Abstract
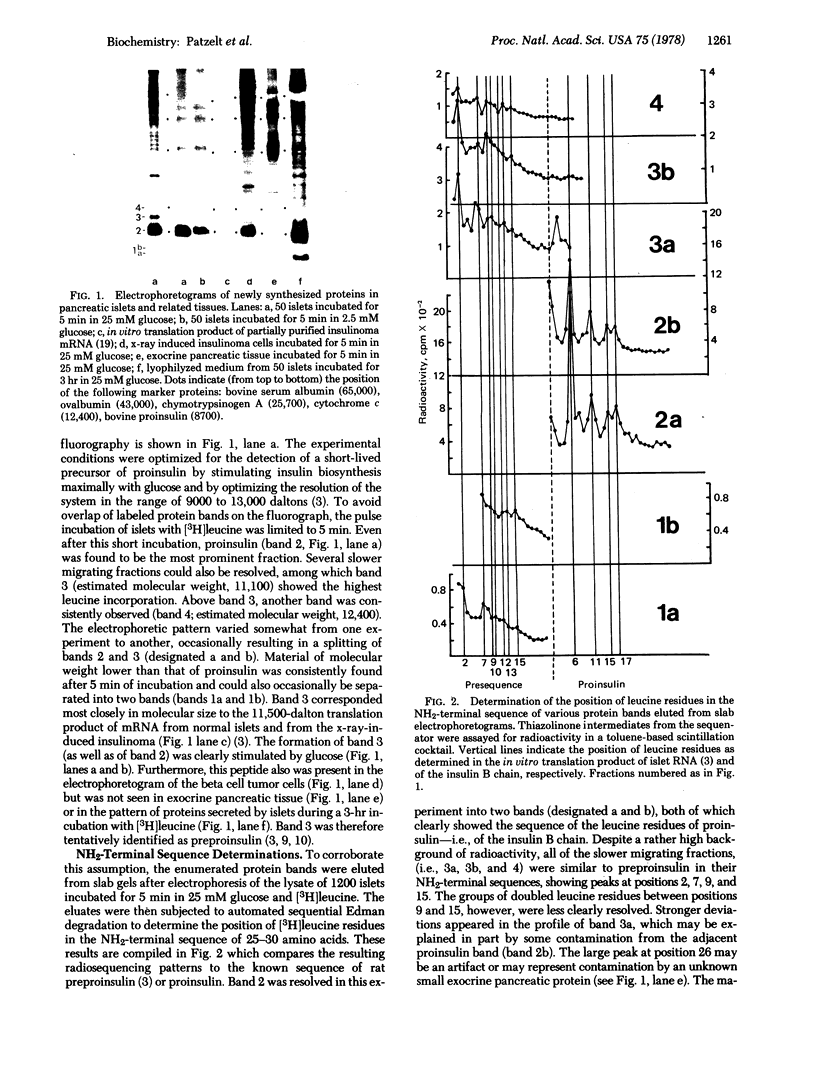
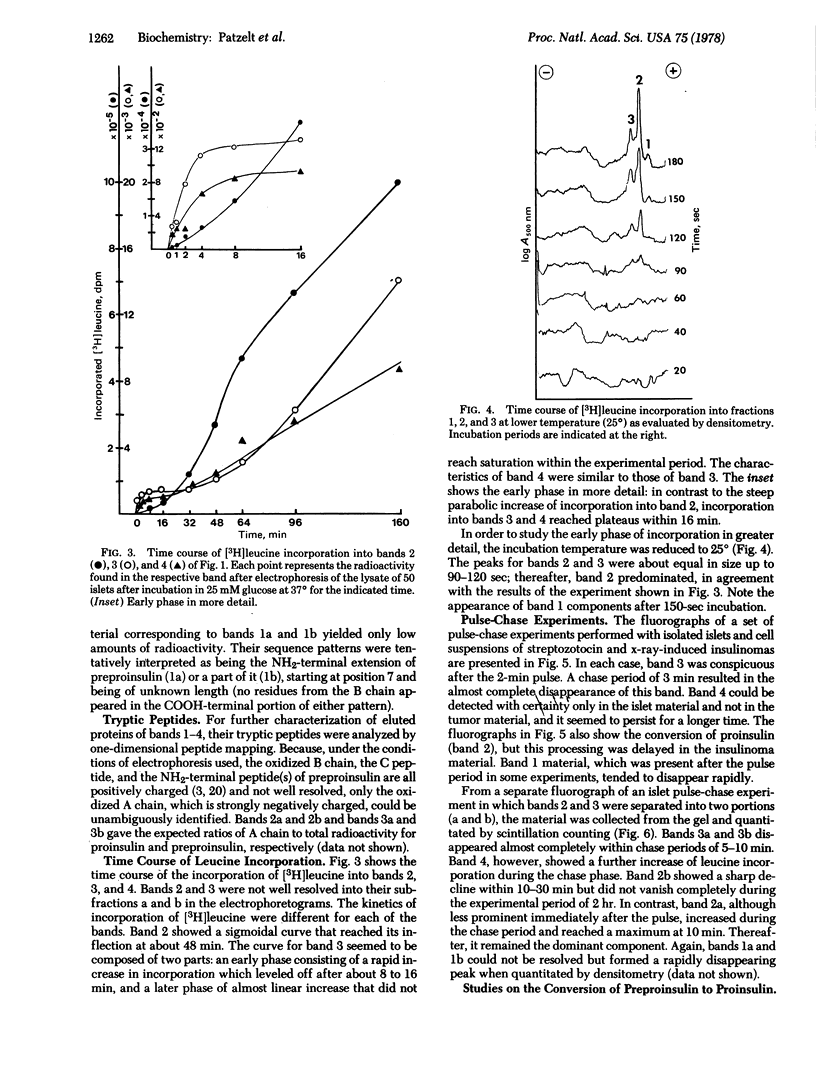
Newly synthesized rat islet proteins have been analyzed by polyacrylamide slab gel electrophoresis and fluorography. A minor component having an apparent molecular weight of 11,100 was identified as preproinsulin by the sensitivity of its synthesis to glucose, the pattern of NH2-terminal leucine residues, and the rapidity of its appearance and disappearance during incubation of islets or islet cell tumors. A small amount of labeled peptide material which may represent the excised NH2-terminal extension of preproinsulin or its fragment was also detected. The kinetics of formation and processing of the preproinsulin fraction were complex, consisting of a rapidly turning over component having a half-life of about 1 min and a slower minor fraction that may have bypassed the normal cleavage process. The electrophoretic resolution of the preproinsulin and proinsulin fractions into two bands each is consistent with the presence of two closely related gene products in rat islets rather than intermediate stages in the processing of these peptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borgese N., Mok W., Kreibich G., Sabatini D. D. Ribosomal-membrane interaction: in vitro binding of ribosomes to microsomal membranes. J Mol Biol. 1974 Sep 25;88(3):559–580. doi: 10.1016/0022-2836(74)90408-2. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Grollman A. P., Huang M. T. Inhibitors of protein synthesis in eukaryotes: tools in cell research. Fed Proc. 1973 Jun;32(6):1673–1678. [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone. Evidence for an early biosynthetic precursor of proparathyroid hormone. J Biol Chem. 1976 Jul 10;251(13):3893–3899. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Chan S. J., Steiner D. F., Saunders G. F. Immunological and chemical characterization of bovine preproinsulin. J Biol Chem. 1977 Nov 25;252(22):7971–7978. [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Walker M. O., Lacy P. E. The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes. 1971 May;20(5):257–265. doi: 10.2337/diab.20.5.257. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Biesbroeck J., Chyn R., Boime I., Szczesna E., McWilliams D. Isolation of a biologically active messenger RNA: preparation from fish pancreatic islets by oligo(2'-deoxythymidylic acid) affinity chromatography. Ciba Found Symp. 1976;41:97–116. doi: 10.1002/9780470720233.ch6. [DOI] [PubMed] [Google Scholar]
- Rakieten N., Gordon B. S., Beaty A., Cooney D. A., Davis R. D., Schein P. S. Pancreatic islet cell tumors produced by the combined action of streptozotocin and nicotinamide. Proc Soc Exp Biol Med. 1971 May;137(1):280–283. doi: 10.3181/00379727-137-35561. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Adams J. M., Harris A. W. Detection of a possible precursor of immunoglobulin light chain in MOPC 41 A plasmacytoma cells. FEBS Lett. 1975 Apr 15;53(1):95–98. doi: 10.1016/0014-5793(75)80691-0. [DOI] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wold J. S., Longnecker D. S., Fischer L. J. Species dependent pancreatic islet toxicity produced by cyproheptadine: alterations in beta cell structure and function. Toxicol Appl Pharmacol. 1971 Jun;19(2):188–201. doi: 10.1016/0041-008x(71)90106-2. [DOI] [PubMed] [Google Scholar]