Summary
In this prospective study of patients with relapsed and/or refractory multiple myeloma (MM) treated with lenalidomide and dexamethasone, relationships between markers of endothelial stress and drug administration and incidence of venous thromboembolism (VTE) were assessed. Of 33 enrolled patients, laboratory and treatment data were available for 32 patients. Of these, 23 received pulsed dexamethasone (40 mg/day on days 1–4, 9–12, and 17–21 of each 28-day cycle) and 9 received weekly dexamethasone (40 mg/day on days 1, 8, 15, and 21 of each cycle). The overall incidence of VTE was 9%. A decreasing trend in markers values was observed with intercellular adhesion molecule (P = 0·05), fibrinogen (P = 0·008), plasminogen activator inhibitor-1 (P < 0·001), homocysteine (P = 0·002), and P-selectin (P < 0·001) during therapy. Compared with weekly dexamethasone, pulsed dexamethasone was associated with significantly greater variation in mean adjusted relative values of fibrinogen, P-selectin, and vascular endothelial growth factor (P < 0·001 for all comparisons), although there was no apparent association with VTE incidence. Lenalidomide plus dexamethasone affects endothelial stress marker levels in patients with advanced MM. The higher variation seen with pulsed dexamethasone suggests greater endothelial stress with this approach.
Keywords: multiple myeloma, venous thromboembolism, dexamethasone, lenalidomide, endothelial markers
Introduction
Cancer patients experience increased venous thromboembolism (VTE), and those with haematological malignancies, including multiple myeloma (MM), have a particularly high risk (Blom et al, 2005; Sallah et al, 2002). In patients with MM, VTE is associated with significant morbidity and greater risk of death (Jacobus et al, 2008). The exact mechanism by which VTE occurs in MM patients is unknown, but individual risk appears to be influenced by a combination of hereditary-, disease-, and treatment-related factors (Sallah et al, 2002; Palumbo et al, 2008).
When given as monotherapy in patients with MM, dexamethasone is associated with a relatively low rate of VTE (approximately 3–5%, based on the results of recent clinical trials) (Dimopoulos et al, 2007; Rajkumar et al, 2006; Weber et al, 2007). Lenalidomide is also associated with relatively low rates of VTE when used as a single agent and with appropriate thromboprophylaxis (≤ 2%) (Bennett et al, 2006; Richardson et al, 2006). However, the combination of lenalidomide and dexamethasone, which is approved for the treatment of relapsed, or relapsed and refractory MM, is associated with an increased VTE risk of approximately 11–15% (Dimopoulos et al, 2007; Weber et al, 2007).
How the combination of these agents induces a state of hypercoagulability is not yet fully understood. Recent evidence from a large randomized study suggested that the dose and schedule of dexamethasone may influence the risk of VTE when used in combination with lenalidomide (Jacobus et al, 2008; Rajkumar et al, 2010). We therefore conducted an observational study to evaluate changes in markers of endothelial stress and coagulation during treatment with combination lenalidomide and dexamethasone, and assessed the relationship between these findings and the dose and schedule of dexamethasone.
Methods
Patients and treatment
Between January and August 2006, 33 patients with relapsed, or relapsed and refractory MM were treated prospectively with lenalidomide (25 mg/day on days 1–21 of each 28-day cycle) plus dexamethasone. Low-dose dexamethasone was defined as a total monthly dose of 0–160 mg, and high-dose dexamethasone was defined as a total monthly dose of 160–480 mg. Dexamethasone schedules were categorized as pulsed (40 mg/day on days 1–4, 9–12, and 17–21of each 28-day cycle) or weekly (weekly dose of 40 mg on days 1, 8, 15, 22 of each 28-day cycle). Patients who switched from pulsed to weekly schedules (n = 4) were included in the pulsed schedule. Combination lenalidomide and dexamethasone treatment was continued until disease progression.
Eligibility criteria included age >18 years, a diagnosis of MM that had progressed after at least 2 cycles of prior anti-myeloma treatment or had relapsed with progressive disease after completion of treatment, with measurable levels of myeloma paraprotein in serum (≥5.0 g/l) or urine (≥0·2 g/ 24 h), and an Eastern Cooperative Oncology Group performance status score of ≤2. Patients with any serious medical condition that placed them at unacceptable risk were excluded. Patients were also excluded if their absolute neutrophil count was <1.0 × 109/l; platelet count was <75 × 109/l and bone marrow contained <50% plasma cells, or their platelet count was <30 × 109/l and bone marrow contained ≥50% plasma cells; serum creatinine level was >221 μmol/l; serum glutamic oxaloacetic transaminase or serum glutamic pyruvic transaminase levels were >3.0 × the upper limit of normal; total bilirubin level was >2·0 34.2 mmol/l; neuropathy of grade ≥2; or they had known hypersensitivity to thalidomide or dexamethasone.
Bisphosphonates were permitted as concomitant treatment. Other therapies considered necessary by the physician for the patient’s supportive care were also allowed, including antibiotics, antihistamines, growth factor support, and blood product transfusions. Erythropoietin was recommended if patients had chronic anaemia.
As combination therapy with lenalidomide and dexamethasone is known to increase the risk of VTE, the use of aspirin was strongly encouraged (Palumbo et al, 2008; Rajkumar et al, 2005). If a patient was unable to tolerate aspirin, the use of a low-molecular-weight heparin (LMWH) or warfarin was recommended.
Concomitant use of other anti-myeloma therapies was prohibited. Radiation was allowed to treat pathological fractures or post-fracture pain, and if initiated prior to study enrolment to pre-existing disease. Radiation due to other reasons was considered to be a treatment failure, and patients were taken off study.
Marker assessment
Blood samples were obtained at baseline and/or within 14 days of starting cycle 1, serially on day 1 of each subsequent cycle, or as directed for any dose modification or interruption for up to 8 cycles. The samples were collected in citrated tubes, processed immediately and frozen within 24 h. Any clinical evidence of VTE that developed during the study period was recorded.
The following markers of coagulation, endothelial injury, and angiogenesis were assessed: fibrinogen [normal (nl), 1.5–4.0 g/l] ; plasminogen (nl, 60–250 mg/l); homocysteine (nl, 5–15 μmol/l); von Willebrand factor (VWF) (nl, 300–1800 u/l); factor VIII (nl, 50–200%); factor V Leiden; protein S (nl, 66–140%); prothrombin (DNA-based test); tissue factor pathway inhibitor (TFPI) (nl, 75–120 μg/l); antithrombin (nl, 19·7–262 g/l); protein C (nl, 66–140%); plasminogen activator inhibitor-1 (PAI-1) (nl, 4–43 μg/l); cathepsin G (nl, <15 u/ml); intercellular adhesion molecule (ICAM) (nl 115–306 μg/l); vascular cell adhesion molecule (VCAM) (nl 341–991 μg/l); tumour necrosis factor-α (TNF-α) (nl, 829–3170 ng/l); P-selectin (nl, 22–53 μg/l); interleukin-6 (IL-6) (nl, 0·428–9·96 μg/l); vascular endothelial growth factor (VEGF) (nl, 0–115 μg/l); and β-fibroblast growth factor (β-FGF) (nl, 1·7–27·8 ng/l). These tests were performed using assays that were based on enzyme-linked immunosorbent assay, activity, thrombin, DNA, prothrombin time (PTT) clotting or chromogenic activity.
Changes in absolute marker value and changes relative to baseline were calculated for each patient across cycles, and by dexamethasone dose and schedule.
Statistical analyses
Both actual marker value and the relative marker value to baseline value were assessed for each patient across cycles. Exploratory analysis was performed with descriptive statistics including means, medians, interquartile range, profile and box plots, and smoothing curves across cycles. A linear mixed effect model with first-degree auto-correlation as covariance structure was used to test for trends in absolute marker values across cycles, treating participants as a random effect for both intercept and slope. Relative marker values were used to compare variations between patients under different dexamethasone doses (low vs. high) and schedules (pulsed vs. weekly). The F test was used to compare variations at each cycle, as well as in mean adjusted relative marker values from all cycles by ignoring the potential correlation among cycles. Influence of potential outliers was checked by performing analysis with and without outliers. Due to the exploratory nature of this study, no adjustments for multiple comparisons were made. Given the relatively low incidence of VTE in this study, investigation of a correlation between VTE and markers of endothelial stress and coagulation was not possible.
Results
A total of 33 patients were enrolled in the study. Women comprised 30% (10/33) of the study population (Table I). Overall, 31 patients (94%) were white, 1 patient (3%) was black, and 1 patient (3%) was Hispanic. The median age at enrolment was 60 years (range, 45–83 years). Patients were equally distributed across Durie-Salmon stages, with 33% of patients (11/33) at stage I, 30% (10/33) at stage II, and 36% (12/33) at stage III.
Table I.
Patient baseline characteristics.
| Total no. of patients (N = 33) | |
|---|---|
| Median age, years (range) | 60 (45–83) |
| Sex, n (%) | |
| Female | 10 (30) |
| Male | 23 (70) |
| Race, n (%) | |
| White | 31 (94) |
| Black | 1 (3) |
| Hispanic | 1 (3) |
| Durie-Salmon stage, n (%) | |
| I | 11 (33) |
| II | 10 (30) |
| III | 12 (36) |
| Type of prior therapy, n (%) | |
| Alkylators | 20 (60) |
| Anthracyclines | 8 (24) |
| Stem cell transplantation | 12 (36) |
| Bortezomib | 20 (61) |
| Vinca alkaloid | 8 (24) |
| Thalidomide | 31 (94) |
| Dexamethasone | 31 (94) |
| Bisphosphonate | 30 (91) |
| Other | 8 (24) |
| Median number of prior lines of therapy*, (range) | 5 (3–9) |
Most lines of therapy involved combinations of drugs.
All patients received anticoagulation, with 21 patients (64%) receiving aspirin alone; 19 patients received aspirin 325 mg/day and 2 patients received 81 mg/day. Five patients (15%) were on warfarin alone, of which 2 patients were on treatment dose and 3 patients were on 1–2 mg/day. Two patients (6%) used a LMWH, of which 1 patient was on treatment dose and 1 patient was on prophylactic dose. A total of 5 patients (15%) were on a combination of the above; 3 patients were on full-dose anticoagulation and 2 patients were on prophylactic dose. All patients were relapsed or relapsed and refractory, and thus had received multiple lines of prior anti-myeloma therapy, with the median number of previous lines of treatment being 5.
Incidence of VTE
The total incidence of VTE was 9%; 2 patients developed VTE while on study and 1 patient developed VTE after completing the planned eight cycles of treatment but while still receiving lenalidomide and dexamethasone as part of continuous therapy.
Both patients who developed VTE while on study had been taking 325 mg/day of aspirin. One of these patients, who had received high-dose, pulsed dexamethasone throughout their treatment, developed deep-vein thrombosis (DVT) 3 months after study initiation. The patient was taken off aspirin and given a LMWH as a bridge to warfarin. The patient was subsequently maintained on warfarin throughout the course of the study. The second patient developed DVT 5 months after initiating therapy. This patient was taken off aspirin and treated with full-dose LMWH for the remainder of the study. Likewise, this patient had been on pulsed dexamethasone but a month prior to the DVT event this patient had been switched to weekly, low-dose dexamethasone because of other toxicity associated with the higher dose pulsed schedule. The third patient developed DVT during continued treatment with lenalidomide and dexamethasone after the planned 8 cycles. This patient had been on full-dose warfarin throughout the study and had received weekly low-dose dexamethasone.
Markers of endothelial injury, coagulation, and angiogenesis
Overall, 22 endothelial stress and coagulation markers were tested (Fig 1). Not all patient samples could be analysed for each cycle. Ultimately, analysis included 25 patients at cycle 1, 28 patients at cycle 2, 24 patients at cycle 3, 18 patients at cycle 4, 14 patients at cycle 5, 11 patients at cycle 6, 8 patients at cycle 7, and 2 patients at cycle 8. The linear mixed effect model revealed a significant decreasing trend in absolute values over the course of therapy for the following markers: ICAM (P = 0·05); fibrinogen (P = 0·008); PAI-1 (P < 0·001); homocysteine (P = 0·002); and P-selectin (P < 0·001). The corresponding estimated slopes were −8·49, −10·89, −2·28, −0·34, and −4·39, respectively. Mean ± standard deviation (SD) absolute values at baseline for these markers were: 289·6 ± 100·9 μg/l; 4.095 ± 1.593 g/l; 56·5 ± 17·6 μg/l; 14·1 ± 4·4 μmol/l; and 78·0 ± 31·0 μg/l, respectively. Fig 2 illustrates the decreasing trend observed with PAI-1 and P-selectin, which was apparent both with and without the inclusion of outliers. Although the trend observed was a decrease, there was considerable variance among each patient and with each marker. For example, for PAI-1, there were 2 patients who had levels decrease with each cycle, 3 patients who had levels increase with each cycle, 3 patients who initially had an increase followed by a decrease, 4 patients who initially had a decrease followed by an increase followed by a decrease, 4 patients who initially had a decrease followed by an increase but rarely higher than the original value, 5 patients who initially had an increase followed by a decrease followed by an increase but rarely higher than original value, 4 patients who initially had a decrease followed by an increase followed by a decrease followed by an increase, 1 patient who initially had an increase followed by a decrease followed by an increase followed by a decrease, 5 patients who had 5 changes, 3 which ended with a decrease and 2 with an increase, and 1 patient who had 7 changes which ended with a decrease.
Fig 1.

Heatmap plot of 22 markers with values from 33 patients across 8 cycles. Each column represents one marker, each row represents the measurements from one patient under one cycle. Light colours indicate higher values and darker colours (e.g. red) indicate lower values when compared to normal values.
Fig 2.

Profile plot for markers PAI-1 (A) and P-selectin (B). Each line represents one patient, and each circle represents the marker value for that particular treatment cycle. The broken red line is a smoothing curve for all patients, and represents the absolute value of the studied marker over treatment cycles. Similar graphs were generated for ICAM, fibrinogen, and homocysteine.
Similar graphs were generated for ICAM, fibrinogen, and homocysteine. Given the relatively low incidence of VTE in this study, investigation of a correlation between VTE and markers of endothelial stress and coagulation was not possible.
Dexamethasone dose and schedule
Treatment and laboratory data were available for 32 patients. Overall, 23 patients (72%) received the pulsed schedule of dexamethasone and 9 (28%) received the weekly schedule; 1 patient with baseline data only was not included (Fig 3). Compared with the weekly schedule, the pulsed schedule was associated with significantly greater variation in mean adjusted relative marker values for the following markers: fibrinogen (SD 0·42 vs. 0·26); P-selectin (SD 0·45 vs. 0·23); and VEGF (SD 0·69 vs. 0·35) (P < 0·001 for all comparisons). Fig 4 illustrates how the variation in VEGF values was greater with the pulsed schedule than with the weekly schedule. Similar patterns were seen for fibrinogen and P-selectin.
Fig 3.

Schedule of dexamethasone used for each patient according to treatment cycle. Each row represents one patient and each column represents one treatment cycle.
Fig 4.
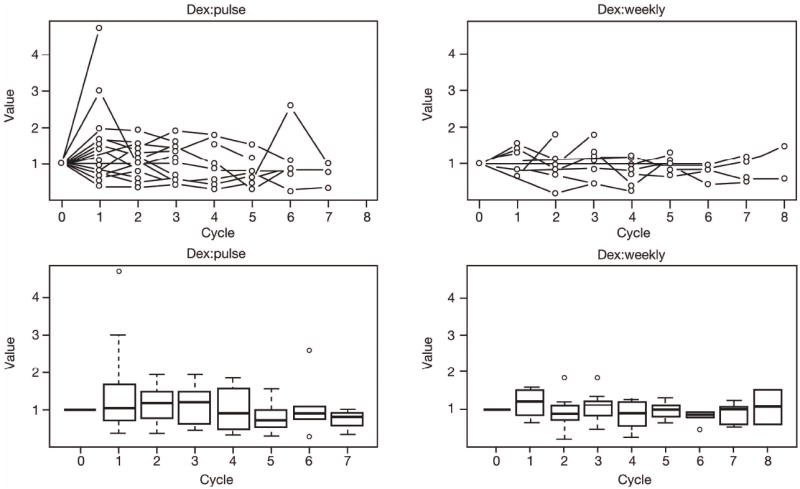
Profile plots (top) and box plots (bottom) showing the degree of variation in VEGF values in patients treated with the pulsed (left) or weekly (right) schedule of dexamethasone (Dex). Similar graphs were seen for fibrinogen and P-selectin.
No clear pattern was observed with regards to marker variation and the dose of dexamethasone. Notably, the majority of patients who started on high-dose dexamethasone required dose reductions for expected toxicity associated with this agent and this may have confounded the results.
Discussion
This study is one of the most comprehensive assessments to-date of markers of coagulation and endothelial stress in patients with MM treated with lenalidomide and dexamethasone, and provides novel insights into the mechanism of VTE in this setting. Our main findings suggest that the schedule of dexamethasone influences the level of endothelial stress, and that the conventional, pulsed schedule of dexamethasone induces a greater degree of endothelial injury than the weekly schedule. This effect may contribute to the increase in risk of VTE seen with pulsed administration of dexamethasone when given in combination with lenalidomide.
Fig 5 illustrates possible mechanisms by which the combination of lenalidomide and dexamethasone may contribute to endothelial injury in MM patients and so activate other putative downstream effects that could lead to thrombosis. Lenalidomide and dexamethasone are likely to have direct and indirect effects on endothelial cells that could stimulate production of endothelial stress markers (e.g. ICAM and PAI-1), and pro-angiogenic factors, such as VEGF. Treatment may also induce the coagulation cascade, leading to increases in fibrinogen, P-selectin, and homocysteine, which may in turn contribute to a hypercoagulable state beyond the pro-thrombotic effects of the disease itself. The considerable variance that we observed in patients’ endothelial makers including initial increases followed by decreases may reflect the changes caused by both treatment effect and subsequent response; thus examining the effects overall (as we did) are likely to be more informative given these potential confounders.
Fig 5.

Potential mechanism by which dexamethasone and lenalidomide may promote the formation of venous thromboembolism. Treatment may have direct effects on endothelial cells, resulting in upregulation of intercellular adhesion molecule (ICAM) and plasminogen activator inhibitor-1 (PAI-1) expression. Dexamethasone and lenalidomide therapy may also induce the coagulation cascade, resulting in increased levels of fibrinogen, P-selectin, and homocysteine. VEGF, vascular endothelial growth factor.
We found no clear relationship between dexamethasone dose and marker variation. However, the fact that most patients treated with high-dose dexamethasone required dose reductions confounds these results. Similarly, no association was found between the incidence of VTE and markers of endothelial injury and coagulation due to the relatively low incidence of VTE in this patient cohort (9%). Other analyses have reported VTE incidence rates up to 24% in patients with relapsed or refractory MM treated with lenalidomide and dexamethasone in the absence of prophylactic anticoagulation (Bennett et al, 2006). In two randomized controlled trials evaluating lenalidomide plus dexamethasone in this setting, 11–15% of patients experienced VTE although, importantly, thromboprophylaxis was not required in these studies, which may partly explain the higher incidence seen compared to our study (Dimopoulos et al, 2007; Weber et al, 2007).
Due to the small number of participants, this study was underpowered to detect the potential influence of anticoagulant use on endothelial stress markers. We recommend this be the subject of future analyses.
The profile of endothelial stress identified in this study may be different in newly diagnosed, previously untreated MM patients or in patients treated with a different schedule of dexamethasone. However, it is well recognized that patients with newly diagnosed MM have a greater prothrombic profile than patients with relapsed or relapsed and refractory MM, making this observation all the more valid (Palumbo et al, 2008). As an example, in the E4A03 study of newly diagnosed MM, lenalidomide plus low-dose weekly dexamethasone resulted in longer median survival than did lenalidomide plus high-dose pulsed dexamethasone (1-year survival rate: 96·5% vs. 86·0%, respectively) as a result of excess toxicity in the high-dose group, including thromboembolism, which in turn led to more frequent treatment interruptions and high rates of disease progression (Rajkumar et al, 2010). Subsequently, Gandhi et al (2010) examined the pharmacological activity of lenalidomide and dexamethasone in various MM cell lines, and confirmed that although dexamethasone enhances the anti-proliferative effects of lenalidomide on MM cells, it also inhibits its immunostimulatory effects on T cells and natural killer cells. They suggested that the use of lower doses of dexamethasone may facilitate lenalidomide in killing MM cells without interfering with its immunomodulatory effects (Gandhi et al, 2010). Similar benefits may be achieved in terms of safety. In the E4A03 trial, the incidence of VTE was 26% in patients who received lenalidomide plus high-dose pulsed dexamethasone (considered standard treatment at the time), compared with 11% in those treated with lenalidomide plus low-dose, weekly dexamethasone (40 mg/day on days 1, 8, 15, and 22 of each 28-day cycle) (Jacobus et al, 2007; Rajkumar et al, 2010). Initially, routine thromboprophylaxis was recommended but not required. After the first 264 patients were enrolled, the protocol was amended to require aspirin thromboprophylaxis in all patients. In addition, more potent thromboprophylaxis with either warfarin (target international normalized ratio 2–3) or a LMWH was recommended for patients assigned to the then standard high-dose, pulsed dexamethasone schedule. Rates of VTE, however, did not differ significantly before and after the protocol was amended, supporting the notion that pulsed, high dose dexamethasone was still deleterious in this respect. The findings from our current study may also help inform more effective approaches to both steroid dose/schedule and thromboprophylaxis, based on the molecular effects of lenalidomide and dexamethasone on endothelial cells and coagulation pathways.
The immunomodulatory drug pomalidomide has also been evaluated in patients with relapsed and relapsed and refractory MM with generally reported low rates of VTE reported (Streetly et al 2008; Lacy et al, 2009; Lacy et al, 2010; Lacy et al, 2011; Leleu et al, 2010, Vij et al, 2012). In one phase I study, DVT was observed in 3 of 19 patients (16%) who received at least 4 weeks of treatment with pomalidomide with or without dexamethasone; prophylaxis was not mandated (Schey et al, 2004). In another phase I study, using alternate-day dosing of pomalidomide and no prophylaxis, none of the 20 patients developed VTE (Streetly et al, 2008). In phase II studies of the combination of pomalidomide and dexamethasone that mandated prophylaxis, VTE were low (0–4.3%) (Lacy et al, 2009; Lacy et al, 2010; Lacy et al, 2011; Leleu et al, 2010). Notably, pomalidomide treatment was shown to have no significant effect on markers of endothelial function (VCAM) haemostasis (prothrombin), and fibrinolysis (D-dimers) in a 4-week study in patients with relapsed/refractory MM patients (Streetly et al, 2005). These results suggest that the combination of pomalidomide and dexamethasone may have less effect on endothelial stress activation than the combination of lenalidomide and dexamethasone, although it is important to note that dexamethasone dosing was uniformly weekly and low dose (versus pulsed) in these pomalidomide-based combination studies, again supporting the notion that dexamethasone dose and schedule is important in this setting.
Results from this relatively small observational study do, of course, require validation in larger prospective studies in order to determine if these markers can help identify MM patients at higher risk of developing VTE during treatment with lenalidomide and dexamethasone. If these results are validated, prospective, randomized controlled trials are warranted to evaluate improving the efficacy of thromboprophylaxis in modifying these markers and preventing VTE. Clinical application of this information also depends, in part, on the ability to assess markers of coagulation and endothelial stress rapidly and reliably. Therefore, new diagnostic technology must be developed in conjunction with the clinical trials proposed above to facilitate the assessment of key markers related to thrombotic risk. The ThromboPath system (Instrumentation Laboratory Company, Bedford, MA, USA), for example, is a new chromogenic assay that can be used to assess prothrombotic risk factors that affect the protein C pathway (Toulon et al, 2009). This technology may allow for rapid, “real time” assessment of thrombotic risk so that appropriate prophylaxis may be initiated for at-risk patients in a timely manner.
In summary, these findings provide greater insight into the effects of lenalidomide and dexamethasone on pathways of endothelial cell injury and coagulation, which may contribute to the increased risk of VTE observed in MM patients treated with this regimen. They also lend support to further clinical evaluation of new strategies aimed at reducing the risk of VTE in MM patients—through more effective thromboprophylaxis (including newer agents, such as defibrotide which specifically targets endothelial stress and has shown promise in this setting) (Mitsiades et al, 2009; Palumbo et al, 2010); low-dose, weekly schedules of dexamethasone; or the use of lenalidomide in combination with other novel agents, such as bortezomib (Richardson et al, 2007; Richardson et al, 2009; Richardson et al, 2010).
Acknowledgments
Support for this project was provided by the Rick Corman Multiple Myeloma Research Foundation and Celgene Corporation. The authors gratefully acknowledge Michelle Maglio for her extensive administrative support; Jacob Laubach MD for his review of the manuscript and contributions to the discussions; and the assistance of the Medical Arts Core, DFCI, Boston, MA, USA.
The authors also received editorial support in the preparation of this manuscript, funded in part by Celgene Corporation. The authors are fully responsible for the content, writing, and editorial decisions for this manuscript.
Footnotes
Conflict of interest
None: R. Rosovsky, F. Hong, D. Tocco, B. Connell, CS. Mitsiades, D. Warren, G. Bradwin, M. Doyle, R.J. Soiffer, R.S. Schlossman, J. Laubach, M. Maglio and E. Weller.
Instrumentation Laboratory (employee): M. Doyle.
Celgene (Advisory Board, Research Support): I. Ghobrial, N.C.Munshi, K.C. Anderson and P. Richardson.
References
- Bennett CL, Angelotta C, Yarnold PR, Evens AM, Zonder JA, Raisch DW, Richardson P. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. Journal of the American Medical Association. 2006;296:2558–2560. doi: 10.1001/jama.296.21.2558-c. [DOI] [PubMed] [Google Scholar]
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Journal of the American Medical Association. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, Corso A, Masliak Z, Olesnyckyj M, Yu Z, Patin J, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. New England Journal of Medicine. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinoso L, Fang W, Xu S, Hampton G, Bartlett JB, Schafer P. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Current Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- Jacobus S, Kumar S, Callander NS, Abonour R, Fonseca R, Siegel D, Greipp P, Rajkumar SV. Effect of Venous Thrombotic Events on Overall Survival in Multiple Myeloma: Analysis of Thrombotic Events Occurring in E4A03A Randomized Trial of Lenalidomide Plus High-Dose Dexamethasone (RD) Versus Lenalidomide Plus Low-Dose Dexamethasone (Rd) in Newly Diagnosed Multiple Myeloma, a Trial Coordinated by the Eastern Cooperative Oncology Group (ECOG). Blood; ASH Annual Meeting Abstracts; 2008. Abstract 1750. [Google Scholar]
- Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Kyle RA, Fonseca R, Bergsagel PL, Roy V, Mikhael JR, Stewart AK, Laumann K, Allred JB, Mandrekar SJ, Rajkumar SV. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. Journal of Clinical Oncology. 2009;27:5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Mikhael JR, Stewart AK, Laumann K, Allred JB, Mandrekar SJ, Rajkumar SV, Buadi F. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Stewart AK, Laumann K, Mandrekar SJ, Reeder C, Rajkumar SV, Mikhael JR. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of two dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leleu X, Attal M, Moreau P, Duhamel A, Fermand JP, Michalet M, Traulle C, Marit G, Roussel M, Kolb B, Arnulf B, Garderet L, Mathiot C, Macro M, Odile Petillon M, Pegourie B, Stoppa AM, Brechiniac S, Royer B, Benboubker L, Caillot D, Escoffre-Barbe M, Hulin C, Harousseau J-L, Avet-Loiseau H, Facon T. Phase 2 study of 2 modalities of pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. IFM 2009-02. Blood. 2010;116 abstract 859. [Google Scholar]
- Mitsiades CS, Rouleau C, Echart C, Menon K, Teicher B, Distaso M, Palumbo A, Boccadoro M, Anderson KC, Iacobelli M, Richardson PG. Preclinical studies in support of defibrotide for the treatment of multiple myeloma and other neoplasias. Clinical Cancer Research. 2009;15:1210–1221. doi: 10.1158/1078-0432.CCR-08-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, Harousseau J, Zonder JA, Cavo M, Zangari M, Attal M, Belch A, Knop S, Joshua D, Sezer O, Ludwig H, Vesole D, Blade J, Kyle R, Westin J, Weber D, Bringhen S, Niesvizky R, Waage A, von Lilienfeld-Toal M, Lonial S, Morgan GJ, Orlowski RZ, Shimizu K, Anderson KC, Boccadoro M, Durie BG, Sonneveld P, Hussein MA. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Larocca A, Genuardi M, Kotwica K, Gay F, Rossi D, Benevolo G, Magarotto V, Cavallo F, Bringhen S, Rus C, Masini L, Iacobelli M, Gaidano G, Mitsiades C, Anderson K, Boccadoro M, Richardson P. Melphalan, prednisone, thalidomide and defibrotide in relapsed/refractory multiple myeloma: results of a multicenter phase I/II trial. Haematologica. 2010;95:1144–1149. doi: 10.3324/haematol.2009.017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Gertz MA. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. The Lancet Oncology. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, Schlossman RL, Rajkumar SV, Desikan KR, Hideshima T, Munshi NC, Kelly-Colson K, Doss D, McKenney ML, Gorelik S, Warren D, Freeman A, Rich R, Wu A, Olesnyckyj M, Wride K, Dalton WS, Zeldis J, Knight R, Weller E, Anderson KC. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Hideshima T, Mitsiades C, Anderson KC. The emerging role of novel therapies for the treatment of relapsed myeloma. Journal of the National Comprehensive Cancer Network. 2007;5:149–162. doi: 10.6004/jnccn.2007.0015. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, Mazumder A, Munshi NC, Ghobrial IM, Doss D, Warren DL, Lunde LE, McKenney M, Delaney C, Mitsiades CS, Hideshima T, Dalton W, Knight R, Esseltine DL, Anderson KC. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. Journal of Clinical Oncology. 2009;27:5713–5719. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Avigan DE, Xie W, Ghobrial IM, Schlossman RL, Mazumder A, Munshi NC, Vesole DH, Joyce R, Kaufman JL, Doss D, Warren DL, Lunde LE, Kaster S, Delaney C, Hideshima T, Mitsiades CS, Knight R, Esseltine DL, Anderson KC. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thrombosis and Haemostasis. 2002;87:575–579. [PubMed] [Google Scholar]
- Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. Journal of Clinical Oncology. 2004;22:3269–3276. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- Streetly M, Hunt BJ, Parmar K, Jones R, Zeldis J, Schey S. Markers of endothelial and haemostatic function in the treatment of relapsed myeloma with the immunomodulatory agent Actimid (CC-4047) and their relationship with venous thrombosis. European Journal of Haematology. 2005;74:293–296. doi: 10.1111/j.1600-0609.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. British Journal of Haematology. 2008;141:41–51. doi: 10.1111/j.1365-2141.2008.07013.x. [DOI] [PubMed] [Google Scholar]
- Toulon P, Smirnov M, Triscott M, Safa O, Biguzzi E, Bouziane K, Tripodi A. A new chromogenic assay (HemosIL ThromboPath) is sensitive to major prothrombotic risk factors affecting the protein C pathway. Results of a multicenter study. Thrombosis Research. 2009;124:137–143. doi: 10.1016/j.thromres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Vij R, Richardson PG, Jagannath S, Siegel DS, Baz RC, Srinivasan S, Larkins G, Zaki MH, Hussein MA, Anderson KC. Pomalidomide (POM) with or without low-dose dexamethasone (LoDEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Outcomes in pts refractory to lenalidomide (LEN) and/or bortezomib (BORT) J Clin Oncol. 2012;30(suppl):513s. [abstract 8016] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. New England Journal of Medicine. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]


