Abstract
How to build and maintain a reliable yet flexible circuit is a fundamental question in neurobiology. The nervous system has the capacity for undergoing modifications to adapt to the changing environment while maintaining its stability through compensatory mechanisms, such as synaptic homeostasis. Here, we describe our findings in the Drosophila larval visual system, where the variation of sensory inputs induced substantial structural plasticity in dendritic arbors of the postsynaptic neuron and concomitant changes to its physiological output. Furthermore, our genetic analyses have identified the cyclic adenosine monophosphate (cAMP) pathway and a previously uncharacterized cell surface molecule as critical components in regulating experience-dependent modification of the postsynaptic dendrite morphology in Drosophila.
Proper functions of neuronal circuits rely on their fidelity, as well as plasticity, in responding to experience or changing environment, including the Hebbian form of plasticity, such as long-term potentiation, and the homeostatic plasticity important for stabilizing the circuit (1, 2). Activity-dependent modification of neuronal circuits helps to establish and refine the nervous system and provides the cellular correlate for cognitive functions, such as learning and memory (3, 4). Multiple studies have examined synaptic strength regulation by neuronal activity, whereas to what extent and how the dendritic morphology may be modified by neuronal activity remain open questions (1, 5).
The model organism Drosophila melanogaster has facilitated genetic studies of nervous system development and remodeling (6). Notwithstanding the relatively stereotyped circuitry, flies exhibit experience-induced alterations in neuronal structures and behaviors such as learning and memory (7–10). In our study of experience-dependent modifications of the Drosophila larval CNS, we found that different light exposures dramatically altered dendritic arbors of ventral lateral neurons [LN(v)s], which are postsynaptic to the photoreceptors. Unlike the visual activity–induced dendrite growth in Xenopus optic tectum (11), extending the light exposure of Drosophila larvae reduced the LN(v)s’ dendrite length and functional output, a homeostatic plasticity for compensatory adaptation to alterations in sensory inputs. We further show that the cyclic adenosine monophosphate (cAMP) pathway and an immunoglobulin domain–containing cell surface protein, CG3624, are critical for this sensory experience–induced structural plasticity in Drosophila CNS.
In Drosophila larvae, Bolwig's organ (BO) senses light, and its likely postsynaptic targets are LN(v)s (12). As the major circadian pacemaker, LN(v)s are important for the entrainment to environmental light-dark cycles and larval light avoidance behavior (13, 14). In the larval brain, Bolwig's nerve (BN), the axonal projection from BO, terminates in an area overlapping the dendritic field of LN(v)s (12, 15) (Fig. 1A and fig. S1A). Using the FRT-FLP system [in which DNA sequences flanked by flippase recognition targets (FRT) are snipped out by flippase (FLP)] along with three-dimensional (3D) tracing, we labeled and analyzed the dendritic arbor of individual LN(v) neurons (Fig. 1A). We then demonstrated potential synaptic connections between BN and LN(v)s using the GRASP [green fluorescent protein (GFP) reconstitution across synaptic partners] technique (16) to drive expression of one-half of the split GFP in the BN by means of Gal4/UAS and expression of the other half of the split GFP in LN(v)s via LexA/LexAop. The proximity of putative synaptic connections between BN and LN(v)s’ dendrites reconstituted GFP fluorescence for photo-receptors expressing either rhodopsin 5 (Rh5) or rhodopsin 6 (Rh6) in BO (fig. S2) (15), which suggested that both groups of photoreceptors may have synaptic connections with LN(v)s.
Fig. 1.
Connections between BN and larval LN(v)s. (A) Projected confocal images (left three panels) of a single LN(v) (hs-flp; pdf-Gal4; UAS>CD2>CD8::GFP) marked with an antibody against CD8 (green) and its relation with BN terminals marked with 24B10 (red). (Right) 3D tracing and reconstruction of the BN-LN(v) connection. (B) Schematic diagram of the brain-eye preparation for calcium imaging. Dashed circle indicates the axon terminal of LN(v)s imaged for GCaMP3 signal. VNC, ventral nerve cord. (C) Fluorescence changes of GCaMP3 but not CD2::mCherry coexpressed in LN(v)s before and 1 s after light stimulation. (D) Light responses in LN(v)s require NorpA. Representative traces (gray) and average traces (red) of calcium signal (n > 5) in response to light (488 nm) delivery indicated by arrow. Scale bar, 5 μm.
To test whether LN(v)s can be activated by BN inputs through light stimulation, we performed calcium imaging using GCaMP3 transgenic flies (17, 18) with the larval brain-eye preparation, which included BO, BN, developing eye disks, the larval brain, and ventral nerve cord (Fig. 1B and fig. S1A). Because BO senses blue and green light (15), we used the confocal laser at 488 nm (blue) and 543 nm (green) to stimulate these larval photoreceptors. LN(v)s’ axon terminals displayed a relatively stable baseline of GCaMP3 fluorescence and, upon light stimulation, yielded large calcium responses (Fig. 1, C and D), which increased with the greater intensity and longer duration of the light pulses (fig. S3).
Recent studies suggest that Cryptochrome (CRY) in adult large LN(v)s senses light and elicits neuronal firing (19). In larvae, however, severing BN abolished light-induced calcium responses in LN(v)s (fig. S1B). The loss-of-function mutation of NorpA (no-receptor-potential A), encoding a phospholipase C crucial for photo-transduction (20), also eliminated these calcium responses (Fig. 1D), which indicated that light-elicited responses in LN(v)s are generated via phototransduction in larval photoreceptors rather than as a direct response to light by LN(v)s.
In animals with BO genetically ablated, the dendritic field of LN(v) is absent (12) (fig. S4A). To test whether BO is required for LN(v)s’ dendrite maintenance, we induced the expression of cell death genes rpr and hid in BO after synapse formation and found the LN(v) dendrite length was also greatly reduced (fig. S4B). Whereas physical contacts with BN or growth-promoting factors released from presynaptic axons could be important for LN(v)s’ dendrite maintenance, it is also possible that synaptic activity from BN promotes LN(v) dendrite growth, as suggested by previous studies (12). To explore the latter scenario, we provided newly hatched larvae with different visual experiences through various light regimes—including the standard 12 hours of light and 12 hours of dark daily cycle (LD); constant darkness (DD) for sensory deprivation; constant light (LL) for enhanced light input; 16-hour light and 8-hour dark cycle, mimicking a long day; and 8-hour light and 16-hour dark cycle, mimicking a short day—and we examined the dendrite morphology of LN(v)s of late third instar larvae. Whereas different light exposure had no detectable effects on larval developmental timing, increasing light exposure reduced the total dendrite length of individual LN(v) neurons, with the longest dendrite in constant darkness and the shortest dendrite length in constant light condition (Fig. 2, A and B). Thus, not only is the LN(v) dendrite dependent on the presence of presynaptic nerve fibers, its length is modified by the sensory experience in a compensatory fashion, whereby an increase in sensory inputs causes a reduction in the dendrite length and vice versa.
Fig. 2.
Visual experiences modify LN(v) dendrite morphology. (A) The total dendrite length of LN(v) was reduced with increased light exposure. Representative projected confocal images (top) and 3D tracings of the soma and dendritic arbors (bottom) of individually labeled LN(v)s by hs-flp; pdf-Gal4; UAS>CD2>CD8::GFP with LN(v)s labeled by an antibody against CD8 (green). Scale bar, 10 μm. (B) Quantification of the total dendrite length of individually labeled LN(v) in larvae maintained under different light-dark conditions. **P < 0.01, by analysis of variance (ANOVA). Error bars represent SEM; n > 10 in all groups. (C) Quantification of the total dendrite length of individually labeled LN(v)s from larvae collected 48, 72, 96, or 120 hours after egg laying (AEL) and raised under different light-dark conditions. **P < 0.01, by ANOVA, as compared with the LD group at each time point. Error bars represent SEM; n > 5 in all groups.
Whereas adult LN(v)s alter their axon terminal structures in a circadian cycle–controlled fashion (21), we found no difference in dendrite morphology of LN(v)s from larvae collected at four different time points around the clock (fig. S5), which indicated that circadian regulation is not involved in the light-induced modification of LN(v) dendrites. Under regular light-dark conditions, LN(v) dendrites expanded as the larval brain size increased from the second to the third instar stage (Fig. 2C and fig. S6). However, the dendrite length of the LL group increased at a significantly slower rate than the DD group (Fig. 2C). It thus appears that light exposure retards the growth of LN(v) dendrites throughout the larval development.
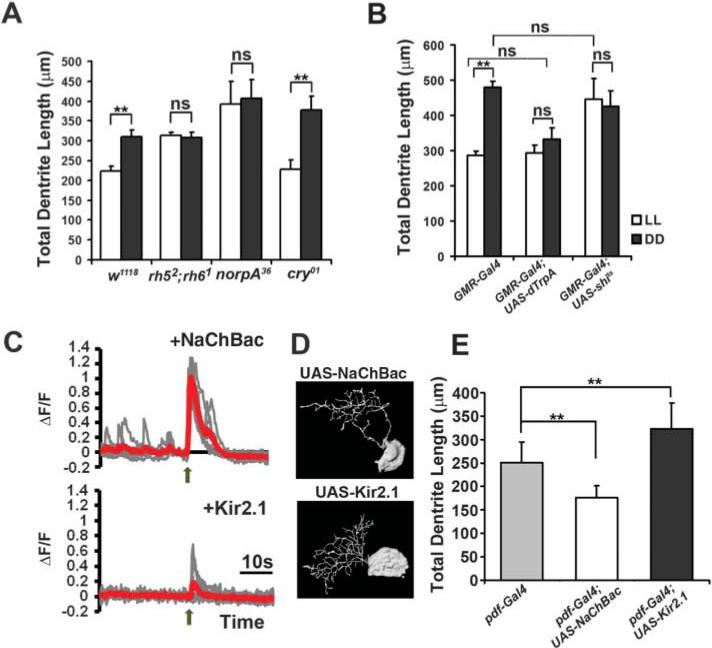
To test the contribution of different light-sensing pathways, we examined loss-of-function mutations of Cry (cry01) (22) or NorpA (norpA36) (20) and of double mutants lacking both Rh5 and Rh6 (rh52;rh61) (15). Although wild-type and cry01 larvae displayed differences in their dendrite length when exposed to constant darkness versus constant light, such light-induced changes were absent in the rh52;rh61 double mutant and the norpA36 mutant (Fig. 3A and fig. S7A). Thus, similar to the calcium response to light (Fig. 1D), light-induced modification of LN(v) dendritic structure requires visual transduction mediated by rhodopsin and NorpA in BO but not Cry function in LN(v)s.
Fig. 3.
Pre- and postsynaptic activity modulates the LN(v) dendrite morphology. (A) Light-induced changes of LN(v) dendrite morphology are mediated through rhodopsin and NorpA, revealed by quantification of the total dendrite length of LN(v)s labeled by pdf-Gal4/UAS-CD8GFP. rh52;rh61 and norpA36 mutants had no significant differences between LL and DD conditions. **P < 0.01; ns, not significant; by Student's t test. Error bars represent SD; n > 8 in all groups. (B) Alteration in BO activity or neurotransmission modifies LN(v) dendrites, revealed by quantification of the total dendrite length of LN(v)s labeled by pdf-lexA/lexAop-CD8tdTomato. Larvae were raised at 29°C. Only the control group (GMR-Gal4) showed significant differences between LL and DD conditions. *P < 0.01; ns, not significant; by ANOVA. Error bars represent SD; n > 9 in all groups. (C) Expressing NaChBac or Kir2.1 in LN(v) modulated its response to light stimulation. Representative traces (gray) and average traces (red) of calcium responses (n > 5) to light (arrow). (D and E) Intrinsic activity of LN(v)s modifies its dendrites. NaChBac expression had significantly reduced, whereas Kir2.1 expression had significantly increased dendrite length, as compared with control groups. (D) Projected 3D tracings of representative images. (E) Quantification of the total dendrite length of individually labeled LN(v)s in larvae with the indicated genotype. **P < 0.01 by ANOVA. Error bars represent SD; n > 9 in all groups.
To manipulate the level of synaptic activity, we either increased the BO excitability by expressing the heat-activated Drosophila transient-receptor-potential A1 (dTrpA) channel (23) or reduced transmitter release from BN through a temperature-sensitive form of the dominant-negative dynamin, Shibirets (Shits) (24). These manipulations eliminated light-induced modification of LN(v) dendrites at 29°C (Fig. 3B and fig. S7B). Reducing BO activity by means of Shits caused dendrite expansion, as if the animal detected no light, whereas increasing BO activity by means of the dTrpA channel resulted in reduction of LN(v) dendrites, a process reminiscent of constant light exposure (Fig. 3B).
We further tested whether intrinsic LN(v) neuronal activity drives modification of its dendrite morphology by expression of the sodium channel NaChBac to increase excitability or the potassium channel Kir2.1 to reduce excitability (25, 26). LN(v)s expressing Kir2.1 showed reduced or no calcium responses upon light stimulation (Fig. 3C). In contrast, LN(v)s expressing NaChBac displayed numerous peaks in GCaMP3 signals in the presence or absence of light stimulation, indicative of elevated spontaneous activities (Fig. 3C). Upon examining LN(v) dendrites, we found that neuronal excitability of the LN(v) was inversely proportional to its dendrite length (Fig. 3, D and E).
These results obtained using genetic approaches agreed with our findings in experiments with different environmental light conditions. They suggested that LN(v)'s dendritic structures are modified according to its neuronal activity, which varies with light-induced synaptic inputs.
To test whether synaptic contacts of BN on LN(v)s are modified by light, we marked synapses formed by BN with EGFP (enhanced green fluorescent protein)–tagged Cacophony (Cac-EGFP), because Cacophony is a calcium channel localized at presynaptic terminals and its distribution correlates with the number of synapses (27). We found close association of Cac-EGFP–expressing structures with LN(v)s’ dendritic arbors (fig. S8). Compared with regular light-dark conditions, constant darkness increased, whereas constant light reduced, the total intensity of Cac-EGFP (fig. S8), which suggested that light modified not only dendritic arbors of LN(v)s but also the number of synaptic contacts impinging on LN(v) dendrites.
Next, using calcium imaging, we examined whether there are light-induced functional modifications of LN(v)s. Increased light exposure caused LN(v)s to be less responsive (Fig. 4, A and B). Conversely, sensory deprivation in constant darkness increased LN(v)s’ sensitivity to light. Thus, in contrast to stable synaptic responses observed in synaptic homeostasis (1, 5), light-induced responses of central neurons post-synaptic to photoreceptors in the Drosophila larval visual circuit have a dynamic range, modifiable by sensory experiences and positively correlated to the dendrite length.
Fig. 4.
Light modulation of LN(v)s calcium responses and molecules important for the structural plasticity of LN(v) dendrites. (A and B) Functional plasticity in LN(v)s induced by different visual experiences. (A) Average traces. (B) Quantification of light-elicited calcium responses in LN(v)s from larvae raised under different light-dark conditions. *P < 0.05, **P < 0.01, by ANOVA. Error bars represent SEM; n > 14 in all groups. (C) The cAMP pathway and CG3624 play critical roles in the light-induced plasticity of LN(v) dendrites, labeled by pdf-Gal4/UAS-CD8GFP (control 1) in dnc1 mutant, or by pdf-lexA/lexAop-CD8tdTomato (control 2) in babos-1 mutant. RNAi knockdown of dnc in LN(v)s and the dnc1 mutant both eliminated the light-induced plasticity. The dendrite length in the babos-1 mutant or in CG3624 RNAi-depleted (knockdown) flies did not increase in DD. *P < 0.05, **P < 0.01, by ANOVA. Error bars represent SEM; n > 6 in all groups. (D) The loss-of-function mutant of CG3624, CG3624[KG05061], exhibited defects in the LN(v) dendrite plasticity specifically in DD conditions. Expression of myc-tagged CG3624 transgene in LN(v)s driven by pdf-Gal4 fully rescued the phenotype. Genotypes are as indicated, with the addition of pdf-Gal4/UAS-CD8GFP in all groups for labeling LN(v) dendrites. **P < 0.01; ns, not significant; by ANOVA. Error bars represent SEM; n > 6 in all groups.
Structural plasticity is also an important mechanism underlying learning and memory (4). To test whether there are shared molecular mechanisms, we examined 24 mutants implicated in learning and memory (7, 28) (table S1). Several mutants of genes associated with cAMP signaling pathways, as well as a mutant named babos-1, have clearly shown modified LN(v)s plasticity. We analyzed two of these mutants further with genetic manipulations.
In dunce1, a loss-of-function mutant of the fly homolog of 3′5′-cyclic nucleotide phosphodiesterase (29), the LN(v)s’ dendrite length was comparable among LD, LL, and DD groups. Reducing dunce gene expression specifically in LN(v)s through RNA interference (dncIR) resulted in a similar indifference of LN(v)s’ dendrite size to the light exposure (Fig. 4C), which implicated a cell-autonomous action of dunce in LN(v) neurons.
To explore the possibility that the elevated cAMP level caused by the dunce mutation interfered with dendrite plasticity, we tested for the involvement of downstream components of the cAMP pathway, including the catalytic subunit of protein kinase A (PKAmc), which up-regulates cAMP signaling (30), and a dominant-negative form of the cAMP response element–binding protein (CREBdn), which inhibits cAMP-induced transcription activation (31). Expression of either transgene specifically in LN(v)s obliterated their ability to adjust dendrite length under different light-dark conditions (fig. S9). Calcium imaging further revealed that the expression of PKAmc or CREBdn eliminated changes of LN(v)s’ light responses produced by different light-dark conditions (fig. S10). Thus, the cAMP pathway regulates both structural and functional plasticity of LN(v)s.
Our screen for mutants with defective LN(v) dendritic plasticity also identified babos-1, a mutant with a P-element insertion near the transcriptional start site of CG3624, a previously uncharacterized immunoglobulin domain–containing cell surface protein (28). The LN(v) dendrite length of babos-1 mutant larvae was comparable to controls in LD and LL but has no compensatory increase in DD (Fig. 4C). Similar phenotypes were found in larvae expressing an RNAi transgene targeting CG3624 in LN(v)s (Fig. 4C). Moreover, flies carrying a hypomorphic allele of CG3624, CG3624[KG05061] (fig. S11), also showed defective light-induced dendritic plasticity, which was fully rescued by expressing the UAS-CG3624 transgene specifically in LN(v)s (Fig. 4D). Thus, the function of this immunoglobulin domain– containing protein in LN(v)s is important for the dendrite expansion in constant darkness.
Bioinformatic analyses suggest that CG3624 is a cell surface protein containing an N-terminal signal peptide, extracellular immunoglobulin domains followed by a transmembrane helix, and a short C-terminal cytoplasmic tail (32). CG3624 is widely expressed in the nervous system throughout development (Flybase and BDGP database). Its specific requirement for the adjustment of LN(v)s’ dendrite length in constant darkness suggests that elevation or reduction of sensory inputs likely invokes separate mechanisms for compensatory modifications of central neuronal dendrites.
A functioning nervous system must have the capacity for adaptive modifications while maintaining circuit stability. Our study of the Drosophila larval visual circuit reveals large-scale, bidirectional structural adaptations in dendritic arbors invoked by different sensory exposure. Whereas the circuit remains functional with modified outputs, this type of homeostatic compensation may modify larval light sensitivity according to its exposure during development and could facilitate adaption of fly larvae toward altered light conditions, such as seasonal changes. Our observations also suggest shared molecular machinery between homeostasis and the Hebbian plasticity with respect to the cAMP pathway (2, 4) and indicate the feasibility of genetic studies of experience-dependent neuronal plasticity in Drosophila.
Supplementary Material
Acknowledgments
We thank C. Desplan, J. Blau, J. Hall, K. Scott, U. Heberlein, and A. Sehgal for reagents and fly lines; S. Barshow for the technical support; and P. Soba and M. Klassen for critical reading of the manuscript. Q.Y. was supported by an Autism Speaks postdoctoral fellowship. Y.X. and Z.Y. are recipients of the long-term fellowship from Human Frontier Science Program. C.H. was supported by a Jane Coffins Child postdoctoral fellowship. This work is supported by NIH grant 2R37NS040929 to Y.N.J. Also, Q.Y., Y.X, C.H., and Z.Y. are postdoctoral associates and L.Y.J. and Y.N.J. are investigators of the Howard Hughes Medical Institute.
References and Notes
- 1.Nelson SB, Turrigiano GG. Neuron. 2008;60:477. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonomano DV, Merzenich MM. Annu. Rev. Neurosci. 1998;21:149. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Wong RO, Ghosh A. Nat. Rev. Neurosci. 2002;3:803. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- 4.Holtmaat A, Svoboda K. Nat. Rev. Neurosci. 2009;10:647. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 5.Davis GW, Bezprozvanny I. Annu. Rev. Physiol. 2001;63:847. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- 6.Jan YN, Jan LY. Nat. Rev. Neurosci. 2010;11:316. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keene AC, Waddell S. Nat. Rev. Neurosci. 2007;8:341. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 8.Sachse S, et al. Neuron. 2007;56:838. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Barth M, Hirsch HV, Meinertzhagen IA, Heisenberg M, Neurosci J. 1997;17:1493. doi: 10.1523/JNEUROSCI.17-04-01493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripodi M, Evers JF, Mauss A, Bate M, Landgraf M. PLoS Biol. 2008;6:e260. doi: 10.1371/journal.pbio.0060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sin WC, Haas K, Ruthazer ES, Cline HT. Nature. 2002;419:475. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 12.Malpel S, Klarsfeld A, Rouyer F. Development. 2002;129:1443. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko M, Helfrich-Förster C, Hall JC. J. Neurosci. 1997;17:6745. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzoni EO, Desplan C, Blau J. Neuron. 2005;45:293. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Sprecher SG, Desplan C. Nature. 2008;454:533. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon MD, Scott K. Neuron. 2009;61:373. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian L, et al. Nat. Methods. 2009;6:875. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang Y, et al. Nature. 2010;468:921. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogle KJ, Parson KG, Dahm NA, Holmes TC. Science. 2011;331:1409. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomquist BT, et al. Cell. 1988;54:723. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 21.Fernández MP, Berni J, Ceriani MF. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolezelova E, Dolezel D, Hall JC. Genetics. 2007;177:329. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamada FN, et al. Nature. 2008;454:217. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphey RK, et al. Development. 2003;130:3671. doi: 10.1242/dev.00598. [DOI] [PubMed] [Google Scholar]
- 25.Nitabach MN, Blau J, Holmes TC. Cell. 2002;109:485. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 26.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. J. Neurobiol. 2005;62:1. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki F, Zou B, Xu X, Ordway RW. J. Neurosci. 2004;24:282. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubnau J, et al. Curr. Biol. 2003;13:286. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 29.Byers D, Davis RL, Kiger JA., Jr Nature. 1981;289:79. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 30.Joiner WJ, Crocker A, White BH, Sehgal A. Nature. 2006;441:757. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 31.Eresh S, Riese J, Jackson DB, Bohmann D, Bienz M. EMBO J. 1997;16:2014. doi: 10.1093/emboj/16.8.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel C, Teichmann SA, Chothia C. Development. 2003;130:6317. doi: 10.1242/dev.00848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.