Abstract
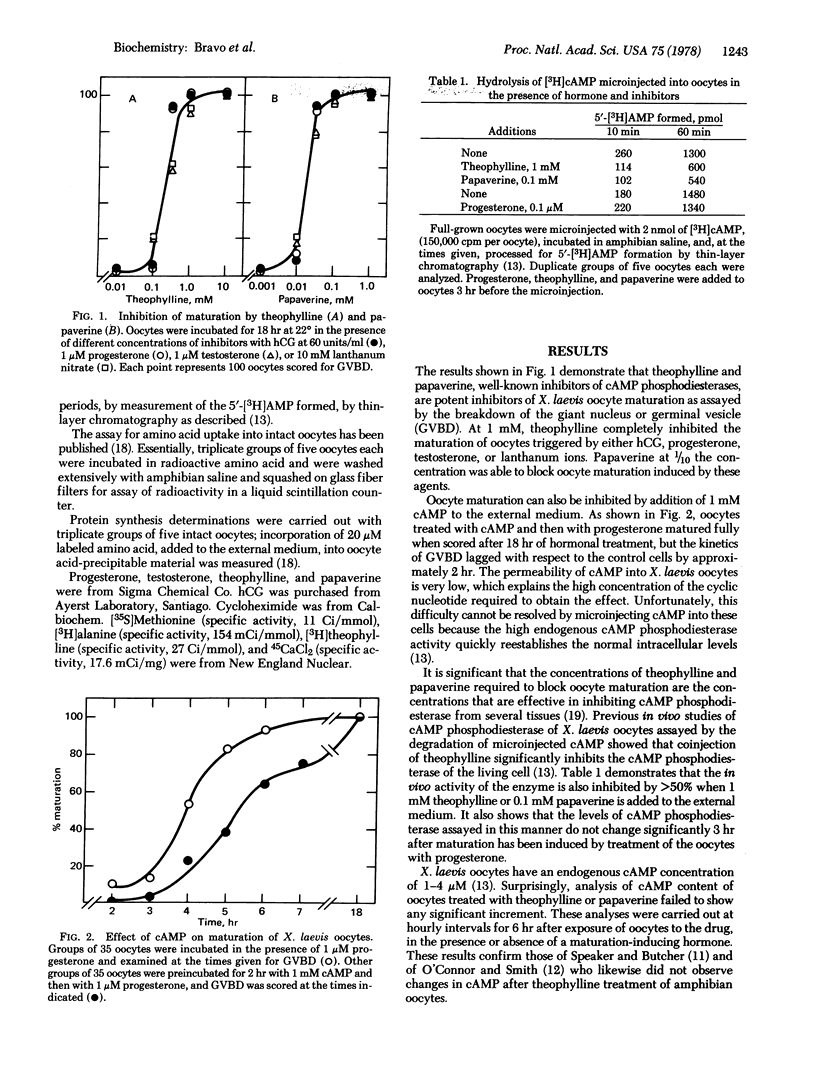
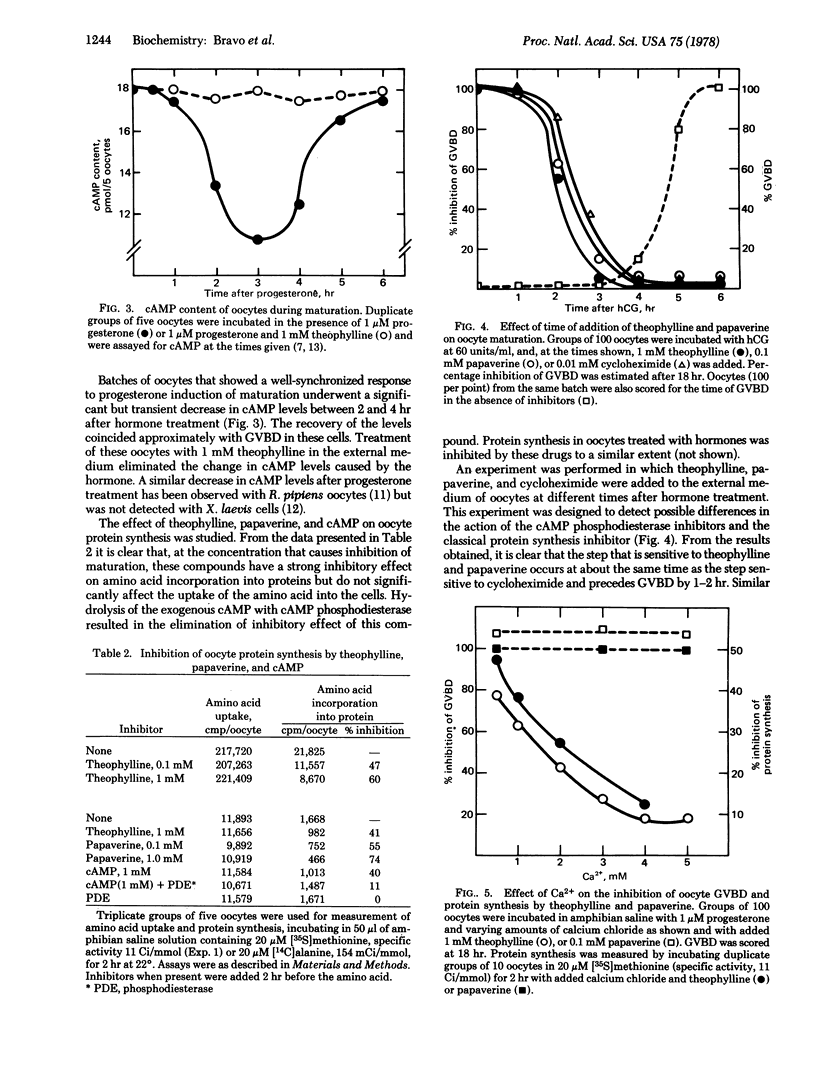
Two inhibitors of cyclic AMP phosphodiesterase (3′:5′-cyclic-AMP 5′-nucleotidohydrolase, EC 3.1.4.17), theophylline and papaverine, inhibit the maturation of Xenopus laevis oocytes induced by four different stimuli: human chorionic gonadotropin, progesterone, testosterone, and lanthanum ions. Addition of 1 mM cyclic AMP to the medium delays maturation by approximately 2 hr. Papaverine, theophylline, and cyclic AMP inhibit amino acid incorporation into oocyte proteins by 50% or more but do not inhibit amino acid uptake. The capacity of theophylline to block maturation and protein synthesis is reversed in a parallel fashion by addition of 1-5 mM calcium ion to the medium. Addition of papaverine, theophylline, and cycloheximide to oocytes at different times after hormonal treatment shows that the step sensitive to blockage by the three drugs is coincident and precedes germinal vesicle breakdown by about 1.5 hr. Theophylline and papaverine do not increase endogenous cyclic AMP levels in oocytes but do block the decrease of cyclic AMP levels observed 3 hr after progesterone treatment. Both drugs inhibit oocyte cyclic AMP phosphodiesterase measured in vivo and severely inhibit the stimulus of calcium uptake caused by progesterone and human chorionic gonadotropin. These results suggest that cyclic AMP, theophylline, and papaverine may block oocyte maturation by inhibiting protein synthesis, possibly via a cyclic AMP-dependent protein kinase as shown in reticulocytes [Datta, A., De Haro, C., Sierra, J. & Ochoa, S. (1977) Proc. Natl. Acad. Sci., USA 74, 1463-1467].
Keywords: hormone induction, progesterone, cyclic AMP phosphodiesterase, meiosis, translational regulation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Bravo R., Allende J. E. Comparison of in vivo and in vitro properties of cyclic adenosine 3':5'-monophosphate phosphodiesterase of amphibian oocytes. J Biol Chem. 1977 Jul 10;252(13):4662–4666. [PubMed] [Google Scholar]
- Brachet J., Baltus E., De Schutter-Pays A., Hanocq-Quertier J., Hubert E., Steinert G. Induction of maturation (meiosis) in Xenopus laevis oocytes by three organomercurials. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1574–1578. doi: 10.1073/pnas.72.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Allende J. E. Conditions affecting protein synthesis in amphibian oocytes. Arch Biochem Biophys. 1976 Feb;172(2):648–653. doi: 10.1016/0003-9861(76)90119-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- Datta A., de Haro C., Sierra J. M., Ochoa S. Role of 3':5'-cyclic-AMP-dependent protein kinase in regulation of protein synthesis in reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1463–1467. doi: 10.1073/pnas.74.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanocq-Quertier J., Baltus E., Brachet J. Induction of maturation (meiosis) in small Xenopus laevis oocytes by injection of maturation promoting factor. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2028–2032. doi: 10.1073/pnas.73.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Merriam R. W. Progesterone-induced maturational events in oocytes of Xenopus laevis. II. Change in intracellular calcium and magnesium distribution at germinal vesicle breakdown. Exp Cell Res. 1971 Sep;68(1):81–87. doi: 10.1016/0014-4827(71)90589-1. [DOI] [PubMed] [Google Scholar]
- Moreau M., Guerrier P., Doree M. Induction of meiosis by injection of heterologous protein kinase and phosphorylase kinase in Xenopus laevis oocytes. J Exp Zool. 1976 Sep;197(3):435–442. doi: 10.1002/jez.1401970317. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Smith L. D. Inhibition of oocyte maturation by theophylline: possible mechanism of action. Dev Biol. 1976 Sep;52(2):318–322. doi: 10.1016/0012-1606(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. Initiation of meiotic maturation in Xenopus laevis oocytes by lanthanum. Nature. 1976 Jul 22;262(5566):289–290. doi: 10.1038/262289a0. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W. Action of hormones on germinal vesicle breakdown in frog (Rana pipiens) oocytes. J Exp Zool. 1967 Dec;166(3):347–354. doi: 10.1002/jez.1401660307. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Speaker M. G., Butcher F. R. Cyclic nucleotide fluctuations during steroid induced meiotic maturation of frog oocytes. Nature. 1977 Jun 30;267(5614):848–850. doi: 10.1038/267848a0. [DOI] [PubMed] [Google Scholar]



