Abstract
Background
Bronchial thermoplasty (BT) has previously been shown to improve asthma control out to 2 years in patients with severe persistent asthma.
Objective
To assess effectiveness and safety of BT in asthma patients 5 years post therapy.
Methods
BT-treated subjects from the Asthma Intervention Research 2 (AIR2) Trial (ClinicalTrials.gov NCT01350414) were evaluated annually for 5 years to assess long-term safety of BT and durability of treatment effect. Outcomes assessed post-BT included severe exacerbations, adverse events, healthcare utilization, spirometry data, and high resolution computed tomography (HRCT) scans.
Results
162/190 BT-treated subjects (85.3%) from the AIR2 Trial completed 5 years of follow-up. The proportion of subjects experiencing severe exacerbations and Emergency Room visits, and the rates of events in each of years 1 to 5 remained low and were less than those observed in the 12 months prior to BT treatment (average 5 year reduction in proportions: 44% for exacerbations and 78% for ER visits). Respiratory adverse events and respiratory-related hospitalizations remained unchanged in Years 2 through 5 as compared to the first year after BT. Pre-BD FEV1 values remained stable between years 1 and 5 after BT, despite a 17% reduction in average daily inhaled corticosteroid dose. HRCT scans from baseline to 5 years after BT showed no structural abnormalities that could be attributed to BT.
Conclusions
These data demonstrate the 5-year durability of the benefits of BT with regard to both asthma control (based on maintained reduction in severe exacerbations and ER visits for respiratory symptoms) and safety. BT has become an important addition to our treatment armamentarium and should be considered for patients with severe persistent asthma who remain symptomatic despite taking ICS (inhaled corticosteroids) and LABA (long-acting-β2-agonists).
Keywords: Bronchial thermoplasty, asthma, Bronchoscopic procedure, Alair System, asthma exacerbation
Introduction
Over 23 million people in the United States suffer from asthma1, 2. Approximately 5% of patients have severe persistent asthma and continue to experience asthma symptoms despite treatment with current state-of-the-art medications3. Poorly controlled and “not-well controlled” asthma remain a significant social and economic burden,2, 4 and lead to increased healthcare utilization with negative impacts on patients' quality of life.
Bronchial Thermoplasty (BT) is a non-pharmacologic treatment for asthma that has been shown to result in significant improvements in a number of asthma control measures in three randomized clinical trials in patients with moderate-to-severe, persistent asthma5-7. The Asthma Intervention Research 2 (AIR2) Trial, a double-blind, sham-controlled, randomized clinical trial of BT in patients with severe asthma, showed a 32% reduction in severe exacerbations, an 84% reduction in emergency room (ER) visits due to respiratory symptoms, a 73% reduction in hospitalizations for respiratory symptoms, and a 66% reduction in time lost from work/school/other daily activities due to asthma symptoms compared to a sham-treated group in the year following the BT treatment period (day of first BT procedure until 6 weeks after the last bronchoscopy, approximately 12 weeks)7. We previously reported safety out to 5 years in moderate-to-severe persistent asthma through extended follow-up of 45/52 BT-treated subjects (86.5%) in the AIR Trial8. Safety and durability of the treatment effect (reduced severe exacerbations and ER visits for respiratory symptoms) were previously reported out to 2 years post-BT in subjects with severe persistent asthma in the AIR2 Trial9. We now describe the long-term safety and durability of BT out to 5 years post-treatment in 162/190 subjects from the AIR2 Trial.
Methods
Study procedures
BT group subjects in the AIR2 Trial were followed to 5 years. The study population and design of the AIR2 Trial have been published7. Data that were collected during the 5-year follow-up and episodes of severe exacerbations were analyzed using a non-inferiority approach to demonstrate that the benefit of BT in the year after the procedure was maintained in each of the subsequent years out to 5 years (ClinicalTrials.gov number, NCT01350414).
Upon completion of the Year 1 evaluation in the AIR2 Trial, subjects in the BT group were instructed to maintain their use of controller medications (unless changes were medically indicated as determined by the investigator) and were contacted via telephone every 3 months. Information on adverse events (AEs – defined as any sign, symptom, illness, clinically significant abnormal laboratory value, or other adverse medical event that appeared or worsened in a patient during the clinical study, regardless of whether or not it is considered related to the procedure used as part of the protocol), hospitalizations, ER visits for respiratory symptoms, and new or increased dosages of oral corticosteroids (OCS) for worsening of asthma symptoms were collected via a specific set of questions. An in-office evaluation was performed annually at Years 2, 3, 4, and 5 at which time the same questions as above were posed and a physical examination and pre- and post-bronchodilator spirometry were performed. Severe exacerbations, ER visits, and hospitalizations for the year prior to BT were subject-reported. One-hundred (100) subjects in the BT group who had a HRCT scan at baseline and Year 1 underwent repeat HRCT scan at Years 3 and 5.
Evaluation Periods
In order to facilitate a comparison of the durability of treatment effect over matched periods of time, the post-treatment evaluation period for the purposes of these analyses consisted of 52 week windows beginning at 6 weeks after the last BT bronchoscopy. An additional analysis of the annualized rate of exacerbations and emergency room visits beginning at the time of randomization, including the three bronchoscopies, was also performed.
Evaluation of HRCT
Baseline and Year 5 follow-up HRCT images for 93 evaluable pairs were read by an independent pulmonary radiologist who was blinded to time point (Baseline or Year 5) (J.H.M.A, over 33 years of thoracic CT experience). Upon completing this assessment, the radiologist was unblinded and assessed whether findings in follow-up images were new observations, improvements from baseline, or deteriorations from baseline. The radiologist's findings were reviewed by an independent pulmonologist (N.N.J, 24 years of pulmonology experience) who attributed a clinical significance to each finding based on subject information including lung function and AE profiles, as well as occurrence and timing of respiratory events and severe exacerbations.
Statistical Analyses
All statistical processing was performed using SAS® software Version 9.1.
Severe Exacerbations
Point estimates and 95% confidence intervals for the proportion of subjects (number of subjects with events over the total number of subjects evaluated in the period) experiencing severe exacerbations during each of the 12-month evaluation periods were calculated. The definition of severe exacerbations was derived from the definition originally utilized in the parent trial7 and consisted of treatment with oral or intravenous corticosteroids, OR a doubling of the baseline inhaled corticosteroid [ICS] dose for at least 3 days, OR any temporary increase in the dosage of OCS for subjects taking maintenance OCS at entry into the AIR2 Trial. Additionally, the upper 95% confidence limit for the difference in proportions between 12-month follow-up periods and the first year was calculated. A non-inferiority margin of 20% was used to demonstrate that the proportions were not substantially worse during each of the subsequent evaluation periods (i.e. the upper 95% confidence limit for the difference in proportions is less than 20%). The number of subjects who completed follow-up visits for each particular year was used as the denominator to calculate the proportions of subjects with severe exacerbations during each year. No imputations were made for missing data. A subject that terminated during the follow-up was still counted in those years that the subject provided data.
Hospitalizations and ER Visits for respiratory symptoms
Descriptive statistics with 95% CI were tabulated for the event rates (events/subject/year) and the proportions of subjects experiencing respiratory adverse events, ER visits for respiratory symptoms, and hospitalizations for respiratory symptoms for each 12-month period starting 6 weeks after the last treatment bronchoscopy.
Maintenance medications
Changes in the ICS dose from baseline to Year 5 were analyzed with the Sign test. Medication change was defined as an increase or decrease of 50% or more in daily dosage.
Sub-Group Analyses
Responder analysis based on improvements in the Asthma Quality of Life Questionnaire (AQLQ) scores at Year 1 following BT in this group showed that 79% of the subjects achieved a minimally important difference (MID) of 0.5 or more. In the absence of a control group during the long-term follow-up, key parameters were evaluated for the responders (subjects achieving an AQLQ score change of ≥0.5) and non-responders (subjects not achieving an AQLQ score change of ≥0.5).
Ethics
Written, informed consent was obtained from all participating subjects after the AIR2 Trial was approved by the Institutional Review Boards/Ethics Committees at each participating institution. The study was conducted in accordance with the principles of the Declaration of Helsinki (200410, 200811).
Results
Of the 190 subjects who underwent BT treatment in the AIR2 Trial, 162 subjects (85.3%) completed the 5 year follow-up. The number of BT subjects completing annual follow-up at Years 1, 2, 3, 4, and 5 was 181, 165, 162, 159, and 162, respectively. Twenty-eight (28) BT subjects (14.7%) did not complete the Year 5 evaluation (18 were lost to follow-up, 4 were withdrawn by the investigators (terminal illness: 1; non-compliance with physician instructions: 3), 5 were withdrawn for nonmedical reasons, and 1 died in a motor vehicle accident). Four subjects missed the Year 4 visit, but remained in the study.
Demographics and Clinical Characteristics
The baseline demographics and clinical characteristics of the 190 subjects enrolled in the BT group in the AIR2 Trial, the 162 subjects completing follow-up at 5 years, and the 28 subjects who did not complete follow-up at 5 years are summarized in Table 1. There was no difference in baseline characteristics between the subjects completing the 5 year follow-up compared to the subjects not completing follow-up at 5 years, or the original cohort of 190 subjects at enrollment, except for age, with the cohort not completing follow up at year 5 being younger (p=0.019). At Baseline, 32% of the subjects were not-well controlled and 68% were poorly controlled according to the NAEPP EPR-3 (2007) guidelines despite their maintenance asthma medication.
Table 1. Demographics and Clinical Characteristics.
| All BT Subjects at Baseline (n= 190) | BT Subjects completing 5 Year Follow-up (n= 162) | BT Subjects Not completing 5 Year Follow-up (n= 28) | |
|---|---|---|---|
| Age (years) | 40.7 ± 11.9 | 41.5 ± 11.8 | 35.8 ± 11.3d |
|
| |||
| Gender | Male: 81 (42.6%) | Male: 68 (42.0%) | Male: 13 (46.4%) |
| Female: 109 (57.4%) | Female: 94 (58.0%) | Female: 15 (63.6%) | |
|
| |||
| Race | |||
| Caucasian | 151 (79.5%) | 134 (82.7%) | 17 (60.7%) |
| African American/Black | 19 (10.0%) | 13 (8.0%) | 6 (21.4%) |
| Hispanic | 6 (3.2%) | 4 (2.5%) | 2 (7.1%) |
| Asian | 4 (2.1%) | 3 (1.9%) | 1 (3.6%) |
| Other | 10 (5.3%) | 8 (4.9%) | 2 (7.1%) |
|
| |||
| Weight (kg) | 81.7 ± 18.4 | 81.4 ±17.1 | 83.4 ± 24.6 |
|
| |||
| ICS Dose (μg)a | 1960.7 ± 745.2 | 1958.9 ±757.9 | 1900 ± 551.6 |
|
| |||
| LABA Dose (μg)b | 116.8 ± 34.4 | 120.8 ± 47.7 | 108.9 ± 23.8 |
|
| |||
| Symptom-Free Days (%) | 16.4 ± 24.0 | 16.1 ± 24.1 | 18.4 ± 24.1 |
|
| |||
| Asthma Control Questionnaire (ACQ) Score | 2.1 ± 0.87 | 2.1 ± 0.84 | 2.3 ± 1.02 |
|
| |||
| Asthma Quality of Life Questionnaire (AQLQ) Score | 4.30 ± 1.17 | 4.32 ± 1.17 | 4.23 ± 1.16 |
|
| |||
| Emergency Room Visits for Respiratory Symptoms in prior 12 months c No. Events (No. Subjects) | 141 (55) | 115 (47) | 26 (8) |
|
| |||
| Hospitalizations for Respiratory Symptoms in prior 12 months c No. Events (No. Subjects) | 10 (8) | 10 (8) | 0 (0) |
|
| |||
| Seasonal allergies (n[%])c | |||
| Yes | 103 (54.5%) | 85 (52.8%) | 18 (64.3%) |
| No | 86 (45.5%) | 76 (47.2%) | 10 (35.7%) |
|
| |||
| Lung Function Measures | |||
|
| |||
| Pre-bronchodilator FEV1 | 77.8 ± 15.65 | 77.8 ± 15.84 | 78.0 ± 14.75 |
|
| |||
| Post-bronchodilator FEV1 | 86.1 ± 15.76 | 85.9 ± 15.83 | 87.1 ± 15.57 |
|
| |||
| Morning PEF (L/min) | 383.8 ± 104.3 | 380.9 ± 106.0 | 400.7 ± 93.8 |
|
| |||
| Methacholine PC20 (mg/ml) Geometric mean [range] | 0.27 [0.22, 0.34] | 0.27 [0.21, 0.35] | 0.29 [0.15, 0.54] |
Definition of abbreviations: BT = Bronchial Thermoplasty; ICS = Inhaled Corticosteroid; LABA = Long-Acting β2-Agonist; PEF = Peak Expiratory Flow; FEV1 = Forced Expiratory Volume in 1 second Values are mean ± SD except when indicated otherwise.
Beclomethasone or equivalent;
Salmeterol or equivalent;
Patient reported;
p=0.019 comparing subjects completing 5 year follow-up versus subjects not completing 5 year follow-up (t-test)
Treatment Parameters
The average number of activations (± standard error of the mean) for the 3 treatment procedures were 44 ± 1.2 (Procedure 1, right lower lobe), 47 ± 1.2 (Procedure 2, left lower lobe), and 60 ± 1.6 (Procedure 3, both right and left upper lobes) with coverage of all accessible airways between 3 and 10mm in diameter. For the 162 patients that completed follow up at 5 years, the total number of activations for the 3 procedures was 151.
Severe Exacerbations
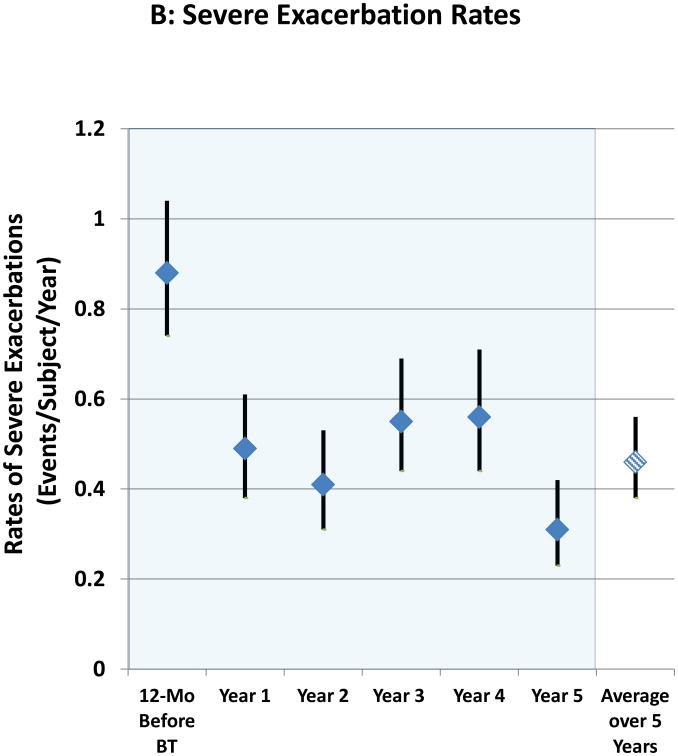
The proportion of subjects experiencing severe exacerbations (>97% of which were based on systemic corticosteroid administration) in each of Years 1 to 5 are shown in Figure 1A with the period constituting Year 1 beginning at 6 weeks after the last BT bronchoscopy. The proportion of subjects having severe exacerbations in each subsequent year (Years 2, 3, 4, and 5) compared to the first year after BT were not significantly different. In addition, the reduction in proportion of subjects experiencing severe exacerbations in the year following BT (30.9%) compared to the 12 months before BT (51.6%) was maintained for the entire 5 year follow up period with an average decrease of 44% over this period. Matched pairs analysis comparing the 162 subjects completing the Year 5 evaluations to the same group in previous years showed similar proportion of subjects having a severe exacerbation in Years 1-5 (30.9%, 23.5%, 34.0%, 36.4%, and 21.6%, respectively), representing a persistent reduction compared to the 12 months before BT when 53.1% of subjects experienced one or more exacerbations. The decrease in severe exacerbation rates that was achieved in the post-treatment period following BT in Year 1 was maintained out to 5 years (Figure 1B). Compared to the 12 months prior to BT treatment, the average reduction over 5 years in the rate of severe exacerbations was 48%. The upper 95% confidence limit for the difference in percentages for Years 2, 3, 4, and 5 compared to Year 1 (Subsequent Year – Year 1) was 0.5, 11.3, 14.0, and -1.6, respectively. All were less than the pre-defined non-inferiority margin of 20%. The rates of severe exacerbation during Years 2 through 5 were also low when compared to the annualized exacerbation rate during the approximately 64-week “Year 1” period that included both the treatment period (approximately 12-week period from the first bronchoscopy until 6 weeks after the third bronchoscopy) and post-treatment period (52-week period beginning 6 weeks after the last bronchoscopy) (online supplement, Figure 1).
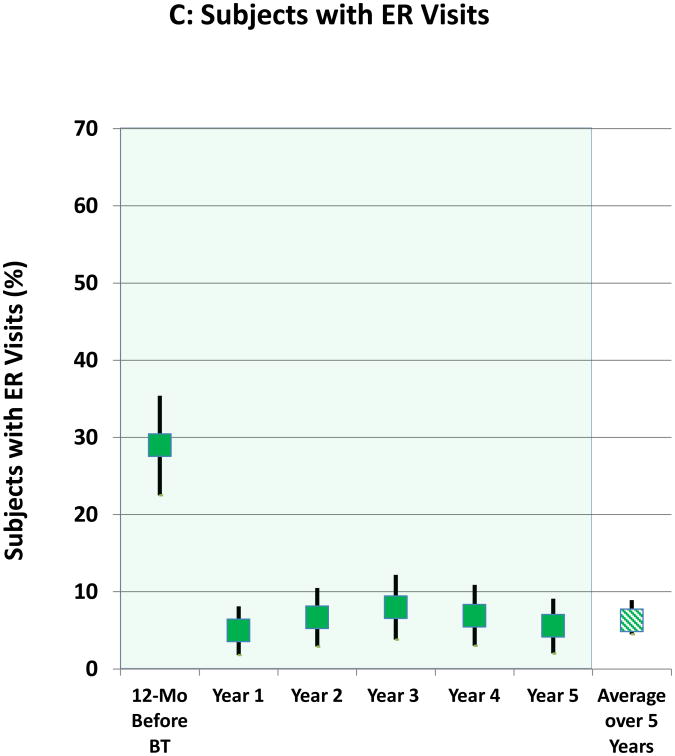
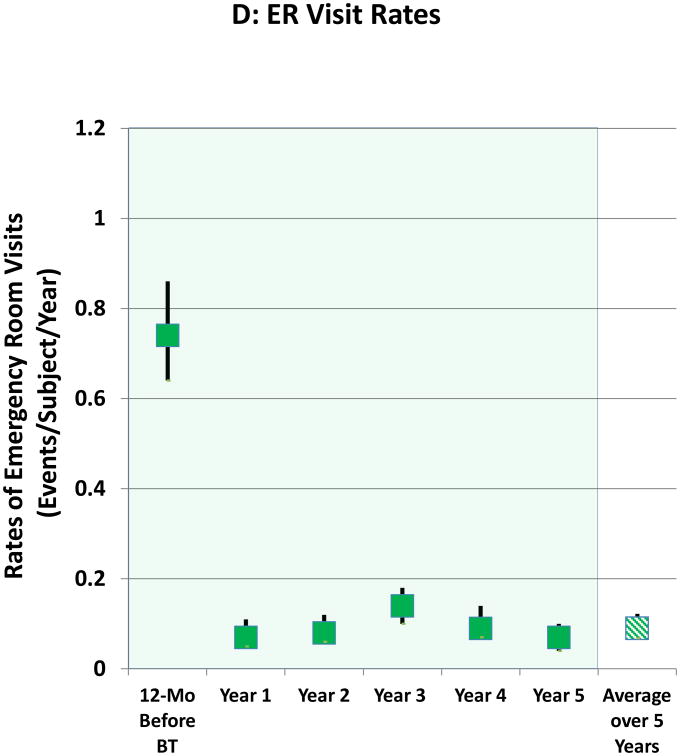
Figure 1. Severe Exacerbations and Emergency Room Visits In the 5 Years Following BT.
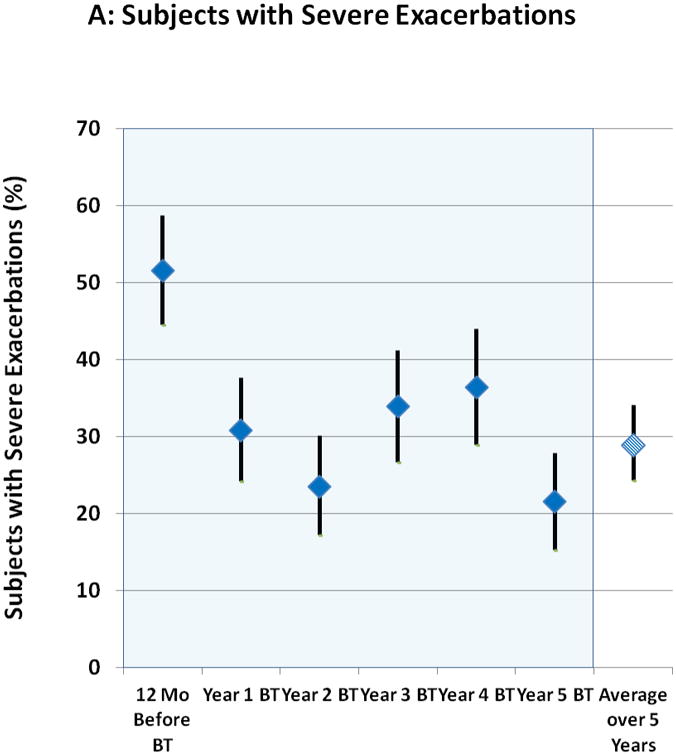
A: Proportion of subjects with severe exacerbations; B: Severe exacerbation rates; C: Proportion of subjects with Emergency Room visits for respiratory symptoms; D: Emergency Room visit rates. Values are point estimates with 95% upper and lower CIs. The 365 day period constituting Year 1 began at 6 weeks after the last BT bronchoscopy.
There was no difference in the average proportion of subjects experiencing severe exacerbations over 5 years between subjects reporting seasonal allergy (29.3%) versus those with no allergies (29.5%). On average, patients with both FEV1 60-70% predicted as well as those with FEV1 >70% predicted had sustained improvements in exacerbations over the 5 year period (data not shown).
Safety
The proportions of subjects having ER visits for respiratory symptoms and the yearly rates of ER visits over the 5 years after BT are shown in Figures 1C and 1D, respectively. The decrease in the proportion of subjects experiencing ER visits for respiratory symptoms that was achieved following BT in Year 1 was maintained out to 5 years. Compared to the 12 months before BT, the average reduction over the 5 years in proportion of subjects having ER visits for respiratory symptoms was 78%. The decrease in rates of ER visits that was achieved following BT in Year 1 was maintained out to 5 years (Figure 1D). Compared to the 12 months prior to BT treatment, the average reduction over 5 years in the rate of ER visits was 88%. The rates of ER visits during Years 2 through 5 were lower when compared to the annualized rate of the approximately 64-week Year 1 period that included both the treatment period (approximately 12-week period from the first bronchoscopy until 6 weeks after the third bronchoscopy) and post-treatment period (52-week period beginning 6 weeks after the last bronchoscopy)-(online supplement, Figure 1).
The proportion of subjects experiencing any respiratory AEs, asthma (multiple symptoms) AEs, and hospitalizations for respiratory symptoms did not increase over 5 years (online supplement Table 1). The reduction in respiratory adverse events and asthma (multiple symptoms) adverse events that was observed at one year persisted through the 5 years of follow-up, with no increase in rate from years 1 through 5 (online supplement Table 1). Respiratory adverse events that occurred at an incidence rate of ≥3.0% of subjects in any of the years 1 through 5 included sinusitis, asthma (multiple symptoms), bronchitis, cough, lower respiratory tract infections, influenza, nasopharyngitis, pneumonia, rhinitis, upper respiratory tract infections, and wheezing. There were no incidences of pneumothorax, intubation/mechanical ventilation, cardiac arrhythmias, or death as a result of BT treatment over the 5 years of follow-up. The proportion of subjects experiencing hospitalization and the rate of hospitalization for respiratory symptoms was low at baseline and remained unchanged over the 5 years after BT.
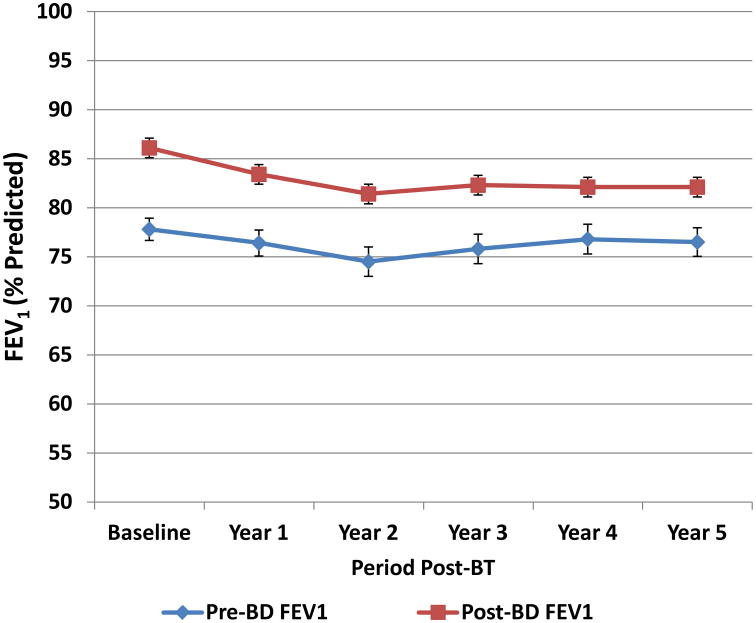
Lung Function
The percent predicted pre-bronchodilator FEV1 values remained unchanged over the 5 years after BT and bronchodilator responsiveness was maintained over the 5 years after BT (Figure 2).
Figure 2. Pre- and Post-bronchodilator FEV1 over 5 Years (% Predicted).
Percent predicted pre- and post-bronchodilator FEV1 values (mean ± SEM) for subjects completing follow-up during each year. The percent predicted pre-bronchodilator FEV1 values remained unchanged over the 5 years after BT and bronchodilator responsiveness was maintained over the 5 years after BT.
Maintenance Medication Changes
At baseline, 116 of the 162 subjects (72%) who completed evaluations at 5 years were prescribed 2 maintenance asthma medications (i.e. high dose ICS (>1000μg beclomethasone equivalent) + LABA), and 45 of the 162 subjects (28%) were prescribed 3 or more maintenance asthma medications. At 5 years following BT, 27% of subjects (44/162) had decreases of 50% or more of their ICS maintenance medications, with half of this group (21/162) having reduced their daily ICS dose to equal to or less than 500 μg/day beclomethasone equivalent. Nine of the 162 subjects (5%) subjects had an increase of 50% or greater of their ICS maintenance medications. Of those subjects with changes in ICS doses of 50% or greater, significantly more subjects had a decrease compared to those with an increase (p<0.001). There was an overall reduction of 17% in the average ICS dose at 5 years. Twenty of the 162 subjects (12%) were completely weaned off LABA, 9% (15/162) were weaned off ICS and LABA maintenance medications, and 7% (12/162) were no longer taking any maintenance asthma medications.
High Resolution Computed Tomography
Of the 93 evaluable HRCT pairs at Year 5, 82% showed either no radiological changes or improvement from baseline. At 5 years post-BT as compared to baseline, 71% of the HRCT pairs showed no radiologic changes of clinical significance. A similar proportion of subjects had improvements or deteriorations of clinical significance (improvements in 14% and deteriorations in 15%) and represented predominantly changes in gas trapping, bronchial wall thickening, or consolidation. Over the 5 year period, 3 subjects (3%) were noted to have increased or new bronchiectasis: one involved worsening of pre-existing bronchiectasis; one with mild bronchiectasis in two lobes, including the right middle lobe that had not been treated with BT; and one subject with newly identified bronchiectasis by HRCT at 3 years. Unfortunately, no 5 year HRCT for this subject was obtained, but the subject was clinically stable. There was no evidence of bronchial stricture, bronchiolitis obliterans or new pulmonary emphysema in any of the HRCT pairs evaluated at Year 5.
Sub-Group Analyses
The event rates (events/subject/year) averaged over Years 2 through 5 were higher in the non-responders compared to the responders: severe exacerbations, 0.720 versus 0.389; respiratory AEs, 1.487 versus 1.012; asthma (multiple symptoms) AEs, 0.745 versus 0.376; ER visits for respiratory symptoms, 0.214 versus 0.068; and hospitalizations for respiratory symptoms, 0.079 versus 0.051, respectively (online supplement Table 2).
Discussion
In this study, we examined the long-term follow-up of patients that underwent BT in the AIR2 Trial7 via an open-label observation of post-therapy events. Previously published data have demonstrated the persistent benefits of BT out to 2 years in patients with severe persistent asthma9. This study demonstrates an improvement in asthma control as measured by a maintained reduction in the proportion of subjects experiencing severe exacerbations that persists out to at least 5 years post-BT. There was minimal loss to follow up with 85.3% of subjects completing the evaluations at Year 5. A 44% average reduction in the proportion of subjects experiencing severe exacerbations over a 5-year period may be associated with a substantial reduction in the use of systemic corticosteroids in these patients and provide a meaningful improvement in quality of life.
Consistent with the persistent reduction in severe exacerbations, the data also demonstrate a persistent reduction in ER visits for respiratory symptoms, with an average decrease in the proportion of subjects with ER visits over 5 years of 78% compared to the 12 months before BT. The absence of an increase in respiratory AEs and asthma (multiple symptoms) AEs over a 5 year period provides further support for the long-term effectiveness of BT. These improvements with BT were noted in the presence of reduced use of maintenance medications. Collectively, these data raise the possibility that BT may be a disease modifying therapy. Further work will be needed to test this intriguing hypothesis.
The safety of BT over the long-term is supported by the absence of any decline in lung function (no deterioration of FEV1), the lack of increase from the low baseline rate of hospitalizations, and the absence of any significant structural changes in the airways (from HRCT review) over the course of 5 years of follow-up. These data confirm the previously established safety profile6-9, 12-14.
The potential for a transient increase in AEs (including severe exacerbations) around the time of BT procedures compared with sham-control subjects7 should be considered in seeking to achieve a sustained improvement in asthma control defined by maintained reduction in severe exacerbations and ER visits out to at least 5 years following BT. The long-term benefits of BT, including a reduction in severe exacerbations and ER visits reported here, are consistent with the stated goals of asthma control as defined by the National Asthma Education and Prevention Program (NAEPP)15. Unlike other currently available therapies for asthma, BT appears to provide long term (years) asthma control for many patients following a one-time treatment comprising three procedures. Physicians must consider these short-term risks of the procedure along with the long-term safety and efficacy described here to assess the appropriateness of this therapy for their individual patients.
Follow-up out to 5 years in this large cohort of severe asthma subjects treated with BT addresses many concerns previously expressed regarding long-term safety of this novel therapy. Furthermore, the stable lung function as assessed by FEV1 over 5 years and the absence of any unexpected structural alterations in HRCT scans in 93 matched HRCT pairs from subjects with severe asthma evaluated at 5 years is reassuring and consistent with findings previously reported in subjects with mild to moderate asthma following BT12. The observed radiological improvements or deteriorations of gas trapping, bronchial wall thickening, or consolidation at 5 years following BT represent findings that are commonly associated with severe asthma and are often temporary and transient in nature16, 17, and have been shown in cross-sectional surveys to correlate with indicators of airway obstruction by spirometry and lung volume measurements18. The 3 cases of bronchiectasis are of particular interest; one had existing bronchiectasis which would currently be considered a contraindication to BT treatment, the second case developed bronchiectasis in the lingula and the untreated right middle lobe making a cause and effect relationship with BT treatment unlikely. The third case represents the only case of bronchiectasis that is theoretically possibly related to BT treatment within the study population. Gupta et al19 have previously reported a baseline prevalence of bronchiectasis in asthma of ∼31% when compared to healthy controls (∼12.5%), so it is not clear in the present cases if the development of bronchiectasis is due to the underlying severe asthma or BT. The approximate incidence of less than 0.2% per annum in the present study is reassuring and suggests that BT does not cause bronchiectasis. While the main purpose of this study was to assess long term (5-year) durability and safety follow-up in a cohort of patients who underwent BT, as in other long term studies of therapies for severe asthma, a limitation of this study is the lack of sham-control group beyond one year, including the lack of HRCT scans for the sham-group beyond one year. Collecting meaningful 5-year study data without confounding would have required maintaining the study blind for the entire 5-year period in both treatment and sham groups and this was felt to be unethical in this study population. On the other hand, maintaining sham patients in the follow-up study after breaking the blind and requiring them to continue the same treatment regimen despite poor control was deemed neither ethical nor practical and likely to result in poor patient retention, thus leading to further difficulty in study result interpretation due to missing data and confounding. Because of these concerns, the sham group exited the study at the end of the first year and was not followed in the long-term extension study
While comparison to historical controls has not been possible due to the lack of studies with long-term (greater than one year) follow-up of patients with severe asthma on current standard-of-care therapy, one potential approach to address this in the present study was to compare the outcomes of those subjects who improved post-BT and those that did not improve. The analysis of the data for responders and non-responders following BT treatment (based on an AQLQ score improvement of ≥0.5 (responders) and <0.5 (non-responders)) provided insight into the subsequent course of these two groups and is consistent with previously published literature suggesting AQLQ is linked with healthcare utilization20; over the 5 years of follow-up, severe exacerbation rates, respiratory AE rates, asthma (multiple symptoms) AE rates, and rates of ER visits and hospitalizations for respiratory symptoms remained higher in the non-responders compared to the responders.
Despite the absence of the sham-control group comparison at 5 years, the present data are meaningful as the benefits in the BT group demonstrated in the first year after BT were maintained at 5 years. The effects of BT that were reported for the first year after the treatment were based on a mean of 151 total number of activations for the full treatment7 and were not different for the 162 subjects that completed follow-up at 5 years. It has not been possible to demonstrate a dose response that defines a minimal number of activations that may be necessary for producing an effect at one year. The intent of bronchial thermoplasty remains to treat all accessible airways reachable by the bronchoscope and therefore activations will vary by patient airway anatomy.
The question of phenotyping to define responders cannot be addressed from the present data as assessments of exhaled nitrous oxide (FeNO), sputum eosinophils, or other biomarkers were not performed at baseline as part of the AIR2 Trial. However, there was no difference in outcomes based on the subjects' self-reported allergy status (allergic versus non-allergic). Describing the phenotypes that benefit most from this therapy remains an area of considerable interest. BT may benefit a heterogeneous group of patients with severe asthma who remain symptomatic despite standard care. These patients are identified at Steps 5 or 6 of the NAEPP guidelines15 by the need for high dose inhaled corticosteroids and long-acting beta-agonists but who are still experiencing break through asthma symptoms. Although patients in this study were reasonably stable (i.e. FEV1>60%, no more than 3 hospitalizations in the prior year and 8 or fewer puffs of rescue medications per day on average) and able to undergo bronchoscopy, the experience of the patients with severe refractory asthma in the RISA Trial5 (i.e. no limit on previous hospitalizations or rescue medication use) provides assurance that more severe patients may also benefit from BT.
These data demonstrate that BT is an effective and safe therapy. The improvements in asthma control in the post-treatment period at one year based on reduction in severe exacerbations and ER visits compared to the Sham control group7 are maintained for at least 5 years in the BT group of patients with severe persistent asthma. A single BT treatment comprising 3 procedures provides long-term benefit to at least 5 years. Whether BT is a disease-modifying therapy will depend upon results of future appropriately designed clinical studies. BT has become an important addition to our treatment armamentarium for patients with severe persistent asthma who remain symptomatic despite taking ICS and LABA.
Supplementary Material
Online Supplement Figure 1: Annualized severe exacerbation rates (top panel) and emergency room visit rates (bottom panel) in the AIR2 Trial over the 5 year evaluation periods. The data for Year 1 in these figures for both the Sham (white bars) and BT groups (blue bars) are standardized to 52 weeks. The solid bars in both graphs represent the Post-Treatment Period, and the hashed bars represent the Treatment Period.
Online supplement Table 1s: Respiratory Adverse Events, Asthma (multiple symptoms) AEs, and Hospitalizations In the 5 Years Following BT
Online supplement Table 2s: Event Rates: Responders versus Non-Responders (Events/Subject/Year)
Clinical Implications.
With 5 years of data demonstrating safety and durability of effect, bronchial thermoplasty should be considered for severe persistent asthma patients who remain symptomatic despite taking inhaled corticosteroids and long-acting-β2-agonists.
*.
Members of The AIR2 Trial Study Group were as follows: Australia: Royal Adelaide Hospital: M. Holmes, H. Jersmann, P. Robinson, H. Greville. L. Milazzo, J. McGrath; Sir Charles Gairdner Hospital: M. Phillips, C. Read, K. Broughton; Brazil: Irmandade Santa Casa de Misericórdia de Porto Alegre: A.S. Rubin, M. Cavalcanti, P. Soares, F. Spilimbergo; Hospital São Lucas da PUCRS: J. Fiterman, F. Kahan, C. Reck, D. Cavalet Blanco; Faculdade da Medicina do ABC: E. Fiss, M. S. Lapa, MA Neis, E Felix, CR Gomes, Jr., N Cristina, S. Squassoni; Hospital Universitario Clementino -Fraga Filho: J.R. Lapa e Silva, M. d Andrade Lima, L de Carvalho Rodrigues; Canada: Hospital Laval, Laval University: M. Laviolette, L-P. Boulet, L. Trėpanier; Montreal Chest Institute: R. Olivenstein, J. Bourbeau, C. Fugere; St. Joseph's Healthcare, Hamilton: G. Cox, G. Travis, S. Goodwin; the Netherlands: Universitair Medisch Centrum Groningen: N.H.T. ten Hacken, DJ. Slebos, K. Klooster; UK: Wythenshawe Hospital: R. Niven, T. Pickering, G. Fletcher; Gartnavel General Hospital, University of Glasgow: N. Thomson, S. Bicknell, M. Spears, R. Chaudhuri, J. Lafferty; Glenfield General Hospital: I. Pavord, J. Beer, N. Goodman, A. Charalambou; Chelsea &Westminster Hospital: P.L. Shah, S. Singh, D. Lai, G. Davies, C. Caneja; Birmingham Heartlands Hospital: A.H. Mansur, L. Webber; United States: University of Pennsylvania Health System: D. Sterman, M. Sims, B. Russell; Henry Ford Medical Center: M. Simoff, E. Zoratti, J. Diaz, C. Ray, R-Rolando Almario; University of Chicago: I. Noth, K. Hogarth, M. Strek, C. Brown; Duke University Medical Center: M. Kraft, D. Beaver; Baylor College of Medicine: N. Hanania, P. Alapat, M. Atik; Brigham and Women's Hospital, Harvard Medical School: M. Wechsler (now at National Jewish Health), E. Israel, K. Zheng; Pulmonary Associates of Northern Virginia: D. Duhamel, J. Hales, S. Zimmet, M. Obeid; Washington University School of Medicine: K. Sumino, M. Castro, J. Tarsi, T. Koch; Cleveland Clinic Foundation: S. Erzurum, A. Mehta, T. Gildea, M. Aronica, D. Culver, R. Dweik, D. Laskowski, E. Cleggett; Keck School of Medicine of the University of Southern California: R. Barbers, A. Baydur; University of Iowa: J. Kline, K. Sprenger, J. Keating; Swedish Medical Center: B. Louie, E. Vallieres, R. Aye, S. McHugh, D. Iriarte, J. Gorden; HealthPartners Specialty Center: C. McEvoy, K. Graven, A. Adams, K. Ham, N. Woodruff; Veritas Clinical Specialties: W. Leeds, S. Roeder, L. Ludlow; Statisticians - QST Consultations, Ltd: J. Quiring, B. Armstrong
The authors are grateful to Dr. Nizar Jarjour (N.N.J., Division of Pulmonary and Critical Care Medicine, University of Wisconsin, Madison, Wisconsin) for reviewing all the HRCT observations to determine the clinical significance of any radiologic findings.
Database Management and Data Analysis: The database for this study was managed by, and all statistical analyses were performed by Brian Armstrong, MS and John Quiring, PhD (QST Consultations, Ltd., Allendale, MI).
Data and Safety Monitoring Board — William Busse, MD, Robert Schellenberg, MD, Scott Berry, PhD, and Arthur S. Slutsky, MD (Chair)
Role of Sponsor: The sponsor (Boston Scientific Corporation) was responsible for the conception and design of the study and the analysis of the data. The sponsor's role in the development of the manuscript is as described for the author contributions.
Sponsored by: Boston Scientific Corporation, Sunnyvale, CA
Abbreviations List
- AEA
Adverse Event
- AIR2
Asthma Intervention Research 2
- AQLQ
Asthma Quality of Life Questionnaire
- BD
Bronchodilator
- BT
Bronchial Thermoplasty
- CI
Confidence Interval
- ER
Emergency Room
- FEV1
Forced Expiratory Volume in 1 second
- HRCT
High Resolution Computed Tomography
- ICS
Inhaled Corticosteroid
- LABA
Long-acting beta-agonist
- MID
Minimal Important Difference
- PEF
Peak Expiratory Flow
- OCS
Oral Corticosteroids
Author contributions
Dr. Wechsler had full access to the data and vouches for the integrity of the data and the accuracy of the data analysis, including adverse events.
Dr. M.E. Wechsler: contributed to the acquisition and interpretation of the data, writing and critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M. Laviolette: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. AS Rubin: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. J Fiterman: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. JR Lapa: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. P Shah: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. E Fiss: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. R Olivenstein: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. NC Thomson: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. R Niven: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. I Pavord: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M Simoff: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. J Hales: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. C McEvoy: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. DJ Slebos: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M Holmes: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M Phillips: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. S Erzurum: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. N Hanania: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. K Sumino: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M Kraft: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. G Cox: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. D Sterman: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. K Hogarth: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. J Kline: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. A Mansur: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. B Louie: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. W Leeds: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. R Barbers: contributed to the acquisition and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. J Austin: contributed to the conception and design of HRCT evaluations, performed the review of all the HRCTs, and contributed to the analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. N Shargill: contributed to the conception and design of the study, interpretation of the data, writing and critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. J Quiring: contributed to the conception and design of the study, analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Mr. B Armstrong: contributed to the conception and design of the study, analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published.
Dr. M Castro: contributed to the conception and design of the study, interpretation of the data, writing and critical revision of the article for important intellectual content, and final approval of the version to be published.
The authors have reported the following conflicts of interest
Dr. M.E. Wechsler: In the past 3 years, MEW's institution has received financial support for industry sponsored clinical trials from Asthmatx, GlaxoSmithKline and Cephalon, and he has received compensation for consulting and/or speaking fees from Asthmatx, Boehringer Ingelheim, Boston Scientific, Cytos, GlaxoSmithKline, Merck, NKT Therapeutics, Novartis, Sunovion and Teva.
Dr. M. Laviolette: In the past 3 years, ML has received financial support for industry-sponsored clinical trials from AstraZeneca, GlaxoSmithKline, Centocor and Boston Scientific Corporation, and $3,000 from Merck Frosst in lecture fees and for attending an international meeting.
Dr. AS Rubin: In the past 3 years, ASR has undertaken research funded by Novartis, Boheringer Ingelheim, Takeda, Intermunne, Europharma and Gilead, has participated in continuing medical education activities sponsored in whole or in part by GlaxoSmithKline, AstraZeneca, Novartis, Boheringer Ingelheim and Ache, and has participated in advisory boards for Boheringer Ingelheim.
Dr. J. Fiterman: In the past 3 years, JF has undertaken research funded by Cephalon, Novartis and Takeda, has participated in continuing medical education activities sponsored in whole or in part by AcrE, Takeda and Novartis.
Dr. JR Lapa e Silva: In the past 3 years, JRLS has undertaken research funded by National Institute of Health (NIH), Brazilian Research Council (CNPq), Rio de Janeiro State Research Council (FAPERJ), Higher Education Coordination Agency (CAPES), has participated in continuing medical education activities sponsored in whole or in part by Nycomed/Takeda, and has received industry-sponsored grants from Asthmatx/Boston Scientific.
Dr. P Shah: In the past 3 years, PLS has received compensation for consulting with Olympus, has undertaken research funded by Olympus, PneumRx, Pulmonx, Boston Scientific, has participated in continuing medical education activities sponsored in whole or in part by Olympus Keymed, Immotech, Erbe, Pulmonx, Cook Medical, Boston Scientific, and PneumRx.
Dr. E Fiss: In the past 3 years, EF has undertaken research funded by Pfizer, GlaxoSmithKline, Nycomed, AstraZeneca, Novartis, MSD, Roche, Genentech, Teva, Ache, Libbs, Boehringer, and Sanofi.
Dr. R Olivenstein: In the past three years RO has received compensation for consulting with Merck, has undertaken research funded by Novartis, MedImmune, AstraZeneca and Asthmatx, has participated in whole or in part in continuing medical education activities sponsored by AstraZeneca, GlaxoSmithKline, Merck, Novartis, Nycomed and Boston Scientific, has consulted for or participated in advisory boards for AstraZeneca, GlaxoSmithKline, Merck, Novartis and Nycomed and has not received any industry sponsored grants.
Dr. NC Thomson: In the past 3 years, NCT has received compensation for consulting with Asmacure, has undertaken research funded by Glaxo SmithKline, has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca, Chiesi, GlaxoSmithKline, Novartis, has consulted for, or participated in advisory boards for Boston Scientific, Chiesi, Respivert, and has received industry-sponsored grants from Aerovance, Asthmatx, AstraZenica, GlaxoSmithKline, MedImmune, Novartis, and Synairgen.
Dr. R Niven: In the past 3 years, RN has received NO compensation for consulting, has undertaken research funded by GlaxoSmithKline, Vectura, Roche and Synairgen, has participated in continuing medical education activities sponsored in whole or in part by GlaxoSmithKline, Boston Scientific, Astra Zeneca, Novartis and Chiesi has participated in advisory boards for GlaxoSmithKline, Vectura, Boston Scientific and has received industry-sponsored grants from Novartis and Astra Zeneca.
Dr. I Pavord: In the last 5 years IDP has received speaker's honoraria for speaking at sponsored meetings from Astra Zeneca, Boehringer Inglehiem, GlaxoSmithKline. He has received honoraria for attending advisory panels with; Almirall, Astra Zeneca, Boehringer Ingelheim, GlaxoSmithKline, MSD, Schering-Plough, Novartis, Dey, Napp. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GlaxoSmithKline, Astra Zeneca and Napp. He is Chief Medical Advisor to Asthma UK, a member of the UK Department of Health Asthma Strategy Group, a member of the BTS SIGN Asthma guideline group and joint editor in chief of Thorax. Neither IDP nor any member of his family has any shares in pharmaceutical companies.
Dr. M Simoff: In the past 3 years MS has been involved in the Lung Cancer Guidelines, 3rd edition, by the American College of Chest Physicians as a Chapter Editor, all relationships for research, consulting, and advisory boards has been reviewed regularly by the American College of Chest Physicians. He is on the Medical Advisory Board for Varian Medical with all honorarium donated to charitable organizations as per agreements with the American College of Chest Physicians. Industry sponsored grants have come from Boston Scientific – Asthmatx, for ongoing work in bronchial thermoplasty, and is part of a grant from Varian Medical for $100,000.00 analyzing tissue samples in radiation therapy treated lung cancer patients for molecular markers, which suggest improved result. No other conflict to report.
Dr. J Hales: In the past 3 years, JBH has received compensation for consulting with GlaxoSmithKline and Boston Scientific International, has undertaken research funded by Asthmatx, has participated in continuing medical education activities sponsored by University of Virginia, and has participated in advisory boards for Covidien.
Dr. C McEvoy: In the past 3 years CM has not received any compensation for consulting, has undertaken research funded by NHLBI, GlaxoSmithKline, Boston Scientific, Pfizer and HealthPartners Research Foundation, has participated in continuing medical education activities sponsored in whole or in part by HealthPartners and American Lung Association, has consulted for, or participated in advisory boards for American Lung Association of Minnesota and Institute for Clinical System Improvements (ICSI), and has received industry-sponsored grants from GlaxoSmithKline, Boston Scientific, and Pfizer Pharmaceuticals.
Dr. DJ Slebos: In the past 3 years, DJS has received compensation for consulting with Boston Scientific and has participated in continuing medical education activities sponsored in whole or in part by Boston Scientific.
Dr. M Holmes: In the past 3 years, MH has participated in continuing medical education activities sponsored in whole or in part by Novartis, Astra Zeneca, GlaxoSmithKline, Takeda, Boehringer Ingelheim, Pfizer, and has participated in advisory boards for Novartis.
Dr. M Phillips: In the past 3 years, MP has participated in continuing medical education activities sponsored in whole or in part by Boston Scientific and has participated in a working group on Bronchial Thermoplasty for Boston Scientific. He has no other conflicts of interest.
Dr. S Erzurum: SE has no conflict of interest to declare with this paper apart from payments to her Institution for participating in the AIR2 Trial.
Dr. N Hanania: NAH received honoraria for serving as a consultant or on advisory boards with GlaxoSmithKline, Dey, Sunovion, Pfizer, Boehringer Ingelheim, Genentech and for serving on the speaker bureau of Merck, Genentech, and GlaxoSmithKline. His institution received research support from GlaxoSmithKline, Genentech, Pfizer, Boehringer Ingelheim, MedImmune, Sunovion and Dey.
Dr. K Sumino: In the past 3 years, KS has undertaken research funded by NIH, ALA, VA, has participated in continuing medical education activities sponsored in whole or in part by Astellas, Japan.
Dr. M Kraft: In the past 3 years, MK reported receiving grant support from Merck, GE Healthcare, Asthmatx, Novartis, N30, Genentech, and GlaxoSmithKline; board membership for the American Thoracic Society; royalties from Elsevier; and payment for continuing medical education activities.
Dr. G Cox: In the past 3 years, GC has received compensation for consulting with Boston Scientific, has undertaken research funded by Boston Scientific, has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca and Boerhinger-Ingelheim, and has participated in advisory boards for InterMune and Roche.
Dr. D Sterman: In the past 3 years, DS has received compensation for consulting with Olympus Medical Corporation and Broncus Technologies, Inc., and has undertaken research funded by National Cancer Institute. He has not participated in continuing medical education activities sponsored by industry, nor has he participated in advisory boards for or received industry-sponsored grants.
Dr. K Hogarth: In the past 3 years, KH has received compensation for lecturing for Boston Scientific, has undertaken research funded by Boston Scientific has participated in continuing medical education activities sponsored in whole or in part by Boston Scientific.
Dr. J Kline: In the past 3 years, JK has received no compensation for consulting, sponsored continuing medical education activities, or advisory boards; he has undertaken research funded by the NIH, Boston Scientific, GlaxoSmithKline, TEVA Pharmaceuticals, Forest, Novartis, Cephalon, and MedImmune.
Dr. A Mansur: AM has no conflict of interest to declare with this paper apart from payments to his Institution for participating in the AIR2 Trial.
Dr. B Louie: BL has no conflicts relevant to this manuscript to report.
Dr. W Leeds: In the past 3 years, WL has received compensation as a speaker for GlaxoSmithKline and Forest Pharmaceuticals, Inc.; has undertaken research funded by Aeris Therapeutics, Inc., Apnex Medical, Inc., BioCryst Pharmaceuticals, Inc., Boehringer-Ingelheim Pharmaceuticals, Inc., Broncus Technologies, Inc., Forest Research Institute, Inc., GlaxoSmithKline, Merck, Sharpe & Dohme Corp., on behalf of Schering Corp., Novartis Pharmaceuticals, Inc., PulmonX, Inc., Rox Medical, Inc., and Sunovion Pharmaceuticals, Inc.; has participated in continuing medical education activities sponsored by the Kansas Association of Sleep Professionals, St. Catherine Hospital, Kansas Medical Education Foundation, and Veterans Affairs Administration; and has participated on advisory boards for Washburn University and Xyrem Pharmaceuticals.
Dr. R Barbers: In the past 3 years, RB has undertaken research and has received industry-sponsored grants funded by GlaxoSmithKline and Genentech.
Dr. J Austin: In the past 3 years, JHMA has received compensation for consulting with Boston Scientific on this study. He has no other potential conflicts of interest, including other funded research, continuing medical education activities, and consultation for or participation in advisory boards. He has no received industry-sponsored grants.
Dr. N Shargill: NS is a full time employee of Boston Scientific Company, and holds stock ownership or options in Boston Scientific.
Dr. J Quiring: JQ is an employee of QST Consultations which was paid more than $100,001 from Asthmatx/Boston Scientific in consultancy fees.
Mr. B Armstrong: BA is an employee of QST Consultations which was paid more than $100,001 from Asthmatx/Boston Scientific in consultancy fees.
Dr. M Castro: MC reported consultancies with Schering, Asthmatx, Genentech, IPS, Pulmogen, and Sanofi-Aventis; grants pending from American Lung Association, Asthmatx, Amgen, Ception, Genentech, MedImmune, Merck, Novartis, GlaxoSmithKline, and Sanofi-Aventis; lecture fees from Boehringer, Pfizer, Merck, GlaxoSmithKline, Genentech, Asthmatx; and royalties from Elsevier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michel Laviolette, Email: Michel.Laviolette@fmed.ulaval.ca.
Adalberto S. Rubin, Email: arubin@terra.com.br.
Jussara Fiterman, Email: fiterman@pucrs.br.
Jose R. Lapa e Silva, Email: Jrlapa.ntg@terra.com.br.
Pallav L. Shah, Email: pallav.shah@ic.ac.uk.
Elie Fiss, Email: eliefiss@uol.com.br.
Ronald Olivenstein, Email: Ronald.Olivenstein@mcgill.ca.
Neil C. Thomson, Email: Neil.Thomson@glasgow.ac.uk.
Robert M. Niven, Email: robert.niven@uhsm.nhs.uk.
Ian D. Pavord, Email: ian.pavord@uhl-tr.nhs.uk.
Michael Simoff, Email: msimoff1@hfhs.org.
Jeff B. Hales, Email: jhales@pmaofnova.com.
Charlene McEvoy, Email: Charlene.E.McEvoy@HealthPartners.com.
Dirk-Jan Slebos, Email: d.j.slebos@umcg.nl.
Mark Holmes, Email: mark.holmes@health.sa.gov.au.
Martin J. Phillips, Email: Martin.Phillips@health.wa.gov.au.
Serpil C. Erzurum, Email: erzurus@ccf.org.
Nicola A. Hanania, Email: Hanania@bcm.tmc.edu.
Kaharu Sumino, Email: KSUMINO@DOM.wustl.edu.
Monica Kraft, Email: monica.kraft@duke.edu.
Gerard Cox, Email: coxp@mcmaster.ca.
Daniel H. Sterman, Email: sterman@mail.med.upenn.edu.
Kyle Hogarth, Email: dhogarth@uchicago.edu.
Joel N. Kline, Email: Joel-kline@uiowa.edu.
Adel H. Mansur, Email: Adel.mansur@heartofengland.nhs.uk.
Brian E. Louie, Email: Brian.Louie@swedish.org.
William M. Leeds, Email: dr.leeds@psakansas.com.
Richard G. Barbers, Email: barbers@usc.edu.
John H.M. Austin, Email: jha3@columbia.edu.
Narinder S. Shargill, Email: Narinder.shargill@bsci.com.
John Quiring, Email: jquiring@qstconsultations.com.
Brian Armstrong, Email: barmstrong@qstconsultations.com.
Mario Castro, Email: castrom@wustl.edu.
References
- 1.National Health Interview Survey, National Center for Health Statistics, CDC. [Accessed 12/10/10];2009 at http://www.cdc.gov/nchs/fastats/asthma.htm.
- 2.American Lung Association. Epidemiology & Statistics Unit, Research and Program Services. Trends in Asthma Morbidity and Mortality. 2007 Nov; [Google Scholar]
- 3.Moore W, Bleecker E, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chipps BE, Zeiger RS, Dorenbaum A, et al. the TENOR Study Group. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) observational cohort. Curr Respir Care Rep. 2012;1(4):259–269. doi: 10.1007/s13665-012-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavord ID, Cox G, Thomson NC, et al. the RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185–1191. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 6.Cox G, Thomson N, Rubin A, et al. for the AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 7.Castro M, Rubin AS, Laviolette M, et al. for the AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson NC, Rubin AS, Niven RM, et al. for the AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011 Feb 11;11:8. doi: 10.1186/1471-2466-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro M, Rubin A, Laviolette M, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Annals Allergy Asthma Immunol. 2011;107(1):65–70. doi: 10.1016/j.anai.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association Declaration of Helsinki, as most recently amended by the 52nd Annual WMA General Assembly, Edinburgh, Scotland, October 2000, and the note of clarification on Paragraph 29 added by WMA General Assembly, Washington, DC, 2002 and the note of clarification on Paragraph 30 added by WMA General Assembly, Tokyo, 2004
- 11.World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects, 59th WMA General Assembly, Seoul, October, 2008.
- 12.Cox G, Miller J, Goodwin S, Fitzgerald JM, et al. Long-term follow-up of bronchial thermoplasty for asthma: safety results at 5 years. Am J Respir Crit Care Med. 2008;177:A567. [Google Scholar]
- 13.Cox G, Laviolette M, Rubin A, Thomson N the AIR and RISA study groups. Long term safety of bronchial thermoplasty (BT): 3 year data from multiple studies. Am J Respir Crit Care Med. 2009;179:A2780. [Google Scholar]
- 14.Pavord ID, Laviolette M, Thomson N, et al. 5-year safety of bronchial thermoplasty demonstrated in patients with severe refractory asthma: Research in Severe Asthma (RISA) trial. Am J Respir Crit Care Med. 2011;183:A6382. [Google Scholar]
- 15.Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute; 2007. National Asthma Education and Prevention Program. Available from www.nhlbi.nih.gov/guidelines/asthma/asthgdlin.htm. [Google Scholar]
- 16.Lee YM, Park JS, Hwang JW, et al. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest. 2004;126(6):1840–1848. doi: 10.1378/chest.126.6.1840. [DOI] [PubMed] [Google Scholar]
- 17.Sorkness RL, Bleecker ER, Busse WW, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104(2):394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 18.Busacker A, Newell JD, Jr, Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135(1):48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Siddiqui S, Halder P, et al. Qualitative analysis of High-Resolution CT scans in severe asthma. Chest. 2009;136:1521–1528. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatz M, Zeiger R, Mosen D, Vollmer W. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care. 2008;14:206–211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement Figure 1: Annualized severe exacerbation rates (top panel) and emergency room visit rates (bottom panel) in the AIR2 Trial over the 5 year evaluation periods. The data for Year 1 in these figures for both the Sham (white bars) and BT groups (blue bars) are standardized to 52 weeks. The solid bars in both graphs represent the Post-Treatment Period, and the hashed bars represent the Treatment Period.
Online supplement Table 1s: Respiratory Adverse Events, Asthma (multiple symptoms) AEs, and Hospitalizations In the 5 Years Following BT
Online supplement Table 2s: Event Rates: Responders versus Non-Responders (Events/Subject/Year)