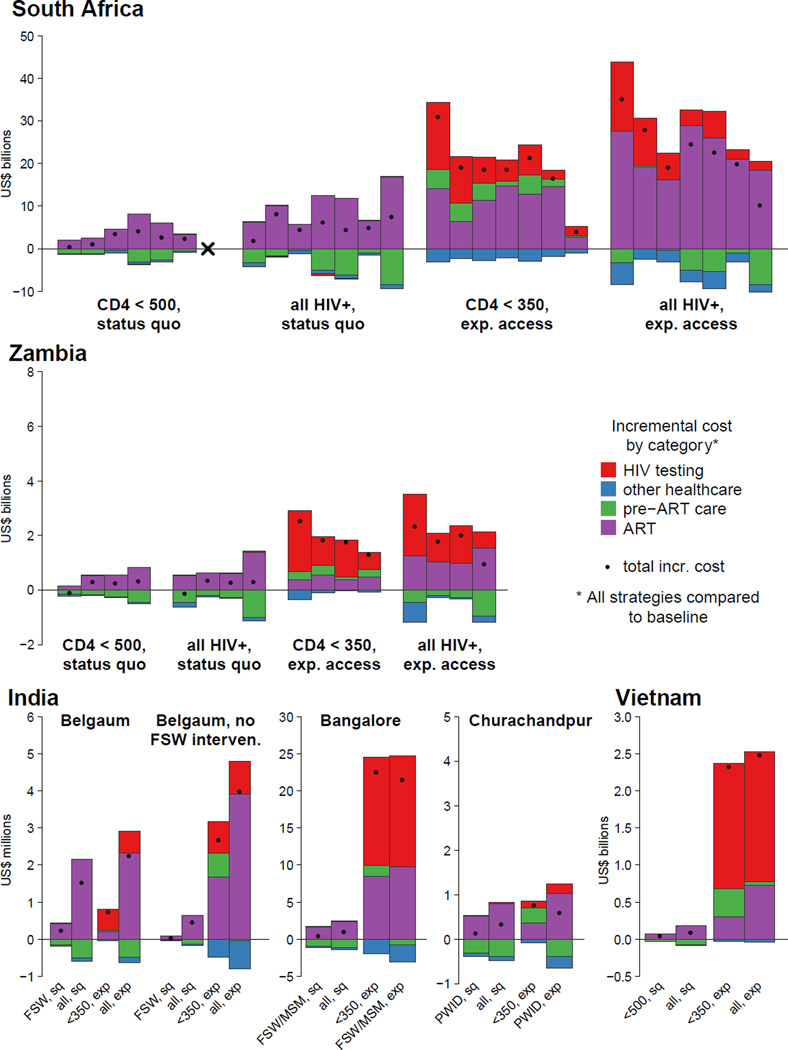
In both countries, the additional costs of strategies that expand access are much higher than the costs of strategies that only change eligibility (Figure 3). Initiating persons already attending clinic earlier has a relatively low incremental cost because the cost of the additional years of ART are partially offset by savings in pre-ART monitoring and other averted healthcare costs. In contrast, the incremental costs associated with strategies that expand treatment access include additional costs for HIV testing and additional costs for pre-ART monitoring and ART costs for those diagnosed through the expanded testing.
Figure 3.
The incremental costs for different ART eligibility and access strategies compared to continuation of 2010 eligibility guidelines and status quo access to care, summed over 20 years. Costs are undiscounted, and reported in 2012 US dollars. Costs underneath the horizontal axis represent cost savings. Total incremental costs are indicated by solid dots. Strategies are indicated by ‘eligibility, access’. In generalized epidemic settings (South Africa, Zambia), ‘expanded access’ refers to expanded access for the general population. In concentrated epidemic settings (India, Vietnam), ‘expanded access’ refers to expanded access for all high-risk groups (FSW, MSM, PWID; see Table 1). For South Africa and Zambia, within each strategy each bar represents a model in the same sequence as the bars in Figure 1. ‘x’ indicates that the CD4 ≤500 cells/µL strategy is not simulated by Menzies. The models for Belgaum and Vietnam also simulated expanded access to the general adult population, which are not illustrated (see Table 3 and Supplementary Information, Figures S5–S7 and Tables S9–S10).
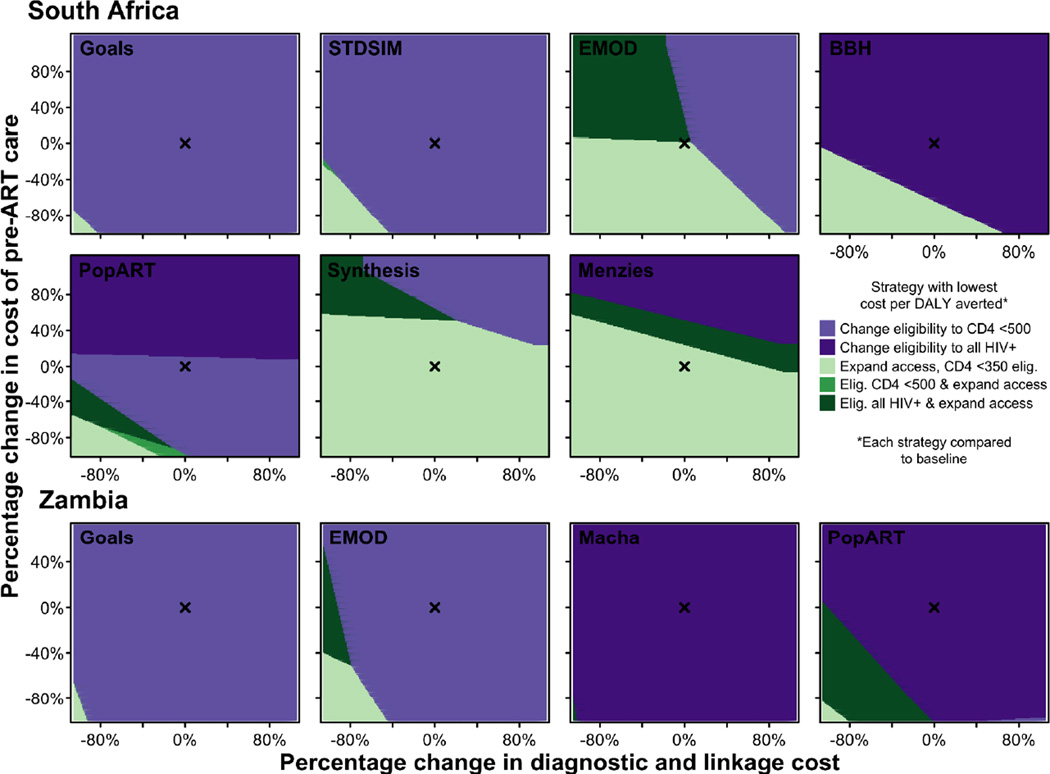
If the objective is to maximise the health returns per dollar spent, as an initial step of program expansion, countries could prioritise the strategy that has the lowest cost per DALY averted (Figure 4). All models for Zambia indicate that expanding eligibility has the lowest cost per DALY averted. This result is robust to alternative assumptions about the relative costs of HIV testing and linkage, pre-ART monitoring, and ART provision. Four of seven for South Africa indicate the same, but three models instead suggest that expanding access would have the lowest cost per DALY averted. Overall, this suggests that in settings with moderate to high coverage, expanding eligibility may be the preferred initial strategy. But expanding access with CD4 ≤350 cells/µL eligibility may be a preferred initial strategy in settings with lower coverage, especially if testing and pre-ART monitoring costs are low compared to the costs of providing ART. Ultimately, both forms of expansion (i.e. eligibility and access) would be considered cost-effective relative to benchmarks—if a country were to proceed by initially expanding in one way, it would still be cost-effective to extend services in the other subsequently.
Figure 4.
Threshold analysis depicting the strategy associated with the lowest cost per DALY averted for given percentage change in the baseline cost assumed for pre-ART care (vertical axis) and for HIV diagnostic testing and linkage to care (horizontal axis). All strategies are compared to the baseline strategy assuming continuation of CD4 ≤350 cells/µL eligibility guidelines and status quo access to care. ‘x’ indicates the baseline cost estimated for pre-ART care and diagnostic and linkage to care (Table 2).
(iv) The costs and benefits of expanding access to HIV care in concentrated epidemic settings
Whereas in generalised epidemics testing and linkage campaigns were implemented in the general population, in concentrated epidemics it may be preferable to focus resources to specific populations. In this section we use the model results to assess this alternative.
In Belgaum, India, providing immediate ART eligibility for FSW, eligibility for all HIV-positive adults, or for all HIV-positive adults with expanded HIV testing and linkage to treatment among FSW would all be very cost-effective. The more extensive of these strategies would lead to greater reductions in new infections, albeit at a greater cost per DALY averted (Table 3). However, undertaking intervention to expand access for all adults in the general population resulted in an ICER of $5648/DALY, which would not be cost-effective, although it could lead to the largest impact on HIV incidence (53% infections averted). Each of these interventions had lower ICERs in the simulated scenario that did not include the effect of the prevention programmes in Belgaum (Table 3).
Table 3.
Health impacts and cost of expanded access to key populations over 20 years, compared to 2010 eligibility guidelines and status quo health access (all costs in 2012 US dollars)
| Percent of infections averteda |
DALYs averted (000s)b |
Additional cost (millions)c |
ICERd | |
|---|---|---|---|---|
| India | ||||
| Belgaum | ||||
| FSW, status quo access | 13% | 3.5 | $0.2 | $85 |
| all HIV+, status quo access | 21% | 9.0 | $1.6 | $268 |
| all HIV+, prioritised FSW | 29% | 11.0 | $2.3 | $395 |
| all HIV+, universal access | 52% | 33.8 | $123.9 | $5,648 |
| Belgaum, no condom | ||||
| FSW, status quo access | 1% | 0.9 | $0.1 | $73 |
| all HIV+, status quo access | 1% | 2.2 | $0.5 | -- |
| all HIV+, prioritised FSW | 41% | 37.6 | $4.0 | $123 |
| all HIV+, universal access | 66% | 108.9 | $138.7 | $2,054 |
| Vietnam | ||||
| Prevtool | ||||
| FSW, status quo access | 2% | 41.5 | $5.9 | $161 |
| MSM, status quo access | 5% | 146.2 | $37.1 | -- |
| PWID, status quo access | 5% | 149.1 | $36.8 | -- |
| CD4 ≤500, status quo access | 4% | 175.6 | $47.5 | -- |
| all HIV+, status quo access | 12% | 367.1 | $96.4 | $305 |
| CD4 ≤500, prioritised FSW, MSM, PWID | 30% | 1497.5 | $2,442.6 | -- |
| all HIV+, prioritised FSW, MSM, PWID | 52% | 2082.5 | $2,485.7 | $1,586 |
| CD4 ≤500, universal access | 37% | 2544.5 | $25,692.5 | -- |
| all HIV+, universal access | 63% | 3278.2 | $25,725.4 | $21,550 |
Percentage of infections averted over 20 years compared to CD4 ≤350 cells/µL eligibility with status quo access (undiscounted).
Cumulative disability-adjusted life-years averted compared to CD4 ≤350 cells/µL eligibility with status quo access (undiscounted).
Cumulative additional cost over 20 years compared to CD4 ≤350 cells/µL eligibility with status quo access (undiscounted).
Incremental cost per DALY averted over 20 years relative to previous undominated strategy, ‘--‘ indicates a dominated strategy (either weak or strong). Costs and health outcomes discounted at 3% per annum.
For Vietnam, results were qualitatively similar to Belgaum (Table 3); whereas expanding eligibility for the whole population and intervening to expand access for FSW, MSM and PWID would be cost-effective, interventions to expand access for the whole population would not (ICER $21,549).
Discussion
In all settings and across all models, extending adult ART eligibility to those already in care with CD4 ≤500 cells/µL or to all HIV-positive adults was found to be very cost-effective over a twenty-year period. These findings reflect the relatively low cost of providing additional years of ART to persons in care and the assumption that expanded access to ART will reduce HIV transmission in the whole population, adding to the well-established clinical benefits of ART to reduce morbidity and improve survival of HIV-positive individuals.
In the generalised epidemic settings we examined, all models suggested that immediate implementation of the new WHO clinical recommendations for patients with CD4 ≤500 cells/µL to initiate treatment would be cost-effective, even in settings where testing and access to care are still being increased to achieve universal access under the 2010 guidelines (persons are eligible if their CD4 cell count is ≤350 cells/µL). However, the models also highlight that in settings where ART coverage is incomplete, changing ART eligibility criteria alone without an increase in healthcare access, though cost-effective, would have a smaller health impact than would be achieved by increasing ART coverage among those with a CD4 cell count ≤350 cells/µL. Our modelling did not consider cases in which resources are severely constrained, resulting in waiting lists of patients with low CD4 cell counts, or situations where earlier ART eligibility would reduce ART access for patients with the greatest therapeutic need. The WHO guidelines recommend that, in this case, treatment be prioritised for patients with CD4 ≤350 cells/µL.1
In concentrated epidemic settings, we estimated that extending ART eligibility to all HIV-positive adults or those with CD4 ≤500 cells/µL already in care would be very cost effective. We also estimated that increases in HIV testing to achieve universal access to immediate ART among members of specific populations including FSW, MSM, and PWID would be very cost-effective in India, and cost-effective in Vietnam. In contrast, widespread interventions to uniformly expand access to treatment services for the general population did not appear to be cost-effective in concentrated epidemic settings. Other testing strategies not considered in our analyses, such as provider initiated testing, might be more efficient at identifying HIV positive adults, and could potentially be cost-effective in these settings.
Our results also highlight that investments in earlier ART eligibility should be considered as long-term population health investments. Although up-front costs are high, the health benefits generated by expanded eligibility increase over time (Figure S6), such that the cost of averting ill health and premature death becomes progressively lower as cost and benefits are considered over longer time periods (Table S7–S10). However, in contrast to the conclusions of earlier analyses5,12,19 we do not find that the highest impact interventions will be cost-saving over a twenty-year period.
This analysis brought together many independent models to examine the same policy question, and their collective findings were in general agreement about the cost-effectiveness of earlier ART eligibility. The variation in some aspects of the model results serves to highlight existing uncertainties and key directions for further data collection. Factors contributing to this variation include different fundamental representations of the underlying epidemiology of HIV transmission and different expectations about future patterns of treatment uptake and effectiveness. Several on-going studies will provide further data about other key assumptions that directly underlie our conclusions—particularly for the therapeutic benefits of earlier ART,20,21 that the individual prevention benefits of ART can be scaled to the population level,22 how earlier ART affects risk behaviours, and that ART reduces transmission risk similarly among MSM23 and PWID. It is useful to compare model predictions with observational data. The epidemiologic consequences of high ART coverage in high-income country settings have seemingly been mixed,24–29 but one observational study in rural South Africa has found the risk of HIV infection to be lower for individuals living in areas with higher ART coverage,30 and studies have not found increases in sexual risk behaviour amongst persons initiating early ART31,32 or the general population.33 As in all scientific endeavours, the conclusions of this analysis should be re-evaluated in the light of new data as they become available.
The paucity of data on the cost of managing and supporting front-line services, the cost of scaling and maintaining testing programmes beyond current levels, and the flow of patients through care services also add uncertainty to our estimates. Growing evidence points to reductions in unit costs as service provision sites expand and mature.34–36 However it is not clear how these relationships translate to scale-up within a national program context, which would likely involve expansion of existing sites as well as creation of new treatment sites and potentially novel care platforms. Therefore, the experience in settings that rapidly adopt earlier treatment and achieve high coverage will provide better information on the epidemiologic and economic consequences that may be experienced by other countries.
We assessed cost-effectiveness following a convention that approximates the social willingness-to-pay to achieve health gains using a country’s per capita GDP. Interventions found to be cost-effective according to this benchmark can be taken to be a reasonable investment, given a country’s current level of income.16 However, this does not mean that the current level or distribution of health spending is optimal, and there could be other interventions (for HIV or other health concern) that would produce greater health gains per dollar spent. For example, recent analyses of medical male circumcision suggest that expanding circumcision access may have a lower ICER than expanding ART access,37 and may even be cost-saving over the long term.38 National policy-making will require explicit comparison of alternative spending portfolios, which may include other interventions as well as a broader array of ART and HIV testing strategies. Similarly, countries will need to weigh affordability and feasibility when considering large expansions in ART access or eligibility. Implementing these strategies may require large one-time investments in the years immediately following policy change. Given these costs and the uncertainties involved, some countries—especially those with low current coverage—may decide to take a gradual approach to ART eligibility policy change.
For this analysis we adopted an analytic approach that considers total health attainment (total DALYs averted) and is indifferent to how these health benefits are distributed. For this reason, our results do not reflect other considerations for decisions-makers, such as equity of treatment access. The conclusions of this analysis may differ from a narrower analysis focused only on the health benefits for those receiving ART, especially while on-going studies seek to better determine whether there is a direct health benefit from ART initiation at high CD4 cell count, compared with initiation at CD4≤350 cells/µL.39 For the economic analysis we adopt a health systems perspective, which excludes some economic outcomes that may be valued by decision-makers, such as reduced orphanhood, improved productivity, and survival of working age adults. For all these reasons, the general guidance from the four country case-studies undertaken in this analysis should be considered as inputs into a decision-making process that weighs all locally-relevant considerations, rather than prescribing a particular policy outcome.
Revised WHO recommendations have required decision makers to reconsider policies around ART eligibility and access, even while trials and demonstration projects are being undertaken to quantify the consequences of expanded HIV treatment.20–22 As a result, uncertainties persist about key outcomes of these policies.40 However, informed by currently available epidemiological, biological, and economic information, the consensus finding of this study is that extending ART eligibility to all those with CD4 ≤500 cells/µL, and potentially all HIV-positive adults, would be cost-effective and should be considered among other high priority health interventions competing for health budgets in low- and middle-income settings.
Supplementary Material
Research in Context.
Systematic Review
Recently the World Health Organization (WHO) issued revised guidance for antiretroviral therapy (ART) use, which include the recommendation that HIV-positive adults initiate antiretroviral therapy when their CD4 cell count falls below 500 cells/µL.1 Countries must now decide whether to adopt and implement these recommendations. Reductions in HIV infectiousness for persons initiating ART earlier3 means that both the individual therapeutic benefits and prevention benefits must be considered when evaluating the public health benefits of earlier ART eligibility.
Many mathematical models have been developed to examine the population level health impact and costs of different ART strategies in low- and middle-income country settings, and previous work has shown that there can be wide variation in results from different analyses.6 This suggests that considering results across different models and epidemic settings is essential for determining the impact and cost-effectiveness of earlier ART eligibility to inform policy decisions.
Interpretation
Expanding ART eligibility to persons with CD4 cell count less than 500 cells/µL or to all HIV-positive adults was estimated to be cost-effective over 20 years in low- and middle-income country (LMIC) settings, relative to conventional WHO cost-effectiveness benchmarks. Adoption of these recommendations should be considered among other high-priority health interventions in LMIC settings.
In generalised HIV epidemic settings, broad expansions of HIV testing and linkage to care to achieve high levels of programme access was found to be cost-effective and should be considered by policy-makers. In concentrated epidemic settings, increased HIV testing and linkage to care amongst key populations at risk of transmitting HIV was highly cost-effective and should be considered where this is possible. Widespread HIV testing programmes aimed at the entire adult population did not appear cost-effective in concentrated epidemic settings, suggesting that health resources may be better allocated elsewhere.
Acknowledgements
We thank Ellen McRobie and Annick Borquez from Imperial College London for coordinating the HIV Modelling Consortium ART Eligibility Guidelines modelling project. We thank Mary Mahy from UNAIDS for providing additional information about UNAIDS country-level epidemiological estimates. We thank Maaya Sundaram from the Clinton Health Access Initiative, Emmanuela Gakidou and Herbert Duber from the Institute for Health Metrics and Evaluation at the University of Washington for providing information about ART programmes in South Africa and Zambia. We thank Elliot Raises from the U.S. Centers for Disease Control and Joel Kuritsky from USAID for input on ARV supply chain management costs. We thank the Clinton Health Access Initiative and the Division of Global HIV/AIDS of the U.S. Centers for Disease Control for access to unpublished cost estimates.
VC and AP acknowledge the UCL Research Computing Services (Legion Cluster) and input to the Synthesis model from Deborah Ford, Alec Miners, Paul Revill, Fumiyo Nakagawa, and Deenan Pillay. DJK, AB, STC, and BGW thank Bill and Melinda Gates for their active support of this work and their sponsorship through the Global Good Fund. MCB, SM, EM, and MP thank all other members of the Strategic Epi-ART in India Modeling team for their contribution to data and model inputs: Suresh Shastri (Government of Karnataka), Reynold Washington, BM Ramesh (Karnataka Health Promotion Trust); Marissa Becker, Shiva Halli, James F Blanchard, Stephen Moses (University of Manitoba); Michel Alary (Laval University). MCB, SM, EM, and MP acknowledge the Canadian Foundation for AIDS (CANFAR) (Research grant number 023-015) for funding the Belgaum modelling. KMM and HJP acknowledge funding from the Wellcome Trust [086431/Z/08/Z]. TC received funding from the National Institute of General Medical Sciences (#U54GM088558); the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. DPW, CCK, QDP, and LZ acknowledge funding from the World Bank, Australian Research Council, University of New South Wales, and AusAID. QDP acknowledges scholarship support from AusAID. RGW acknowledges research funding from the Medical Research Council (UK) (Methodology Research Fellowship: G0802414 and grant MR/J005088/1) and the Bill and Melinda Gates Foundation (Consortium to Respond Effectively to the AIDS/TB Epidemic: 19790.01, and the TB Modelling and Analysis Consortium: 21675). BEN and DAMCvdV acknowledge research funding from the Aids Fonds (grant 2010-035) -Amsterdam, the Netherlands and the European Union FP7 CHAIN grant (223131). NKM acknowledges funding from a UK National Institute for Health Research Postdoctoral Fellowship
Conflicts of interest
AP has received research funds from Bristol-Myers Squibb and WHO and has received payment for consulting work from Gilead Sciences and GSK Biologicals.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- ARV
antiretroviral
- BMGF
Bill and Melinda Gates Foundation
- CD4
CD4 cell count per micro-litre (µL)
- FSW
female sex worker
- DALY
disability-adjusted life year
- HIV
human immunodeficiency virus
- ICER
incremental cost-effectiveness ratio
- LMIC
low- and middle-income countries
- MSM
men who has sex with men
- PWID
people who injects drugs
- TB
tuberculosis
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- WHO
World Health Organization
- $/DALY
cost (US$) per disability-adjusted life year averted
Footnotes
Author contributions
TBH conceived of the study and was responsible for the overall design of the study. TBH, JWE, MD, NSh, PE, GH, and others contributed to the design of the study. JWE coordinated and analysed the results of epidemiological model simulations. NAM led the design of the economic analysis and analysed cost data. JS, VC, LC, AC, JACH, SH, CCK, DJK, SM, KMM, BEN, and PV led the analysis of the epidemiologic models. RB, TB, AB, DEB, MCB, STC, TC, PJD, CF, CG, JL, NKM, NAM, EM, QDP, MP, AP, LP, CP, HJP, JAS, DAMCvdV, SJdV, BGW, RGW, DPW, and LZ contributed to the development and analysis of epidemiologic models. JB, GMR, BEN, MR, PR, JAS, NSa, FTP, and AV contributed to the development of the economic model and the collation of cost data. All authors approved the final version of the manuscript for submission.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013:269. [PubMed]
- 2.World Health Organization. Consolidated ARV guidelines 2013: Web annexes. [accessed 4 Aug 2013];2013 http://www.who.int/hiv/pub/guidelines/arv2013/annexes/en/
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane database Syst Rev. 2013;4 doi: 10.1002/14651858.CD009153.pub3. CD009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Eaton JW, Johnson LF, Salomon JA, et al. HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global update on HIV treatment 2013: results, impact and opporunities. 2013:124.
- 8.Rosen S, Fox MP. Retention in HIV Care between Testing and Treatment in Sub-Saharan Africa: A Systematic Review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, et al. Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PLoS One. 2012;7:e37125. doi: 10.1371/journal.pone.0037125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartländer B, Stover J, Hallett T, et al. Towards an improved investment approach for an effective response to HIV/AIDS. Lancet. 2011;377:2031–2041. doi: 10.1016/S0140-6736(11)60702-2. [DOI] [PubMed] [Google Scholar]
- 12.Granich R, Kahn JG, Bennett R, et al. Expanding ART for Treatment and Prevention of HIV in South Africa: Estimated Cost and Cost-Effectiveness 2011–2050. PLoS One. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht R, Stover J, Bollinger L, Muhib F, Case K, di Ferranti D. Financing of HIV / AIDS programme scale-up in low-income and middle-income countries, 2009–31. Lancet. 2010;376:1254–1260. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- 14.Walensky RP, Wood R, Fofana MO, et al. The clinical impact and cost-effectiveness of routine, voluntary HIV screening in South Africa. J Acquir Immune Defic Syndr. 2011;56:26–35. doi: 10.1097/QAI.0b013e3181fb8f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edejer TT-T, Baltussen R, Adam T, et al. Geneva: World Health Organization; 2003. Making Choices In Health: Who Guide To Cost Effectiveness Analysis. [Google Scholar]
- 17.The World Bank. World Development Indicators. [accessed 13 Feb2013];2012 http://data.worldbank.org/indicator/NY.GDP.MKTP.CD. [Google Scholar]
- 18.Boily M, Pickles M, Lowndes CM, et al. Positive impact of a large-scale HIV prevention program among female sex workers and clients in Karnataka state, India. AIDS. 2013;27 doi: 10.1097/QAD.0b013e32835fba81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hontelez JAC, de Vlas SJ, Tanser F, et al. The Impact of the New WHO Antiretroviral Treatment Guidelines on HIV Epidemic Dynamics and Cost in South Africa. PLoS One. 2011;6:e21919. doi: 10.1371/journal.pone.0021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US National Institutes of Health. Strategic Timing of Antiretroviral Treatment (START) [accessed 17 Feb2013]; http://clinicaltrials.gov/ct2/show/NCT00867048.
- 21.French National Agency for Research on AIDS and Viral Hepatitis. [accessed 8 Oct2013];Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-infected Adults (ANRS 12136 TEMPRANO) http://clinicaltrials.gov/ct2/show/NCT00495651. [Google Scholar]
- 22.Boily M-C, Mâsse B, Alsallaq R, et al. HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention. PLoS Med. 2012;9:e1001250. doi: 10.1371/journal.pmed.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodger A, Bruun T, Weait M, et al. Partners of people on ART - a New Evaluation of the Risks (The PARTNER study): design and methods. BMC Public Health. 2012;12:296. doi: 10.1186/1471-2458-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson DP. HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention. PLoS Med. 2012;9:e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Sighem A, Jansen I, Bezemer D, et al. Increasing sexual risk behaviour amongst Dutch MSM: mathematical models versus prospective cohort data. AIDS. 2012 doi: 10.1097/QAD.0b013e3283574df9. [DOI] [PubMed] [Google Scholar]
- 28.Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. Lancet Infect Dis. 2013;13:313–318. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS One. 2013;8:e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science (80-) 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25:1939–1949. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean K, Gabillard D, Moh R, et al. Effect of Early Antiretroviral Therapy on Sexual Behaviors and HIV-1 Transmission Risk Among Adults With Diverse Heterosexual Partnership Statuses in Cote d’Ivoire. J Infect Dis. 2013:1–10. doi: 10.1093/infdis/jit470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath N, Eaton JW, Bärnighausen TW, Tanser F, Newell M-L. Sexual behaviour in a rural high HIV prevalence South African community: time trends in the antiretroviral treatment era. AIDS. 2013;27:2461–2470. doi: 10.1097/01.aids.0000432473.69250.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzies NA, Berruti AA, Blandford JM. The determinants of HIV treatment costs in resource limited settings. PLoS One. 2012;7:e48726. doi: 10.1371/journal.pone.0048726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menzies NA, Berruti AA, Berzon R, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS. 2011;25:1753–1760. doi: 10.1097/QAD.0b013e3283463eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marseille E, Giganti MJ, Mwango A, et al. Taking ART to scale: determinants of the cost and cost-effectiveness of antiretroviral therapy in 45 clinical sites in Zambia. PLoS One. 2012;7:e51993. doi: 10.1371/journal.pone.0051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bärnighausen T, Bloom DE, Humair S. Economics of antiretroviral treatment vs. circumcision for HIV prevention. Proc Natl Acad Sci U S A. 2012;109:21271–21276. doi: 10.1073/pnas.1209017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Njeuhmeli E, Forsythe S, Reed J, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS Med. 2011;8:e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabin CA, Cooper DA, Collins S, Schechter M. Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS. 2013;27:1839–1846. doi: 10.1097/qad.0b013e328360d546. [DOI] [PubMed] [Google Scholar]
- 40.De Cock KM, El-Sadr WM. When to Start ART in Africa - An Urgent Research Priority. N Engl J Med 2013. doi: 10.1056/NEJMp1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Futures Institute. Goals Manual: A Model for Estimating the Effects of Interventions and Resource Allocation on HIV Infections and Deaths. 2011:40. [Google Scholar]
- 42.Hontelez JAC, Lurie MN, Bärnighausen T, Bakker R, Baltussen R. Elimination of HIV in South Africa through Expanded Access to Antiretroviral Therapy: A Model Comparison Study. PLoS Med. 2013;10:e1001534. doi: 10.1371/journal.pmed.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bershteyn A, Klein DJ, Eckhoff PA. Age-dependent partnering and the HIV transmission chain: a microsimulation analysis. J R Soc Interface. 2013;10:20130613. doi: 10.1098/rsif.2013.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cambiano V, Bertagnolio S, Jordan MR, Lundgren JD, Phillips A. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J Infect Dis. 2013;207(Suppl):S57–S62. doi: 10.1093/infdis/jit111. [DOI] [PubMed] [Google Scholar]
- 45.Menzies NA, Cohen T, Lin H-H, Murray M, Salomon JA. Population Health Impact and Cost-Effectiveness of Tuberculosis Diagnosis with Xpert MTB/RIF: A Dynamic Simulation and Economic Evaluation. PLoS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols BE, Boucher Ca B, van de Vijver Da MC. HIV testing and antiretroviral treatment strategies for prevention of HIV infection: impact on antiretroviral drug resistance. J Intern Med. 2011 doi: 10.1111/j.1365-2796.2011.02456.x. [DOI] [PubMed] [Google Scholar]
- 47.Mishra S, Mountain E, Pickles M, et al. Exploring the impact of antiretroviral treatment in south India: the influence of baseline intervention context. AIDS. 2013 doi: 10.1097/QAD.0000000000000109. accepted. [DOI] [PubMed] [Google Scholar]
- 48.Vickerman P, Martin NK, Hickman M. Understanding the trends in HIV and hepatitis C prevalence amongst injecting drug users in different settings--implications for intervention impact. Drug Alcohol Depend. 2012;123:122–131. doi: 10.1016/j.drugalcdep.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Pham QD, Do MH, Kerr C, Wilson DP. Return on investment of HIV prevention in Vietnam: Technical Report for the World Bank and Vietnam Adminstration for AIDS Control. 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.