Abstract
The Scribble polarity complex or module is one of the three polarity modules that regulate cell polarity in multiple epithelia including blood-tissue barriers. This protein complex is composed of Scribble, Lethal giant larvae (Lgl) and Discs large (Dlg), which are well conserved across species from fruitflies and worms to mammals. Originally identified in Drosophila and C. elegans where the Scribble complex was found to work with the Par-based and Crumbs-based polarity modules to regulate apicobasal polarity and asymmetry in cells and tissues during embryogenesis, their mammalian homologs have all been identified in recent years. Components of the Scribble complex are known to regulate multiple cellular functions besides cell polarity, which include cell proliferation, assembly and maintenance of adherens junction (AJ) and tight junction (TJ), and they are also tumor suppressors. Herein, we provide an update on the Scribble polarity complex and how this protein complex modulates cell adhesion with some emphasis on its role in Sertoli cell blood-testis barrier (BTB) function. It should be noted that this is a rapidly developing field, in particular the role of this protein module in blood-tissue barriers, and this short chapter attempts to provide the information necessary for investigators studying reproductive biology and blood-tissue barriers to design future studies. We also include results of recent studies from flies and worms since this information will be helpful in planning experiments for future functional studies in the testis to understand how Scribble-based proteins regulate BTB dynamics and spermatogenesis.
Introduction
In invertebrates and vertebrates, epithelial tissues are arranged as sheets of cells that segregate and protect an organism from changes in its environment. In short, epithelial cells are arranged side-by-side with the apical region of their plasma membranes facing the environment, while the basal area is anchored to the basal lamina (i.e., extracellular matrix, also known as basement membrane in the testis1,2), and the lateral area in contact with each other. This feature is achieved via the development of “polarity” during embryogenesis. Thus, epithelial/endothelial cells of an epithelium/endothelium can be divided into three discrete domains: the apical, the lateral, and the basal domains. Furthermore, at the cell-cell and the cell-extracellular matrix interface, there exist a series of junction complexes. It is noted that an important function of the epithelial barrier is to limit paracellular diffusion from the outside (e.g., environment, blood) to the inside, and this is conferred by tight junctions (TJs) in vertebrates or septate junctions (SJs) in invertebrates. Adhesion between neighboring epithelial cells is conferred by adherens junctions (AJs) lying immediately behind TJs.3,4 Therefore, AJs maintain the epithelial sheet whereas TJs act as a “gate” that seals the intercellular space and permits selective exchange of substances (e.g., ions, sugars, amino acids, electrolytes) across an epithelium. The selective permeability of epithelial cells also relies on the polarized distribution of specialized channel proteins on their cell membranes.
Establishment of epithelial cell polarity, including the blood-testis barrier (BTB) in the seminiferous epithelium of mammalian testes, is critical for maintaining cell morphology and function. This requires targeting of membrane and peripheral proteins precisely to either apical or basolateral membrane domains.5 The asymmetric distribution of proteins in different parts of membranes is an important feature of epithelial cell polarity. Although the signaling network that controls cell polarity has not been elucidated, a number of membrane proteins that regulate cell polarity have been identified based on genetic and biochemical studies in Caenorhabditis elegans, Drosophila melanogaster, and also in mammals during these past several decades. These polarity proteins are organized into several functional complexes that distribute at different membrane domains, some of which are mutually exclusive, facilitating the formation of the apicobasal axis and the assembly and maintenance of epithelial cell junctions.6 The best studied polarity complex thus far is the partitioning-defective (Par) protein complex, which is located at the apical domain and is composed of Par6, Par3 (also known as Drosophila Bazooka) and atypical protein kinase C (aPKC).7,8 This tripartite complex also associates with the small GTPase, Cdc42.9,10 In mammals, Par6 serves as an adaptor, recruiting other proteins to this complex to facilitate TJ assembly (Fig. 1).11,12 The other apical polarity module is composed of the transmembrane protein Crumbs (Crb), the cytoplasmic protein Pals1 [protein-associated with Lin-7, also known as Drosophila Stardust, an adaptor and a member of the membrane-associated guanylate kinase (MAGUK) protein family] and Patj (Pals1 associated tight junction protein) (Figs. 1, 2).13 Recent studies have shown that Crb complex proteins are tumor suppressors besides serving as polarity proteins.6,14 In Drosophila, there is also a basolateral polarity protein complex known as the Yrt/ Coracle group, which is composed of FERM proteins Yurt (Yrt), Coracle (Cora); and the membrane proteins Neurexin IV (Nrx-IV) and Na+, K+-ATPase.15 The Yrt/Cora complex promotes basolateral membrane stability and it also displays negative regulatory interactions with the apical Crb protein module.15
Figure 1.
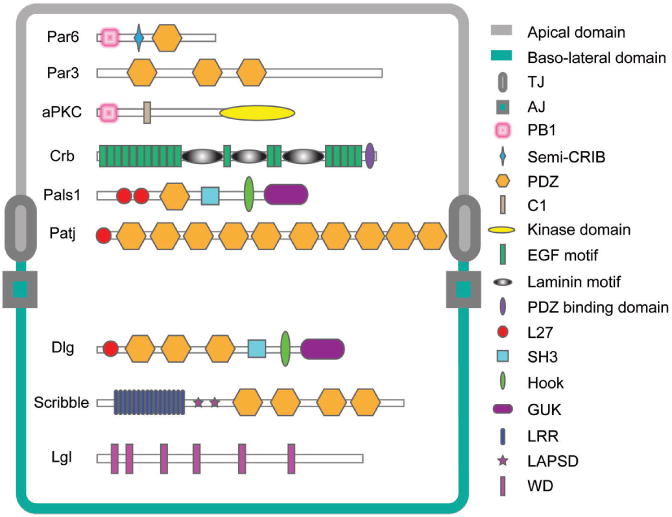
Schematic drawing that illustrates the different functional domains of Scribble complex components in the baso-lateral region versus other apical polarity proteins in the apical region of mammalian epithelial cells. This drawing is also applicable to the testis except that the BTB in the testis lies adjacent to the basement membrane (a modified form of extracellular matrix also known as basal lamina in other epithelia) in the seminiferous epithelium where components of the Scribble protein complex are localized (see Figs. 4 and 5), whereas apical polarity proteins (e.g., Par6) are found both at the Sertoli cell-spermatid interface in the apical region of the seminiferous epithelium and at the BTB.72 Abbreviations used: TJ, tight junction; AJ, adherens junction; PB1, Phox and Bem1p domain; semi-CRIB, semi-Cdc42/Rac interactive binding motif; PDZ, PSD-95/Discs-large/ZO-1 conserved domain; C1, protein kinase C conserved region 1; EGF motif, epidermal growth factor motif; L27, Lin2 and Lin7 binding domain; SH3, Src homology 3 domain; GUK, guanylate kinase homologs conserved domain; LRR, leucine-rich repeats; LAPSD, LAP specific domain; WD, WD-40 repeats.
Figure 2.
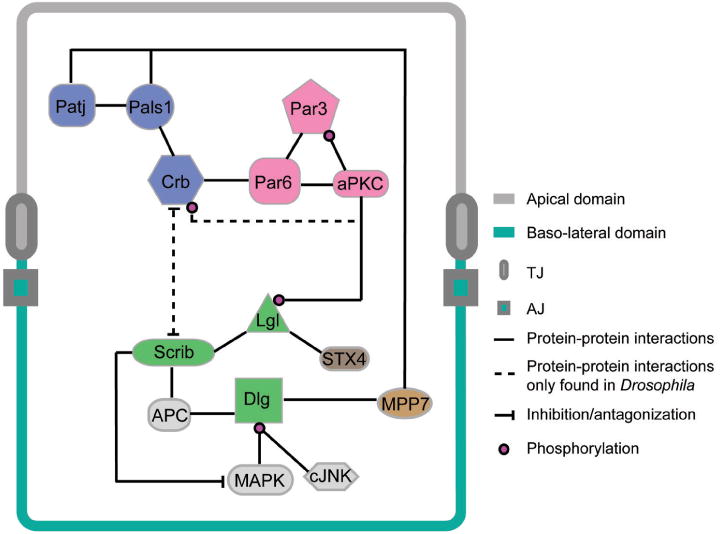
Interactions of Scribble complex components with other polarity or signaling proteins in polarized epithelial cells. The information depicted here is applicable to the seminiferous epithelium of mammalian testes where many components of these proteins have recently been identified. Abbreviations used: Patj, Pals1 associated tight junction protein; Pals1, protein-associated with Lin-7; Crb, Crumbs; Par, partitioning-defective proteins; aPKC, a typical protein kinase C; Scrib, Scribble; Dlg, Discs-large; Lgl, lethal giant larvae; APC, adenomatous polyposis coli; MAPK, mitogen-activated protein kinase; cJNK, c-Jun N-terminal kinase; STX4, Syntaxin 4; MPP7, membrane-palmitoylated protein 7.
In the basolateral domain, there is a protein complex composed of three tumor suppressors: Scribble (Scrib), Lethal giant larvae (Lgl) and Discs large (Dlg), which function cooperatively to regulate cell polarity, junction formation and cell growth in epithelial cells (Figs. 1, 2). This is mediated via mutually exclusive interactions between the Scribble-and the Par-based protein complexes (Fig. 2). Although there is no evidence of physical interaction between the three protein components in the Scribble complex except in Drosophila neuronal synapses, where Scribble associates with Dlg via a protein named GUK holder (Fig. 1),16 the three genes show strong genetic interaction (e.g., the knockout of either Scrib, Lgl or Dlg leads to similar phenotypic changes in mutants versus the wild-type) and these proteins appear to utilize a common pathway to regulate cell architecture and cell proliferation.17 Mutations of these three genes also display similar phenotypes in different epithelia, suggesting that they are components of a fundamental machinery that creates the distinctive architecture of epithelial cells and tissues. In this chapter, we first summarize biochemical features of Scribble complex components. We next focus on their cooperative but distinct roles in regulating epithelial cell junctions and polarity in blood-tissue barriers. We also discuss recent findings on this complex in regulating epithelial barrier functions in different tissues in particularly the BTB in the testis.
Identification and Molecular Structure Of Scribble Complex Components
Members of Scribble complex contain multiple protein–protein interaction domains, in particular PDZ, SH3, and guanylate kinase domains, indicating they can recruit a complex network of proteins to regulate polarity and other cellular functions (Fig. 1). Herein, we focus on the molecular structural features of Scribble, Lgl and Dlg and their functions.
Dlg
The Drosophila discs large tumor suppressor gene, dlg (lethal discs-large), was first discovered in 1972.18 Mutations of this gene were found to display a phenotype similar to that of the lgl mutation.18,19 dlg mutants were found to have imaginal discs fused with each other and to the larval ventral ganglion as they overgrew versus the wild type.20 dlg was shown to be crucial for normal epithelial structure and growth in the embryo,21 indicating the function of this gene is not limited to imaginal discs. Dlg was identified as a member of the membrane-associated guanylate kinase homolog (MAGUK) family, which often locates at cell junctions and contains distinct peptide domains namely PDZ1-3, SH3, HOOK, and GUK (Fig. 1).22,23 Studies using different Dlg derivatives have shown that the subcellular targeting of Dlg to the plasma membrane requires PDZ2 and HOOK domains, leading to the precise localization of Dlg at SJs.21
To date, five mammalian homologs of Drosophila Dlg have been identified (Table 1), with Dlg1 being the best studied Dlg member. The human homolog of Drosophila discs large called hdlg1 was found to contain a C-terminal GUK domain, an SH3 domain, a HOOK domain and three PDZ domains.24 In addition, Dlg1 contains a L-27 domain at its N terminus which can bind to membrane-palmitoylated proteins (MPPs), Lin2/CASK, and Lin7.25-28 In vitro study has shown that PDZ1-2 or HOOK domains of hDlg can function independently to localize exogenous hDlg to the basolateral membrane of cells.29
Table 1. Components of the Scribble complex in different species.
| Drosophila | C. elegans | Mammals | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mr (kDa) | Mr (kDa) | Mr (kDa) | Gene Symbol | |||
| Scribble | 190 | LET-413 | 75 | Scrb1/Crib1/Vartul/hScrib | 250*/175 | SCRB1 |
| Lgl | 127 | – | Lgl1 | 115 | LLGL1 | |
| Lgl2 | 113 | LLGL2 | ||||
| Lgl3/syntaxin-binding protein5 | 127 | LLGL3 | ||||
| Lgl4/syntaxin-binding protein5-like | 130 | LLGL4 | ||||
| Dlg | 107 | Dlg1 | 107 | SAP97/hDlg1 | 130*/100 | DLG1 |
| PSD93/Chapsyn110 | 65 | DLG2 | ||||
| SAP102/NE-Dlg | 90 | DLG3 | ||||
| SAP90/PSD95 | 85 | DLG4 | ||||
| LP-Dlg | 202 | DLG5 | ||||
This apparent Mr was obtained by immunoblot analysis based on SDS-PAGE and the corresponding specific antibodies performed in our laboratory using rat testes as lysates.
The three homology repeats (PDZ domains) in Dlg were first reported in PSD-95 protein from rat forebrain,30 which was also found in the mammalian TJ protein ZO-1.31 Thus, the name PDZ domain (PSD-95, Discs-large, ZO-1) was used to reflect the origin and distribution of this domain.32 In Drosophila, Dlg colocalizes with Scribble via a protein named GUK holder, which can interact with both GUK domain of Dlg and the PDZ2 domain of Scribble (Fig. 1).16
Scribble
Scribble was initially identified in Drosophila where it was found to be a crucial regulator of morphogenesis.33 There is only one Drosophila homolog of Scribble in mammals known as Scrib or Vartul (Table 1).34 Scribble is a LAP [LRR (leucine-rich repeats) and PDZ (PSD-95/Discs-large/ZO-1) domain] protein containing 16 LRRs and 4 PDZ domains (Fig. 1). The first LAP protein, Densin 180, which contains a set of LRRs as well as a PDZ domain, was first isolated in rat brain.35 “LAP” is a collective name33 referring to proteins that contain the LRR and PDZ domains found in fruitflies, worms, rodents and humans.33,36,37 The LAP family has three subfamilies according to the number of PDZ domains, namely LAP0, LAP1 and LAP4.38 The LRRs and PDZ domains of LAP proteins are implicated in mediating protein-protein interactions. Due to their unique polarized localization within the cell membrane, LAP proteins play a key role in regulating subcellular protein distribution.33
LAP family members are strikingly conserved from Drosophila to humans, with respect to their primary amino acid sequence, subcellular localization (basolateral in epithelial cells or postsynaptic in neurons) and protein architecture, suggesting common biological functions.33 A structure-function correlation study on Caenorhabditis elegans LET-413, human Erbin and human Scribble (hScrib) revealed that the LRR domain and the LAPSD (LAP specific domain, a 38-amino acid LRR-like domain located between LRR and PDZ domain) are crucial for anchoring members of this protein family to the plasma membrane.39 In short, LRR domains are necessary and tra to attach Scribble to the plasma membrane, but it is necessary to have the LAPSD domain to organize a polarized epithelium.40
PDZ domains are composed of 80-90 amino acids, folded into two α helixes and six β strands that interact with C-terminal sequences of other proteins.41 Because PDZ domains are frequently found in proteins that regulate cell polarity, it is expected that these motifs are involved in epithelial polarization. However, domain analysis in Scribble has shown that PDZ domains are not indispensable for forming an epithelium, yet they enhance the ability of the LRR to localize proteins to SJs, and to regulate cell proliferation.40
Lgl
The Drosophila tumor suppressor gene, lethal (2) giant larvae (l(2)gl), was first discovered in 1930,42,43 and lgl was cloned and sequenced in the 1980s.44-46 Mutation of lgl in Drosophila caused the imaginal discs to overgrow, lose their epithelial structure and the ability to differentiate, forming solid tumors that continued to grow until the death of larvae.18 lgl encodes a 130-kDa protein that contains stretches of sequences that are similar to cell adhesion proteins.45 Homologs of Lgl have been identified in a variety of organisms, all of which share conserved primary sequences.47 It has been demonstrated that mammalian Lgl proteins (Lgl 1-4, Table 1) contain at least 4-5 putative WD-40 repeat motifs48 (Fig. 1). WD-repeats, each of which comprises a four-stranded anti-parallel β sheet,49,50 are known to contain minimally conserved domains of ∼40-60 amino acids that begin with a glycine-histidine (GH) dipeptide 11 to 24 residues from the N terminus and end with a tryptophan-aspartic acid (WD) dipeptide at the C terminus.51 WD-repeat proteins often function as coordinators of macromolecular protein complexes, such as for protein-protein interaction, receptor-ligand interaction in signal transduction,52-54 pre-mRNA processing,55,56 cell cycle regulation,57 and cytoskeleton assembly.47 Recent studies have illustrated the cellular function of WD-40 repeat motif in Lgl family members. For instance, WD-40 repeats are implicated in promoting the binding of Lgl with the LRR domain of Scribble.58
Relationship Between Cell Junctions and Cell Polarity In Epithelial Cells
Epithelial cell polarity refers to the asymmetric distribution of cellular organelles of different structures and/or functions between the apical and the basolateral domains. This apical-basolateral polarization is essential for epithelial cell function, including cell division (mitosis and meiosis), cell migration, and molecular transport,59-61 and it is intimately related to TJ and AJ dynamics at the cell-cell interface.
TJs that create the limit of the apical and the basolateral domains are composed of three types of proteins: occludins, claudins and junctional adhesion molecules (JAMs). These TJ proteins all bind to the N-terminal PDZ domains of zonula occludens (ZO-1, -2 and -3), members of the MAGUK (membrane-associated guanylate kinase-like homology) family, via their cytoplasmic tails. Since TJs prevent diffusion of membrane proteins between the apical and the basolateral regions in a cell epithelium, it serves as a “fence” or a “barrier” that leads to epithelial cell polarity.
AJs are located immediately underneath TJ in virtually all epithelial barriers.4,62,63 AJs are formed by homophilic interactions between extracellular domain of cadherins and nectins. The cytoplasmic tails of cadherins and nectins associate with a series of intracellular peripheral proteins (e.g., catenins and afadins, which are adaptors), which take part in different biological events, including cytoskeleton organization64-66 and regulation of gene expression.67,68 The interactions between functional polarity complexes, the apical Par- and the Crumb-based modules, and the basolateral Scribble module, generating zones of mutual exclusion around AJs that define the apicobasal axis of epithelial polarity.6
The actin-based cytoskeletal remodeling at the AJ is regulated by Rho, Rac and Cdc42 GTPases.69 However, interactions between AJs and Rho GTPases are bidirectional since AJs also modulate the intrinsic activity of Rho GTPases to adjust cell structure and polarity. Cdc42 is a regulator of actin cytoskeleton and is associated with Par3-Par6-aPKC polarity complex.11 Cdc42, together with Par proteins, were found to regulate AJ stability via their effects on protein trafficking, such as protein endocytosis, in Drosophila melanogaster neuroectodermal epithelium, illustrating functional interactions between cell polarity proteins and endocytosis that are critical to stabilize basolateral AJs.70 Recent studies in the testis have shown that Cdc42 is a component of the Par6-based polarity complex.71,72 Cdc42 is crucial to BTB remodeling during spermatogenesis by regulating endocytic vesicle-mediated protein trafficking,71 since overexpression of a dominant negative mutant of Cdc42 in Sertoli cells that blocks the Cdc42 function can lead to a loss of the ability of TGF-β3 to accelerate protein endocytosis at the Sertoli cell BTB,71 thereby promoting the Sertoli cell TJ-barrier integrity.73,74 Earlier studies have shown that cytokines (e.g., TGF-β2, TGF-β3) are potent regulators of BTB dynamics by perturbing TJ-barrier function, which is mediated via their effects in accelerating the kinetics of clathrin-mediated endocytosis of integral membrane proteins (e.g., N-cadherin, occludin) at the Sertoli cell BTB,75,76 as well as their subsequent endosome-/ubiquitin-mediated degradation.76,77
Scribble Complex in Regulating Cell Junction Dynamics and Cell Polarity
Mutations in scribble were found to cause aberrant cell shapes and loss of epithelial organization in Drosophila embryos.33 Interestingly, mutation in either Lgl or Dlg led to similar phenotypic changes in epithelia versus Scribble mutant.17 Furthermore, Scribble and Dlg colocalized and overlapped with Lgl in embryonic epidermal epithelium, and scribble, dlg and lgl displayed strong genetic interactions.17 These findings thus indicate that these three tumor suppressors share a common role in regulating epithelial polarity and cell growth.
Dlg in Regulation of Cell junctions and Cell Polarity
The product of the Drosophila tumor suppressor gene dlg is a member of the MAGUK family, which is located at the cytoplasmic face of SJs and is necessary for the formation of cell junctions and the maintenance of apico-basal polarity.22,78,79 In Drosophila, the complete loss-of-function of dlg allele (dlgm52) was shown to induce: (i) rearrangement of the cytoskeletal components (e.g., actin and tubulin) and proteins having a polarized distribution (e.g., Ex, Cor), (ii) mis-localization of transmembrane proteins (e.g., fasciclin III, neuroglian), (iii) loss of SJs and growth control, and (iv) a loss of ability to differentiate.79
In mammals, hDlg1 (human homolog of Disc large) colocalizes with E-cadherin at the cell-cell contact site in intestinal and renal epithelial cells.80 A knockdown of hDlg in intestinal epithelial cells by siRNA severely alters AJ integrity and also prevents the recruitment of p85/PI3K to E-cadherin-based cell-cell contact,80 which is required for the association of AJ components with the cytoskeleton and also for AJ assembly.81 This is consistent with findings in C. elegans which illustrated a disrupted AJ associated with a mis-localization of AJM-1 (apical junction molecule, an AJ marker) in epidermis and intestinal epithelial cells after Dlg1 knockdown.82 In short, hDlg1 is a crucial regulator of AJ and TJ assembly in mammalian epithelia, even though the underlying regulatory mechanism(s) remains known.
Scribble in Regulation of Cell Junctions and Cell Polarity
Mutations in Drosophila scribble led to embryos displaying corrugated cuticular surface which was riddled with holes, while the wild type embryonic cuticle formed a smooth, continuous sheet, and hence the name scribble was used.33 Scribble is located basal to Armadillo (Arm, the homolog of vertebrate β-catenin) in the epithelial SJs, and codistributes with Coracle (a SJ marker belongs to protein 4.1 family).33 Thus, Scribble localizes to epithelial SJs in Drosophila, and restricted to the boundary of the apical and basolateral cell surfaces. In vertebrates, however, Scribble is distributed along the entire basal and lateral domains of epithelial cells (Figs. 1 and 2). In embryonic epithelial cells of C. elegans, the homolog of Scribble, Let-413, also localizes in the basolateral region and it is involved in locating AJ components properly at a discrete subapical position.37,83 For instance, a loss of scribble (or let-413 in C. elegans) rendered a disorganization of epithelia, leading to a mis-distribution of apical proteins (e.g., Arm, Sas, Dlt, and Crb) and AJ proteins (e.g., E-cadherin in Drosophila, and HMP-1, JAM-1 in C. elegans embryos) with these proteins moved to the basolateral cell surface, but the localization of basolateral proteins was found to be unaffected.33,83 This preferential loss of apical protein restriction that leads to polarity defects in scrib mutant epithelial cells is the result of mis-distribution of apical proteins.
Although Scribble, together with Dlg,17 are localized to SJs in Drosophila, no protein-protein interaction between either one of these proteins with SJ components was found. During development, Scribble and Dlg are enriched in the membrane before the appearance of SJs,84 so it was assumed that Scribble might predetermine the site of future SJs by recruiting other SJ components to the site for its assembly.40
The biological roles of mammalian Scribble (Scrb1, hScrib) are largely unknown. However, hScrib was localized to cell junctions of MCF10-2A, Caco-2 and MDCK cells, colocalizing with β-catenin.85 hScrib-depleted MCF10A or MDCK cells did not lead to cell polarity defects as seen in Drosophila.86,87 However, hScrib knockdown in MCF10A cells exhibited defects in wound closure and chemotatic movement in vitro. For instance, hScrib silenced cells at the wound edge failed to polarize and to recruit Cdc42 and Rac1 to the leading edge, which is required for the formation of polarized lamellipodia and to facilitate subsequent migration.86 Also, hScrib mutant mice were found to have defect in epidermal wound healing.86 A recent study in mammary epithelial cells demonstrated that depletion of hScrib led to a disrupted 3D acinar morphogenesis by inhibiting the establishment of apicobasal polarity; and, most interestingly, cell apoptosis triggered by activation of c-myc oncogene was blocked in these cells.88 These results thus illustrate the crucial role of hScrib in regulating cell polarity and perhaps cell apoptosis.
In contrast to flies and worms, mammalian Scribble was found to interact with cell junction proteins. For example, co-expression of hScrib and ZO-2, a TJ adaptor protein and a tumor suppressor,89,90 in COS-7 cells has demonstrated a direct interaction between these two proteins, which is mediated by the PDZ3 and PDZ4 domains of hScrib (Fig. 1) and the carboxyl-terminus of ZO-2.91 In MDCK cells, a knockdown of Scribble was found to disrupt E-cadherin-mediated cell adhesion, with these cells acquiring a mesenchymal appearance, migrating more rapidly but with a loss of directionality.87 Interestingly, this adhesion disruption could be partially rescued by overexpressing an E-cadherin-α-catenin fusion protein but not E-cadherin alone. This result thus suggests that Scribble, as a tumor suppressor, it stabilizes the coupling between E-cadherins and catenins, regulating epithelial cell adhesion and migration.87
In addition to epithelial cells, mammalian Scribble is likely involved in planar cell polarity (PCP) in mammals (note: PCP originally refers to the directional polarity of hairs on the wings of fruitflies,92 analogous to the directional alignment of elongating/elongated spermatids with their heads pointing toward the basement membrane in the seminiferous epithelium during spermatogenesis). The study of planar polarization in mammals has demonstrated the role of Scribble in PCP pathway.93 Mutation of Scribble gene in mouse led to defects in the polarization of stereociliary bundles in cochlea, and similar but more severe defects were observed in animals with mutation in Vangle2, a mammalian homolog of the Drosophila PCP gene Strabismus/Van Gogh.93 Moreover, polarization defects in animals heterozygous for Vangl2 and Scrb1 were comparable to Vangl2 homozygotes, demonstrating genetic interactions between these two genes in regulating PCP in mammals.93 A subsequent study in mammalian cells has shown that Scrb1 and Vangl2 interacted with each other through the PDZ domains of Scribble.58 These results illustrate that Scribble is involved in regulating PCP in Drosophila and mammals. However, the involvement of Scribble in spermatid alignment in the seminiferous epithelium during spermiogenesis with their heads pointing to the basement membrane but their tails to the seminiferous tubule lumen, which appears to be a form of planar cell polarity, remains to be established. It is likely that the spermatid PCP in the seminiferous epithelium requires the concomitant contribution of PAR-, Crumbs-, and the Scribble-based modules. This possibility must be carefully evaluated in future studies.
Mutual Inhibition of Lgl and Apical aPKC-Par6 in Regulating Epithelial Cell Polarity
Similar to dlg, lgl was initially identified as a tumor suppressor, because mutation in this gene resulted in tissue-specific tumors in larvae that led to their eventual death.94,95 Studies in Drosophila embryos have shown that Lgl is localized at cell membranes or intercellular matrix of embryonic cells and it is likely involved in cell-cell interactions.45,96 In plasma membrane, Lgl was found to form large aggregates which were resistant to solubilization by nonionic detergents, indicating its involvement in cytoskeletal matrix.97 Lgl also forms homo-oligomers and directly interacts with nonmuscle myosin II.98 Furthermore, Lgl was found to tightly associate with aPKC in the Par protein complex.99 The phosphorylation of Lgl by aPKC leads to a release of Lgl from the plasma membrane, dissociating from nonmuscle myosin II without affecting Lgl homo-oligomerization.100 Additionally, genetic and phenotypic analyses in mutant Drosophila embryos suggested that the lgl product was required in different types of epithelial cells to control cell shape during development in vivo.101 Collectively, these studies illustrate the involvement of Lgl in the formation of cytoskeletal network, and its interaction with cell membrane is mediated via its phosphorylation by aPKC.
The mutual inhibition of Lgl and aPKC has been reported in Drosophila. When Lgl is phosphorylated on three conserved Ser residues by aPKC, Lgl dissociates from the cytoskeleton and becomes inactivated, which, in turn, disrupts proper protein localization to the apical cell cortex.99 Also, phosphorylation of Lgl causes an auto-inhibitory interaction of its N‑terminus with its C-terminus, which prevents the binding of its C-terminus to the cytoskeleton.102 On the other hand, aPKC mutants were found to display a phenotype opposite to that of the lgl mutants, i.e., a reduced aPKC level suppressed both cell polarity and cell proliferation in lgl mutants.103 In Drosophila, the maintenance of Par6 localization to the apical side of epithelial cells requires Lgl, which is active on the basolateral side and excludes Par6 from the cell cortex. While on the apical side, Lgl is inactivated and excluded by aPKC phosphorylation.104 These results suggest that mutual inhibition and exclusion of aPKC and Lgl on opposite sides of an epithelial cell participate in the formation of complementary corticle domains and correct epithelial structure.
In vertebrates, the Lgl homologs, Lgl1 and Lgl2, display similar antagonistic interactions with aPKC-Par3-Par6 complex in regulating epithelial cell polarity and proliferation. In contact-naive MDCK cells caused by the absence of Ca2+ in spent medium, cell adhesion and TJ formation were blocked with Lgl accumulated in punctate cytoplasmic structures. After the addition of Ca2+ to the medium, Lgl was found to associate with the lateral surface following E-cadherin appearance and TJ formation.105 This indicates that the engagement of Lgl to the lateral membrane correlates with the development of a polarized structure in mammalian epithelial cells. This lateral domain restriction of mammalian Lgl (mLgl) is dependent on its phosphorylation within a highly conserved region, because a phosphorylation-resistant recombinant is observed to distribute in a nonpolarized manner.105 In MDCK cells, mLgl competes with Par3 for its interaction with aPKC-Par6 to form a protein complex.106 During epithelial polarization, mLgl temporally colocalizes with aPKC-Par6 at the cell-cell interface and inhibits the formation of TJs, while another complex, Par3-aPKC-Par6, promotes TJ formation. Importantly, the phosphorylation of mLgl by aPKC is involved in its dissociation with aPKC-Par6 during polarization of epithelial cells.106 These findings indicate that aPKC/Par6 selectively associates with either Par3 or Lgl, depending on the intrinsic activity of aPKC, to exerts its regulatory function on epithelial cell polarity.
Using siRNA knockdown, it was shown that mLgl regulated the assembly of apical membrane domains and cell polarization in MDCK cells.107 Utilizing the Ca2+-switch model, MDCK cells cultured in Ca2+-depleted medium were found to have reduced levels of peripheral apical proteins and F-actin due to their redistribution from the cell surface to cell cytosol. However, in the mLgl-knockdown cells, the redistribution of these proteins was significantly suppressed and most of the mLgl-knockdown cells retained the apical proteins and F-actin at the cell periphery. Importantly, overexpression of dominant-negative aPKCλ was found to antagonize the effect of the mLgl knockdown on the redistribution of apical proteins.107 This indicates that in mammalian epithelial cells, aPKC activity also contributes to suppressing the disassembly of apical proteins during cell depolarization, and mLgl counterbalances this aPKC activity to facilitate apical protein disassembly. This study also revealed that the suppression of apical Par3-aPKC-Par6 complex activity by mLgl is mediated through the suppression of an interaction of the aPKC-Par6 complex with Par3 or Cdc42.107 p32, a mLg1 binding protein, was found to form a complex with mLgl and aPKC in regulating cell polarity.108 Overexpression of p32 could rescue the reduced apical actin filaments caused by aPKC inhibitor whereas its knockdown induced defects in cell polarity.108 This study thus suggests that p32 regulates cell polarization by binding to mLgl and aPKC and enhancing phosphorylation of mLgl via aPKC. These results thus illustrates that the mutual suppression between mLgl and aPKC-Par6 also plays an important role in the establishment of mammalian epithelial cell polarity.
Interactions between Scribble Complex and Other Apical Polarity Complexes in Epithelia
As briefly described above, several protein complexes that are involved in epithelial apicobasal polarity have been identified. Using genetic and biochemical approaches, details of the protein-protein interactions that connect these complexes are increasingly clear (Fig. 2). For example, the spatial relationship between Crb complex and Par complex is supported by findings in Drosophila and mammalian cells.109-113 Co-immunoprecipitation in MDCK cells has illustrated direct interaction between Crb3, one of the Crumbs homolog in mammals, and Par6, which is required for the establishment of mature junction structure in epithelial cells.109 In Drosophila, the cytoplasmic tail of Crb that contains two conserved Thr was shown to bind directly to aPKC. Moreover, Dpatj of the Crb complex can regulate the phosphorylation of Crb by aPKC, which induces Dpatj and Scribble to localize to the proper cell membrane domain.110
In addition to the antagonistic interaction between Par3-aPKC-Par6 complex and Lgl, another complex that belongs to apical domain of epithelial cells, the Crb complex, has also been found by genetic screening to have functional interactions with components of Scribble complex in Drosophila.114,115 Crb is an integral apical protein that plays a role in specifying the apical domain of epithelial cells by forming a complex with Stardust (Sdt) and Dpatj in Drosophila.116,117 Overexpression of Crb results in the expansion of the apical domain and a decrease in the basolateral domain, which is consistent with the phenotype caused by impaired activity of basolateral components of Scribble complex.118,119 Interestingly, genetic study revealed that the epithelial defects caused by crb mutation were markedly suppressed by scribble mutation.114,115 Thus, it is likely that the Scribble complex antagonizes Crb in setting the limits of the apicobasolateral domains and in determining the relative distribution of cell junction proteins in these domains.
While no intrinsic catalytic activity is detected in components of the Scribble complex, several protein-protein interaction domains, such as PDZ, SH3 and GUK domains, are found in their primary sequences, illustrating the likely interactions between Scribble/Lgl/Dlg complex and other proteins, which can affect epithelial polarity and cell proliferation. Using biochemical approaches in human epithelial cells, MPP7 (membrane-palmitoylated protein 7), another member of MAGUK family was identified to interact with hDlg1 via its N-terminal L27 domain, and also with Pals1 (MPP5) and Patj, which are components of the Crb complex, via its SH3-HOOK domain.25,120 In Caco2 cells, both hDlg1 and MPP7 displayed partial colocalization with occludin and ZO-1, and shRNA-mediated knockdown of either one of these proteins compromised TJ assembly, indicating the interactions between these polarity complexes affect cell junction function.25 In migrating astrocytes, there is evidence that membrane-associated Dlg1 interacts with microtubule-bound APC (adenomatous polyposis coli, another tumor suppressor) through its PDZ domains to polarize the microtubule cytoskeleton during cell migration, which is required for polarization of astrocytes.121 Furthermore, PKCζ-Par6 complex activated by Cdc42 was shown to control microtubule recruitment of APC and cortical recruitment of Dlg1.121 This suggests a hierarchical biochemical connection exists between the two distinct polarity complexes.
These observations suggest that components of Scribble complex co-operate and compete with the apical polarity proteins to define the apico- and baso-lateral domains by recruiting respective junction proteins to the sites during cell polarity establishment.
Scribble Complex Participates in Cell Signaling Cascades
As summarized above, the Scribble complex and its homologs regulate both cell polarity and cell proliferation. Yet little is known about the underlying mechanism(s) particularly in mammals. However, recent findings have shed lights on the complex signaling network through which Scribble/Dlg/Lgl affects cell polarity and proliferation (Fig. 2).
SAPK3/p38γ is a one of the p38 MAPKs (p38 mitogen-activated protein kinases) which is activated in mammalian cells in response to hyperosmotic stress.122,123 It has a C-terminal sequence that can dock with the PDZ domains of different proteins, one of which is hDlg. Studies by immunofluorescence and co-immunoprecipitation in Hela, PC12 and SH5-SY5Y cells have detected interaction between SAPK3/p38γ and hDlg, revealing specific phosphorylation of hDlg by SAPK3/p38γ in vivo in response to cellular stress.124 In addition, this phosphorylation of hDlg was found to induce its dissociation from guanylate kinase-associated protein (GKAP), which is necessary for its targeting to cytoskeleton.124,125 c-Jun N-terminal kinase (JNK) was also found to induce phosphorylation of hDlg during osmotic stress that contributed to its accumulation within the cell membrane at cell-cell contact site, which was coupled with an increase in E6-induced degradation of hDlg.126 These data thus suggest that the cellular localization, function and proteasome-mediated degradation of hDlg are phosphorylation-dependent events.
Furthermore, the involvement of mammalian Scribble in oncogenic transformation was shown to be mediated via a suppression of MAPK signaling.127 For instance, simultaneous knockdown of Scribble by shRNA and an activation of Ras-signaling in nontransformed human MCF10A mammary epithelial cells were shown to promote cell invasion.127 Moreover, a two-fold increase in the phosphorylation of ERK (extracellular-regulated kinase, an MAPK effector) and an acute hyperphosphorylation of ERK in response to EGF, were also detected in Scribble-depleted cells.127 In Scribble-depleted cells, an activation of Raf-MEK-ERK signaling was found to enhance the defects of Scribble loss. Furthermore, Scribble-overexpressed cells were found to suppress the development of invasive protrusions of Ras-activated cells, and Scribble overexpression also facilitated the restoration of cell polarity in Ras-activated cells.127 These data thus illustrate that Scribble acts as a tumor suppressor by limiting cell invasion, which is mediated downstream of Ras but upstream of MAPK in the signaling pathway.
A recent study has revealed the likely mechanism by which Scribble suppresses the Ras-Raf-MEK-ERK pathway.128 Two ERK binding sites (kinase interaction motif, KIM) were identified on hScrib, and hScrib was found to regulate the ERK signaling pathway in human keratinocytes by inhibiting the phosphorylation and nuclear translocation of ERK.128 In vitro study also revealed the two phospho-acceptor sites of hScrib were putative substrates for PKA and ERK1.128 These results thus support the notion that a loss of Scribble can affect mitotic proliferation in tumors by promoting MAPK phosphorylation and nuclear translocation of ERK.
In mammalian tissue barriers, there is also evidence that Scribble complex components participate in endocytic vesicle-mediated trafficking and membrane fusion events. For example, membrane-associated mLgl from MDCK cells was found to directly interact with Syntaxin 4 (STX4), a component of the exocytic pathway at the basolateral membrane.105 Recently, a study in HaCaT cells has shown that three components of the Scribble complex, hScrib, mLgl and hDlg all display significant degree of colocalization with STX4 at cell membrane.129
The Scribble Module and Mammalian Epithelial Barrier Function
Epithelial monolayers that display apico-basal cell polarity serve as tissue barriers in multiple organisms. Cell polarity and impermeability are two important attributes of these tissue barriers. The polarity protein complexes described above cooperate with each other to confer differential distribution of functional proteins on plasma membrane. On the other hand, junction complexes formed at correct cortical positions help to maintain distinctive cell membrane domains and resist material exchanges between two sides of the epithelial monolayer. Therefore, the development of cell polarity plays an important role in epithelial barrier establishment and function. In this section, we focus on the role of Scribble polarity complex on the formation and function of several mammalian epithelia and the likely underlying mechanism(s).
Scribble Regulates Barrier function of Intestinal Epithelium
In the small intestine, the gut barrier conferred exclusively by TJs of the intestinal epithelium protects the host animal from invasion of pathogens during food digestion and absorption. Although the causal relationship between the establishment of TJs and cell polarity remains unclear, the role of TJs in sealing the paracellular space that creates the gut barrier has been established.130,131
The role of Scribble in TJ assembly was first demonstrated in T84 and SK-CO15 intestinal epithelial monolayers in which Scribble was found to colocalize with TJ proteins occludin and ZO-1.132 This is in contrast to other mammalian epithelia in which Scribble was restricted to the basolateral plasma membrane.91,133 Moreover, similar to the findings in Scribble-depleted MDCK cells,87 a knockdown of Scribble by RNAi was found to significantly delay gut barrier assembly, and to perturb the TJ-barrier function of Scribble-deficient cells.132 Interestingly, Scribble knockdown in SK-CO15 cells did not affect the TJ ultrastructure, nor impeded the development of apicobasal cell polarity.132 These data illustrate Scribble is crucial to the gut barrier function in vitro.
In the intestinal epithelium, Scribble was found to interact directly with ZO-1132 and ZO-2,91 which are cytosolic scaffolds that stabilize integral membrane TJ proteins (e.g., occludins, claudins, JAMs). A study to monitor the subcellular distribution of Scribble in mouse small intestine epithelium showed that Scribble was localized to the basolateral membrane. In addition, Scribble was also detected at the site of apical junction complex.134 Moreover, a distinctive colocalization of Scribble and E-cadherin was detected at the apical junction site, illustrating an apical and basolateral localization of Scribble in mouse intestinal epithelium.134
Although there is little evidence of physical interaction between components of Scribble complex in mammalian epithelia, a recent report in the gut barrier has revealed selective binding between Scribble and Lgl-1, but not Dlg-1, during TJ reassembly.132 More important, a knockdown of Dlg-1, but not Lgl-1, was found to attenuate the gut barrier function.132 Furthermore, interferon (IFN)-γ, a pro-inflammatory cytokine, was found to induce down-regulation of junctional Scribble during TJ disassembly caused by the IFN-γ-induced intestinal inflammation in vivo.132 These findings seemingly suggest the absence of a trimeric Scribble/Lgl/Dlg complex in mammalian intestinal epithelium, and Scribble is likely to play a unique role in gut barrier function.
Scribble Complex Controls Renal Epithelial Polarity
In the kidney, tubular epithelium is composed of polarized epithelial cells with the apical membrane domain facing the tubular lumen, and cell junctions are either restricted to the cell-cell interface or to the cell-basal lamina interface at the basolateral domain. In the kidney, cell polarity and cell junctions in the epithelium of convoluted tubules are necessary to fluid/electrolyte re-absorption and secretion. Studies by confocal microscopy showed that hScrib was located at the cell-cell interface of MDCK (Madin Darby canine kidney) cells, colocalizing with β-catenin. Calcium switch assay also demonstrated hScrib was retained to cell junction upon engagement of E-cadherin by its LRR region in MDCK cells.85 Binding of hScrib to E-cadherin at the lateral side is important for normal adhesion between polarized renal epithelial cells, since knockdown of hScrib was found to increase cell motility and reduce adhesion.87 mLgl was localized to basolateral membranes and it interacted with basolateral exocytic machinery in MDCK cells.105 Further studies in renal epithelium also revealed the interaction between mLgl and aPKC-Par6 complex.106 In hDlg1-deficient mice, abnormalities were found in the renal and urogenital organs. For instance, the kidneys and ureters of hDlg1-defiicent mice became hypoplastic due to reduced cell proliferation in the uretic or renal epithelium.135 These findings thus illustrate the involvement of Scribble complex in the development, cell proliferation, and maintenance of renal epithelial polarity and integrity.
Scribble Complex and the BTB
Based on studies in the field as briefly reviewed and summarized above, it is increasingly clear that the Scribble/Dlg/Lgl polarity protein module, whose components are tumor suppressor genes, is crucial to cell junction dynamics in mammalian epithelial cells. Furthermore, each of the components in this complex can recruit numerous partner proteins via their protein interacting domains (e.g., PDZ domain). As such, a complex network of proteins can be recruited to specific cellular sites/domains and that this complex can regulate a wide range of cellular functions besides cell polarity. Interestingly, even though components of the Scribble protein complex, namely Scribble, Dlg, and Lgl, were discovered ∼3 to 4 decades ago in Drosophila, and their mammalian homologs were since identified and shown to possess similar physiological functions, there is no report in the literature that examined their function in the testis, such as the BTB during spermatogenesis. As shown in Figure 3, Scribble, Dlg1 and Lgl2 were shown to be expressed in the testis, with these proteins expressed by both Sertoli and germ cells. Using an antibody specific to Scribble, its localization in the seminiferous epithelium of the rat testis has also been examined by immunohistochemistry (Fig. 4), illustrating Scribble is localized at the basal compartment of the seminiferous epithelium near the basement membrane, consistent with its localization at the BTB (Fig. 4). More important, the localization and expression of Scribble at the BTB appears to be stage-specific since Scribble is abundantly found at the BTB at Stages VII to VIII of the seminiferous epithelial cycle at the time of BTB restructuring to accommodate the transit of preleptotene spermatocytes at the site (Fig. 4). The localization of Scribble at the BTB has also been confirmed by dual-labeled immunofluorescence analysis, in which Scribble was found to colocalize with putative integral membrane protein occludin and TJ adaptor protein ZO-1 at the BTB (Fig. 5). These findings thus demonstrate unequivocally that Scribble is an integrated component of the BTB. These results also illustrate that functional studies can now be performed to probe the physiological role of the Scribble/Dlg/Lgl at the BTB. Furthermore, it will be important in future studies to examine how these proteins interact with the Par-based polarity proteins, such as Par 6 and 14-3-3, which were shown to regulate spermatid orientation and cell adhesion at the BTB, via their effects on the endocytic vesicle-mediated protein trafficking.71,136,137
Figure 3.
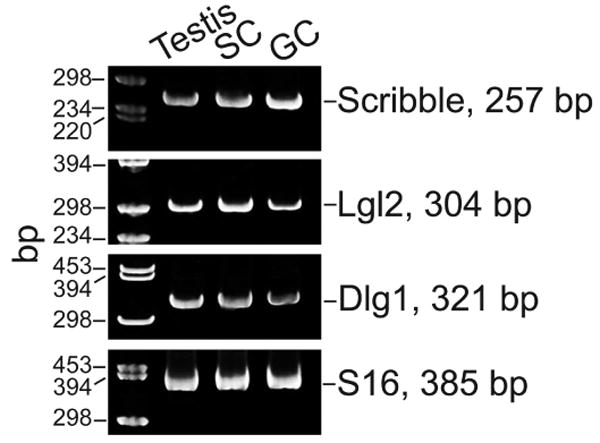
Components of the Scribble protein complex in the testis. Scribble, Lgl2 and Dlg1 were detected in the testis, as well as in Sertoli (isolated from 20-day-old rat testes after 4 days in culture with negligible germ cell contamination) and germ cells (isolated from 90-day-old rats after ∼12-hr in culture) by RT-PCR. Primers used in PCR were as follows: (i) Scribble: sense, 5′-CTGGCACTGCTCACAGATCT-3 (nucleotides 724-743); antisense, 5′-AGCACCTCAAGATGATTCCG (nucleotides 961-980) (GenBank Accession #:XM_002726943.1); (ii) Lgl2: sense, 5′-TCCACCATCTCGAACACTCG (nucleotides 442-461); antisense, 5′-TGCTGGATGACAACAGCCTG (nucleotides 726-745) (GenBank Accession #:NM_001127549.1); (iii) Dlg1: sense, 5′-GTTGACCTCAGAGCTGCAAG (nucleotides 2000-2019); antisense, 5′-CCACCACTCGTCATCAGAAG (nucleotides 2301-2320) (GenBank Accession #:NM_012788.1); which were co-amplified with (iv) S16: sense, 5′-TCCGCTGCAGTCCGTTCAAGTCTT (nucleotides 177-200); antisense, 5′-GCCAAACTTCTTGGATTCGCAGCG (nucleotides 538-561) (GenBank Accession #:XM_001078234). RT-PCR was performed essentially as earlier described.138,139 The cycling parameters for PCR reaction were: denaturation at 94 °C for 1 min, annealing at 57 °C for 2 min, and extension at 72 °C for 3 min, for a total of 21-25 cycles, which were followed by an extension period of 15 min at 72 °C. The identity of the PCR product was confirmed by direct nucleotide sequencing at Genewiz. SC, Sertoli cells; GC, germ cells, bp, base-pair.
Figure 4.
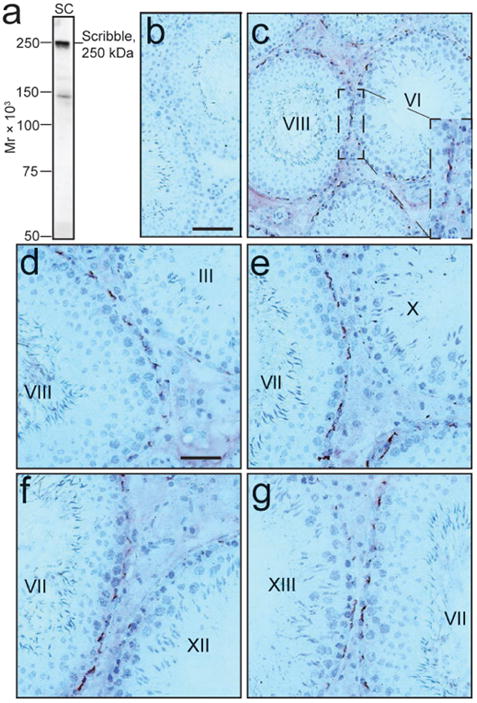
Stage-specific expression of Scribble in the seminiferous epithelium of adult rat testis during the seminiferous epithelial cycle of spermatogenesis. Immunohistochemistry was performed using frozen cross-sections of testes from adult rats and a goat anti-Scribble antibody (Santa Cruz, Cat. # sc-11048) which cross-reacted with Scribble in rats. Using immunoblot analysis and lysates of rat Sertoli cells (SC), this anti-Scribble antibody (at 1:750 dilution for immunoblotting) was found to be specific to Scribble (a). This antibody was used for immunohistochemistry (at 1:50 dilution) (c-g) versus normal goat serum which served as the negative control and shown in (b). Immunoreactive Scribble appears as reddish-brown precipitates (c-g). It is noted that Scribble was localized almost exclusively near the basement membrane in the seminiferous epithelium, consistent with its localization at the BTB (note: this pattern of localization also illustrates its polarized localization) in tubules, most prominently at Stages IV-VIII but it diminished rapidly thereafter to an almost nondetectable level at Stages X-XII but gradually re-appeared from Stage XIII. Bar in b = 80 mm, which applies to c; bar in d = 40 mm, which applies to e-g. Mr, molecular weight. A color version of this figure is available online at www.landesbioscience.com/curie.
Figure 5.
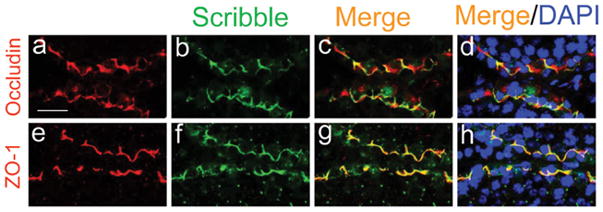
A study by dual-labeled immunofluorescence analysis to assess the colocalization of Scribble with TJ-proteins occludin and ZO-1 at the BTB. In order to further confirm the observations shown in Figure 4 that Scribble is indeed an integrated component of the BTB, dual-labeled immunofluorescence analysis was performed in which Scribble (green fluorescence in b, f) was found to colocalize with either occludin (a TJ-integral membrane protein at the BTB, red fluorescence in a) or ZO-1 (a TJ-associated adaptor protein at the BTB, red fluorescence in e) with the merged images shown in c, d, and g, h, which appear as orange-yellow fluorescence. Cell nuclei were visualized by DAPI staining (d, h). Bar in a = 40 μm, which applies to b-h. A color version of this figure is available online at www.landesbioscience.com/curie.
Conclusion and Future Perspectives
The Scribble/Dlg/Lgl complex has been shown to regulate cell polarity, TJ and AJ dynamics, and cell proliferation in species ranging from fruitflies, worms and mammals (e.g., the gut barrier, the tubular barrier in the kidney) as summarized above. It is anticipated that this polarity complex is a multifaceted functional complex at other blood-tissue barriers, such as the BTB, where components of this protein module were found. Also, it will be important in future studies to assess its functional relationship with the PAR- and the Crumb-based complexes since PAR-6,136 14-3-3,137 and Cdc4271 were shown to be integrated components of the Sertoli cell that regulated BTB dynamics, and how these three polarity modules fine tune the timely restructuring of the BTB during the seminiferous epithelial cycle of spermatogenesis. For instance, it remains to be examined if the Scribble complex regulates endocytic vesicle-mediated protein trafficking at the BTB; and if it does, how this complex works with the PAR-based protein complex to modulate protein endocytosis, transcytosis, recycling, and endosome-/ubiquitin-mediated degradation.
Acknowledgments
Studies in the authors' laboratories were supported by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5 to C.Y.C.; R01 HD056034 to C.Y.C); Hong Kong Research Grants Council (HKU772009 and HKU773710 to W.-Y.L.); and the National Natural Science Foundation of China (81100462, to W.-H.S.).
References
- 1.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 2.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 3.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberts B, et al. Molecular Biology of the Cell. Garland Science; New York: 2002. [Google Scholar]
- 5.Nelson WJ, Yeaman C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 2001;11:483–486. doi: 10.1016/s0962-8924(01)02145-6. [DOI] [PubMed] [Google Scholar]
- 6.Assemat E, Bazellieres E, Pallesi-Pocachard E, et al. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 9.Noda Y, et al. Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells. 2001;6:107–119. doi: 10.1046/j.1365-2443.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- 10.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 11.Joberty G, Petersen C, Gao L, et al. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 12.Lin D, et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 13.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 14.Laprise P. Emerging role for epithelial polarity proteins of the crumbs family as potential tumor suppressors. J Biomed Biotechnol. 2011 doi: 10.1155/2011/868217. 868217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laprise P, et al. Yurt, Coracle, Neurexin IV and the Na(+), K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 16.Mathew D, et al. Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr Biol. 2002;12:531–539. doi: 10.1016/s0960-9822(02)00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 18.Stewart M, Murphy C, Fristrom JW. The recovery and preliminary characterization of X chromosome mutants affecting imaginal discs of Drosophila melanogaster. Dev Biol. 1972;27:71–83. doi: 10.1016/0012-1606(72)90113-3. [DOI] [PubMed] [Google Scholar]
- 19.Murphy C. Cell death and autonomous gene action in lethals affecting imaginal discs in Drosophila melanogaster. Dev Biol. 1974;39:23–36. doi: 10.1016/s0012-1606(74)80005-9. [DOI] [PubMed] [Google Scholar]
- 20.Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- 21.Perrimon N. The maternal effect of lethal(1)discs-large-1: a recessive oncogene of Drosophila melanogaster. Dev Biol. 1988;127:392–407. doi: 10.1016/0012-1606(88)90326-0. [DOI] [PubMed] [Google Scholar]
- 22.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 23.Hough CD, Woods DF, Park S, et al. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lue RA, Marfatia SM, Branton D, et al. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 41. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stucke VM, Timmerman E, Vandekerckhove J, et al. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell. 2007;18:1744–1755. doi: 10.1091/mbc.E06-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnak D, Lee S, Margolis B. Identification of multiple binding partners for the amino-terminal domain of synapse-associated protein 97. J Biol Chem. 2002;277:46730–46735. doi: 10.1074/jbc.M208781200. [DOI] [PubMed] [Google Scholar]
- 27.Bohl J, Brimer N, Lyons C, et al. The stardust family protein MPP7 forms a tripartite complex with LIN7 and DLG1 that regulates the stability and localization of DLG1 to cell junctions. J Biol Chem. 2007;282:9392–9400. doi: 10.1074/jbc.M610002200. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Fan S, Makarova O, et al. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol. 2002;22:1778–1791. doi: 10.1128/MCB.22.6.1778-1791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lue RA, Brandin E, Chan EP, et al. Two independent domains of hDlg are sufficient for subcellular targeting: the PDZ1-2 conformational unit and an alternatively spliced domain. J Cell Biol. 1996;135:1125–1137. doi: 10.1083/jcb.135.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 31.Itoh M, Nagafuchi A, Yonemura S, et al. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy MB. Origin of PDZ (DHR, GLGF) domains. Trends Biochem Sci. 1995;20:350. doi: 10.1016/s0968-0004(00)89074-x. [DOI] [PubMed] [Google Scholar]
- 33.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apperson ML, Moon IS, Kennedy MB. Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family. J Neurosci. 1996;16:6839–6852. doi: 10.1523/JNEUROSCI.16-21-06839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilder D, et al. Collective nomenclature for LAP proteins. Nat Cell Biol. 2000;2:E114. doi: 10.1038/35017119. [DOI] [PubMed] [Google Scholar]
- 37.Legouis R, et al. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol. 2000;2:415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- 38.Santoni MJ, Pontarotti P, Birnbaum D, et al. The LAP family: a phylogenetic point of view. Trends Genet. 2002;18:494–497. doi: 10.1016/s0168-9525(02)02738-5. [DOI] [PubMed] [Google Scholar]
- 39.Legouis R, et al. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 2003;4:1096–1102. doi: 10.1038/sj.embor.7400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeitler J, Hsu CP, Dionne H, et al. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167:1137–1146. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharrer B, Hadorn E. The Structure of the Ring-Gland (Corpus Allatum) in Normal and Lethal Larvae of Drosophila Melanogaster. Proc Natl Acad Sci USA. 1938;24:236–242. doi: 10.1073/pnas.24.6.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadorn E. An Accelerating Effect of Normal “Ring-Glands” on Puparium-Formation in Lethal Larvae of Drosophila Melanogaster. Proc Natl Acad Sci USA. 1937;23:478–484. doi: 10.1073/pnas.23.9.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 1985;4:1551–1557. doi: 10.1002/j.1460-2075.1985.tb03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutzelschwab R, Klambt C, Rossa R, et al. A protein product of the Drosophila recessive tumor gene, l (2) giant gl, potentially has cell adhesion properties. EMBO J. 1987;6:1791–1797. doi: 10.1002/j.1460-2075.1987.tb02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacob L, Opper M, Metzroth B, et al. Structure of the l(2)gl gene of Drosophila and delimitation of its tumor suppressor domain. Cell. 1987;50:215–225. doi: 10.1016/0092-8674(87)90217-0. [DOI] [PubMed] [Google Scholar]
- 47.Baek KH. Structural and functional conservation of the lgl recessive oncogenes (Review) Int J Oncol. 2004;24:1257–1261. [PubMed] [Google Scholar]
- 48.Klezovitch O, Fernandez TE, Tapscott SJ, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sondek J, Bohm A, Lambright DG, et al. Crystal structure of a G-protein beta gamma dimer at 21A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh M, Anthony C, Harlos K, et al. The refined structure of the quinoprotein methanol dehydrogenase from Methylobacterium extorquens at 194 A. Structure. 1995;3:177–187. doi: 10.1016/s0969-2126(01)00148-4. [DOI] [PubMed] [Google Scholar]
- 51.Smith TF, Gaitatzes C, Saxena K, et al. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Roberts R. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell Mol Life Sci. 2001;58:2085–2097. doi: 10.1007/PL00000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Higuera I, et al. Folding of proteins with WD-repeats: comparison of six members of the WD-repeat superfamily to the G protein beta subunit. Biochemistry. 1996;35:13985–13994. doi: 10.1021/bi9612879. [DOI] [PubMed] [Google Scholar]
- 54.Croze E, et al. Receptor for activated C-kinase (RACK-1), a WD motif-containing protein, specifically associates with the human type I IFN receptor. J Immunol. 2000;165:5127–5132. doi: 10.4049/jimmunol.165.9.5127. [DOI] [PubMed] [Google Scholar]
- 55.Dubrovskaya V, et al. Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIF beta (RAP30) and incorporation into the TFIID complex. EMBO J. 1996;15:3702–3712. [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto T, Horikoshi M. Defect in cytokinesis of fssion yeast induced by mutation in the WD40 repeat motif of a TFIID subunit. Genes Cells. 1998;3:347–355. doi: 10.1046/j.1365-2443.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 57.Ohtoshi A, Maeda T, Higashi H, et al. Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex. Biochem Biophys Res Commun. 2000;268:530–534. doi: 10.1006/bbrc.2000.2167. [DOI] [PubMed] [Google Scholar]
- 58.Kallay LM, McNickle A, Brennwald PJ, et al. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem. 2006;99:647–664. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- 59.Delacour D, Jacob R. Apical protein transport. Cell Mol Life Sci. 2006;63:2491–2505. doi: 10.1007/s00018-006-6210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–495. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Fristrom D. The cellular basis of epithelial morphogenesis. A review Tissue Cell. 1988;20:645–690. doi: 10.1016/0040-8166(88)90015-8. [DOI] [PubMed] [Google Scholar]
- 63.Green KJ, Getsios S, Troyanovsky S, et al. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drees F, Pokutta S, Yamada S, et al. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-flament assembly. Cell. 2010;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yonemura S, Wada Y, Watanabe T, et al. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 67.Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 69.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 70.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong EWP, Mruk DD, Lee WM, et al. Regulation of blood-testis barrier dynamics by TGF-b3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lui WY, Wong CH, Mruk DD, et al. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 74.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 75.Xia W, Wong EWP, Mruk DD, et al. TGF-β3 and TNFa perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan HHN, Mruk DD, Lee WM, et al. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su L, Mruk DD, Lee WM, et al. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woods DF, Bryant PJ. Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev Biol. 1989;134:222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 79.Woods DF, Hough C, Peel D, et al. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laprise P, Viel A, Rivard N. Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem. 2004;279:10157–10166. doi: 10.1074/jbc.M309843200. [DOI] [PubMed] [Google Scholar]
- 81.Laprise P, et al. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226–8234. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- 82.Koppen M, et al. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 83.McMahon L, Legouis R, Vonesch JL, et al. Assembly of C elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci. 2001;114:2265–2277. doi: 10.1242/jcs.114.12.2265. [DOI] [PubMed] [Google Scholar]
- 84.Tepass U, Tanentzapf G, Ward R, et al. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 85.Navarro C, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 86.Dow LE, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 87.Qin Y, Capaldo C, Gumbiner BM, et al. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhan L, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chlenski A, et al. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta. 2000;1493:319–324. doi: 10.1016/s0167-4781(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 90.Glaunsinger BA, Weiss RS, Lee SS, et al. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 2001;20:5578–5586. doi: 10.1093/emboj/20.20.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metais JY, Navarro C, Santoni MJ, et al. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 2005;579:3725–3730. doi: 10.1016/j.febslet.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 92.Fanto MMH. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- 93.Montcouquiol M, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 94.Stark MB, Bridges CB. The Linkage Relations of a Benign Tumor in Drosophila. Genetics. 1926;11:249–266. doi: 10.1093/genetics/11.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gateff E. The genetics and epigenetics of neoplasms in Drosophila. Biol Rev Camb Philos Soc. 1978;53:123–168. doi: 10.1111/j.1469-185x.1978.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 96.Klambt C, Schmidt O. Developmental expression and tissue distribution of the lethal (2) giant larvae protein of Drosophila melanogaster. EMBO J. 1986;5:2955–2961. doi: 10.1002/j.1460-2075.1986.tb04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol. 1994;127:1345–1360. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strand D, et al. The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J Cell Biol. 1994;127:1361–1373. doi: 10.1083/jcb.127.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- 100.Kalmes A, Merdes G, Neumann B, et al. A serine-kinase associated with the p127-l(2)gl tumour suppressor of Drosophila may regulate the binding of p127 to nonmuscle myosin II heavy chain and the attachment of p127 to the plasma membrane. J Cell Sci. 1996;109(Pt 6):1359–1368. doi: 10.1242/jcs.109.6.1359. [DOI] [PubMed] [Google Scholar]
- 101.Manfruelli P, Arquier N, Hanratty WP, et al. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development. 1996;122:2283–2294. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- 102.Betschinger J, Eisenhaber F, Knoblich JA. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr Biol. 2005;15:276–282. doi: 10.1016/j.cub.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Rolls MM, Albertson R, Shih HP, et al. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hutterer A, Betschinger J, Petronczki M, et al. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Musch A, et al. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol Biol Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamanaka T, et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 107.Yamanaka T, et al. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci. 2006;119:2107–2118. doi: 10.1242/jcs.02938. [DOI] [PubMed] [Google Scholar]
- 108.Bialucha CU, Ferber EC, Pichaud F, et al. p32 is a novel mammalian Lgl binding protein that enhances the activity of protein kinase Czeta and regulates cell polarity. J Cell Biol. 2007;178:575–581. doi: 10.1083/jcb.200612022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lemmers C, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sotillos S, Diaz-Meco MT, Caminero E, et al. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- 112.Hurd TW, Gao L, Roh MH, et al. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 113.Straight SW, et al. Loss of PALS1 expression leads to tight junction and polarity defects. Mol Biol Cell. 2004;15:1981–1990. doi: 10.1091/mbc.E03-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- 115.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 116.Tepass U. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol. 1996;177:217–225. doi: 10.1006/dbio.1996.0157. [DOI] [PubMed] [Google Scholar]
- 117.Bachmann A, Schneider M, Theilenberg E, et al. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- 118.Wodarz A, Hinz U, Engelbert M, et al. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 119.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 120.Wells CD, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 121.Etienne-Manneville S, Manneville JB, Nicholls S, et al. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goedert M, Cuenda A, Craxton M, et al. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sabio G, et al. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/ PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem J. 2004;380:19–30. doi: 10.1042/BJ20031628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sabio G, et al. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24:1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu H, et al. Intramolecular interactions regulate SAP97 binding to GKAP. EMBO J. 2000;19:5740–5751. doi: 10.1093/emboj/19.21.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Massimi P, Narayan N, Cuenda A, et al. Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6-induced degradation. Oncogene. 2006;25:4276–4285. doi: 10.1038/sj.onc.1209457. [DOI] [PubMed] [Google Scholar]
- 127.Dow LE, et al. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27:5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 128.Nagasaka K, et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene. 2010;29:5311–5321. doi: 10.1038/onc.2010.265. [DOI] [PubMed] [Google Scholar]
- 129.Massimi P, et al. Regulation of the hDlg/hScrib/Hugl-1 tumour suppressor complex. Exp Cell Res. 2008;314:3306–3317. doi: 10.1016/j.yexcr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 130.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 131.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ivanov AI, et al. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Mol Vis. 2005;11:1183–1199. [PubMed] [Google Scholar]
- 134.Yoshihara K, et al. Phosphorylation state regulates the localization of Scribble at adherens junctions and its association with E-cadherin-catenin complexes. Exp Cell Res. 2011;317:413–422. doi: 10.1016/j.yexcr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Iizuka-Kogo A, Ishidao T, Akiyama T, et al. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development. 2007;134:1799–1807. doi: 10.1242/dev.02830. [DOI] [PubMed] [Google Scholar]
- 136.Wong EWP, Mruk DD, Lee WM, et al. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong EWP, Sun S, Li MWM, et al. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Su L, Mruk DD, Lee WM, et al. Drug transporters and blood-testis barrier function. J Endocrinol. 2011;209:337–351. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]


