Abstract
Laparoscopic paraesophageal hernia repair continues to be one of the most challenging procedures facing the minimally invasive surgeon. A thorough understanding of the tenets of the operation and advanced skills in minimally invasive laparoscopy are needed for long-term freedom from symptomatic and anatomic recurrence. These include complete reduction of the hernia sac from the mediastinum back into the abdomen with careful preservation of the integrity of muscle and peritoneal lining of the crura, aggressive and complete mobilization of the esophagus to the level of the inferior pulmonary vein, clear identification of the gastroesophageal junction to allow accurate assessment of the intraabdominal esophageal length and use of Collis gastroplasty when esophageal lengthening is required for a tension-free intraabdominal repair. Liberal mobilization of the phrenosplenic and phrenogastric attachments substantially increases the mobility of the left limb of the crura, allowing for a tension-free primary closure in a large percentage of patients. The following describes our current approach to laparoscopic paraesophageal hernia repair following a decade of refinement in a high-volume center.
Keywords: Hernia, hiatal, Laparoscopy, Gastroesophageal Reflux, Mesh, surgical, Gastroplasty
Introduction
At the thirty-seventh annual meeting of the American Association for Thoracic Surgery in Chicago, IL (May 4–7, 1957), J. Leigh Collis presented “An Operation for Hiatus Hernia with Short Esophagus”. In this address, he noted that “most of the operations which have been suggested for the relief of this condition are large and ill-suited to patients who are frail and often aged … dissatisfaction with the situation this presents had lead many surgeons to return to a palliative line, and to treat such patients by posture and dilatation …” [1] In 2010, the optimal approach to repair of giant paraesophageal hernia continues to be debated. Proponents of an open approach, through either a thoracotomy or laparotomy, highlight a very low rate of reoperation for recurrent hernia, but perioperative morbidity and mortality can be significant. [2–5]
Laparoscopy was introduced in the early 1990’s and was quickly adopted by esophageal surgeons as an opportunity to provide operative repair without the morbidity of the open procedures. Since that time, we and others have established the feasibility and safety of a laparoscopic approach to giant paraesophageal hernia repair. [6–9] When perioperative outcomes are compared directly with open techniques, postoperative morbidity and mortality, blood loss, and hospital length of stay are significantly reduced for the laparoscopic approach. [2,3,10] Despite the immediate benefits of a laparoscopic approach, however, there is still considerable debate as to the technical details of the procedure, such as the need for esophageal lengthening or mesh cruraplasty, as well as concerns for long-term durability of the repair. Attempts to address these questions have been limited by the small numbers of patients reported in most series.
With increasing experience in advanced laparoscopic techniques, laparoscopic repair has become the standard approach to giant paraesophageal hernia at our center. Over the past decade, we have refined the operation and acquired significant experience, which has resulted in excellent long-term outcomes and a recurrence and reoperation rate that is comparable with published open series. [9,11] The following describes our current approach to laparoscopic paraesophageal hernia repair.
Preoperative assessment
Laparoscopic repair of paraesophageal hernia begins with a careful preoperative evaluation. We obtain a careful symptom history, including assessment of typical symptoms of gastroesophageal reflux (heartburn, regurgitation) and dysphagia. Additional symptoms that are common include chest or epigastric pain, recurrent aspiration with or without associated pneumonia, cough, shortness of breath and dyspnea on exertion. Signs of compromised blood flow to the herniated stomach may be subtle, such as iron-deficiency anemia, or present overtly as an acute gastric volvulus with ischemia or frank necrosis. Patients often report knowledge of a ‘hiatal hernia’ for many years.
Next, we obtain a radiographic evaluation by barium swallow. Abnormal esophageal motility is often evident on barium swallow in patients with paraesophageal hernia; associated abnormalities, such as amotile esophagus, achalasia, endoluminal masses or diverticuli, can also be assessed. Barium swallow is useful for identifying the location of the gastroesophageal junction (GEJ), assessing the degree to which the stomach is herniated into the chest and evaluating for evidence of volvulus. Because the esophagus is often foreshortened and tortuous, we rarely perform motility studies using manometry due to difficulties with proper placement of the catheter and the risk of esophageal perforation. We also rarely perform esophageal pH studies. Laboratory studies include assessment of the hemoglobin for occult anemia and serum albumin levels for evaluation of nutritional status. We obtain pulmonary function testing in patients with a complaint of shortness of breath to determine whether the breathing difficulties are due to restriction of lung function due compression of adjacent lung by herniated stomach or to coexisting intrinsic lung disease.
Surgical Procedure
Patient Preparation, Positioning and Port Placement
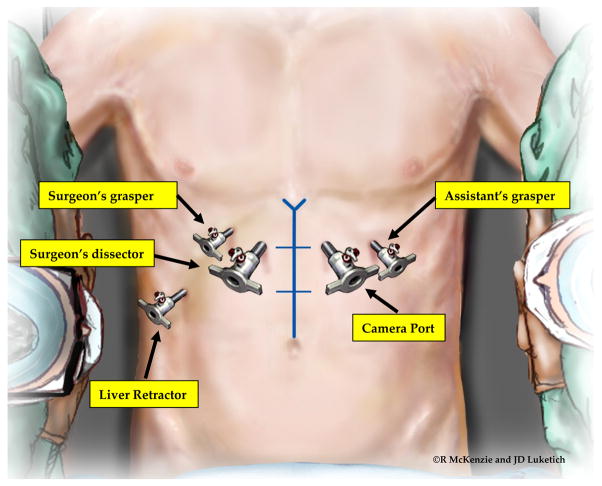
After the preoperative evaluation is complete, the patient is advised of the risks and benefits of the operation, and informed consent is obtained. Once this is accomplished, the patient is taken to the operating room. The preferred position of the patient is supine with the surgeon on the patient’s right side and the assistant on the left. Sequential compression devices are placed on the legs bilaterally. Patients should also receive 5,000 units of heparin subcutaneously prior to induction of anesthesia. [12] A Foley catheter is placed. The patient’s arms are rotated away from the patient, secured to an arm board at a 45° angle from the bed and carefully padded. This angle provides adequate access to the operating table and minimizes the risk of stretch injury to the brachial plexus. A foot stop is placed to facilitate reverse Trendelenburg positioning. After sterile prep and antibiotic prophylaxis, we identify the midline from the xiphoid to the umbilicus and use a skin marker to divide the distance into thirds. (Figure 1) We place the first 10-mm port, using the open Hassan technique, [13] in the right paramedian line approximately one-third of the way from the xiphoid to the umbilicus. Care is taken to avoid dissection into the falsiform ligament. Because of the extensive dissection within the mediastinum, this port must be placed in the upper third of the abdomen. Once we confirm placement within the peritoneal cavity, we insufflate the abdomen to a pressure of 12 to 15 mmHg. We position a second 5- or 10-mm port, for placement of the camera, in the left paramedian line at approximately the same level. We place two 5-mm ports in the mid-clavicular line directly below the costal margin, one on each side, leaving a hands-breath between the paramedian and subcostal ports. We place a final 5-mm port in the far right lateral subcostal position for liver retraction or in the subxiphoid position, depending upon the type of liver retractor.
Figure 1.
Surgeon and port position. Port placement and instrument positions are shown. In a non-obese patient, the ports are positioned one-third of the distance from the xiphoid to the umbilicus. In obese patients, this measure is often inaccurate because of the increased abdominal circumference. In this situation, the patient’s bony anatomy can be used to determine appropriate placement with an imaginary line across the abdomen at top of the anterior superior iliac spines serving as a marker for the normal distance to the umbilicus.
Reducing the Hernia Sac
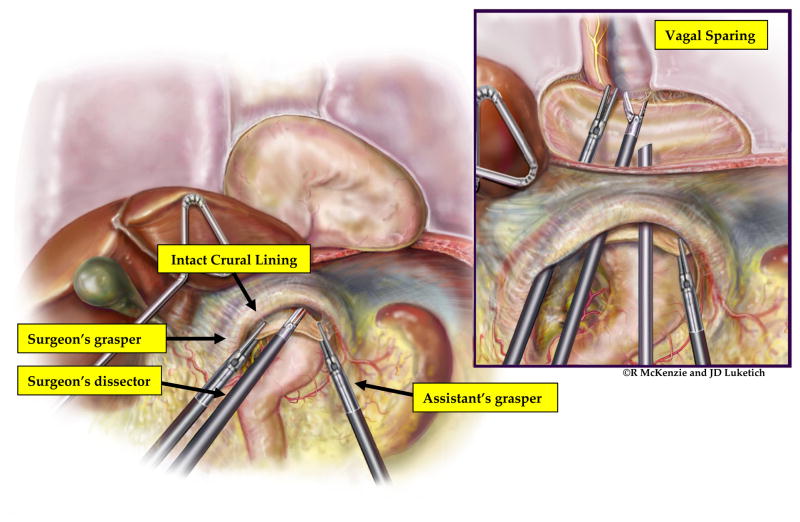
Following port placement and liver retraction, we examine the hiatal hernia. To facilitate visualization of the hiatus, the operating room table is placed in reverse Trendelenburg, allowing the upper abdominal contents to shift toward the patient’s pelvis and away from the hiatus. If necessary, partial reduction of redundant stomach, omentum and bowel is performed upon initial assessment; it is not desirable, however, to then place traction on the stomach as a means of reducing the sac. This causes unnecessary trauma to the stomach and prevents the assistant from being able to assist in the mediastinal dissection. Because the hernia sac is an extension of the peritoneal lining of the cardia of the stomach, reduction of the sac back into the abdomen will, by default, also reduce the stomach. Rather than placing retraction on the stomach, the surgeon and assistant grasp the hernia sac just beyond the diaphragmatic crura at the 12 o’clock position using the surgeon’s left hand and the assistant’s right hand. (Figure 2) Then, we enter the sac using ultrasonic dissection with the harmonic scalpel (Ethicon, Cincinnati, OH) or the ultrasonic shears (US Surgical/Covidien, Mansfield, MA). This allows access to the areolar attachments of the hernia sac to the mediastinal structures. The mediastinal dissection proceeds with sharp ultrasonic dissection. We use blunt dissection sparingly, as this technique can result in bleeding that obscures the operative view. Dissection in the mediastinum proceeds until the entire hernia sac is reduced into the abdomen. (Figure 3) We take care to identify both the anterior and posterior vagus nerves as they traverse the mediastinum and avoid injury to these vital structures. It is also important to identify and maintain intact pleura to avoid hemodynamic instability related to a pneumothorax. Reduction of the sac back into the abdomen, by default, returns the stomach to its anatomic position without the risk of traction injury that can occur with the hand-over-hand technique.
Figure 2.
Reduction of the hernia sac without retraction on the stomach. The operation begins with the surgeon and the assistant everting the hernia sac just beyond the lip of the anterior crura. The sac is incised, allowing entry into the anterior mediastinum. Dissection proceeds using a combination of blunt dissection and coagulation of the fine areolar attachments of the hernia sac to the surrounding mediastinal structures (inset). During this dissection, care is taken to maintain the integrity of the crural lining and avoid damage to the crural muscle, vagus nerves, or pleura. Note the stomach is not being retracted during this dissection. (Video 1)
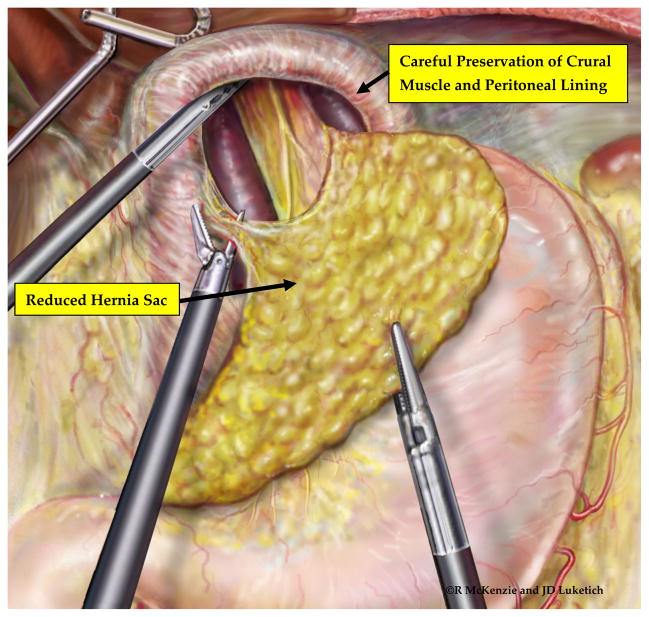
Figure 3.
Establishment of an intraperitoneal stomach after complete reduction of the hernia sac. Complete reduction of the sac and esophageal mobilization may require 1–2 hours of dissection within the mediastinum, but is critical for the long-term success of the operation.
Reestablishing Adequate Intraabdominal Esophageal Length
After reducing the hernia sac, we separate the hernia sac from the crura, taking care to leave the crural peritoneal lining intact. (Figure 4) We believe that strict attention to maintaining the integrity of the peritoneal lining over the crura is critical for the success of primary closure. Without this lining, the crural musculature has no intrinsic strength and, therefore, will not hold suture sufficiently to prevent dehiscence of the crural repair. Throughout the dissection within the mediastinum, therefore, we handle the crura with extreme care and avoid injury as much as possible.
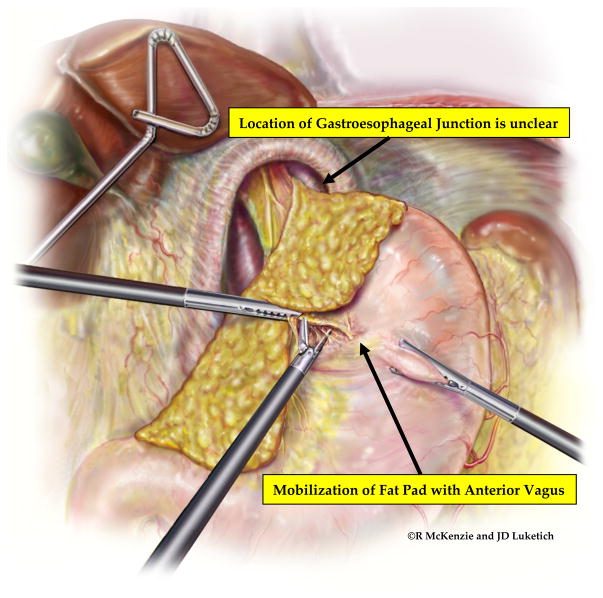
Figure 4.
Mobilization of the esophageal fat pad and identification of the GEJ. To facilitate precise identification of the GEJ and prevent inadvertent placement of the fundoplication around tubularized proximal stomach, the esophageal fat pad is carefully dissected from the anterior surface of the stomach, taking care to preserve the integrity of the anterior and posterior vagal nerves. This dissection is carried posteriorly, staying close to the esophagus, to create a retroesophageal window. (Video 2)
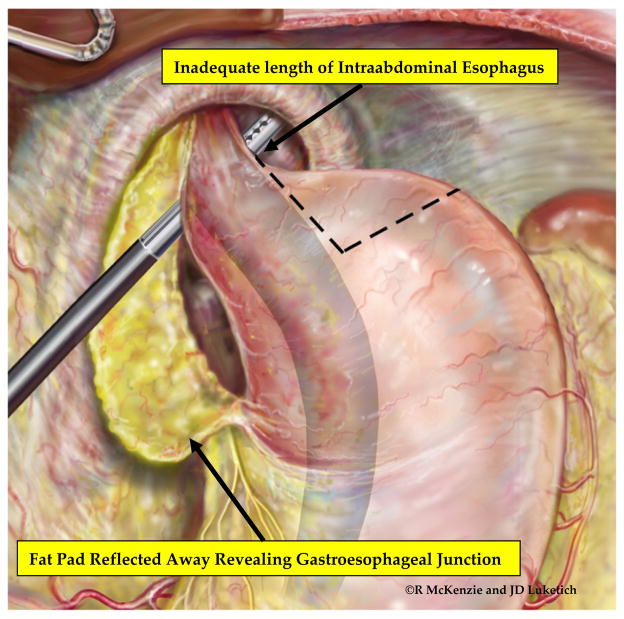
Once we completely reduce the hernia sac and stomach with the crura preserved, we circumferentially mobilize the esophagus within the mediastinum to the level of the inferior pulmonary veins. This mobilization is critical for achieving adequate esophageal length. Throughout this dissection, we identify and carefully preserve the anterior and posterior vagus nerves. At the completion of esophageal mobilization, we divide the short gastric vessels and mobilize the gastric fat pad off the stomach and distal esophagus, using ultrasonic dissection, to allow visualization of the GEJ. We continue the fat pad dissection around the GEJ to create a posterior window between the esophagus and posterior vagus nerve through which to perform the fundoplication. (Figure 5) This allows clear visualization of the longitudinal fibers of the esophagus, which do not have a serosal lining, as they merge with the cardia of the stomach, which does have a serosal lining. Following fat pad mobilization, we assess the location of the GEJ for adequate intraabdominal length in a neutral resting position in the abdomen. If a minimum of 2 cm of tension-free, intraabdominal esophagus is not present, we perform additional mediastinal dissection to further mobilize the esophagus. If esophageal length continues to be inadequate, we perform a Collis gastroplasty using the wedge technique described by Whitson and colleagues. [14] (Figure 6)
Figure 5.
Fully mobilized fat pad provides clear localization of the GEJ and facilitates assessment of esophageal length. The presence of a foreshortened esophagus is determined after creation of the retroesophageal window, assessment of the esophageal length and extensive esophageal mobilization.
Figure 6.
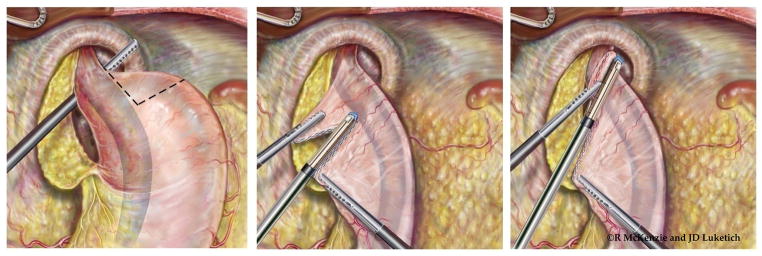
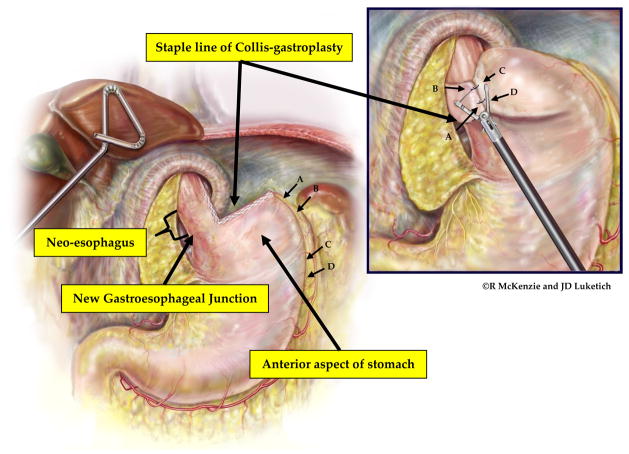
Laparoscopic wedge Collis gastroplasty. If additional esophageal length is needed following extensive esophageal mobilization, the stomach is then grasped at the short gastric vessels and rolled toward the surgeon. The surgeon determines the length of gastroplasty required to create a neoesophagus that will provide at least 2 cm of tension-free intraabdominal esophagus. A 54-french Bougie is placed by the surgeon with direct visualization with the laparoscope to ensure safe passage into the stomach. The surgeon then grasps the stomach just proximal to the planned location of the initial staple line. Depending on the thickness of the stomach, either a 4.8-mm (green load) or 3.5-mm (blue load) cutting endostapler is then applied. Serial fires of the stapler directly perpendicular to the bougie are used to divide the stomach until the staple line reaches the bougie. The surgeon and assistant provide very gentle counter-traction on the proximal and distal aspects of the stomach to draw the lesser curve tight against the bougie. This ensures that the neoesophagus is not patulous. Care must be taken to avoid traction in the cephalad or caudal direction as this can tear the stomach at the crotch of the staple line; this can be difficult to repair and increases the risk of postoperative leak from the Collis gastroplasty. The wedge of stomach is then removed with serial firings of the endostapler parallel to the bougie. (Video 3)
Reestablishing the Antireflux Barrier
Except in rare situations when the surgeon may have concerns regarding the viability of the stomach, patient stability in the operating room, very elderly patients (age 80+) or patients with a significant esophageal motility disorder, we routinely perform an antireflux procedure. Because of the extensive esophageal dissection and complete disruption of the phrenoesophageal ligament, the patient is likely to suffer from significant gastroesophageal reflux postoperatively unless a new antireflux barrier is created. Surgeon preference and preoperative radiographic findings regarding esophageal motility determine whether we perform a circumferential ‘floppy’ fundoplication (2-stitch Nissen over a 54 or 56 bougie) (Figure 7) [15]or a partial fundoplication (Toupet or clam-shell). [16,17] For patients in whom the surgeon decides not to perform fundoplication, many of whom have obstructive symptoms rather than reflux symptoms, the stomach is secured in an intraabdominal position using an extended gastropexy technique. Beginning at the GEJ, serial interrupted horizontal mattress heavy sutures (0-gauge) are used to pexy the stomach to the left crura and anterolaterally to the diaphragm and the anterior abdominal wall. Sutures are placed approximately 2 cm apart over a distance of 10 to 14 cm. This provides multiple points of adherence between the stomach and the abdominal wall and minimizes the risk of large hernia recurrence.
Figure 7.
Creation of ‘floppy, two-stitch’ Collis-Nissen Fundoplication. Maintenance of a proper orientation of the wrap as it passes through the retroesophageal space is critically important for the successful creation of a new antireflux barrier. To begin, a bougie (usually 54-French) is passed into the esophagus under direct vision. An atraumatic instrument is then passed through the retroesophageal window. The line of the short gastric arteries is grasped at the lateral aspect of the gastric cardia (or the proximal fundus if a Collis gastroplasty has been performed; point A) and pulled through the retroesophageal window. If the fundus of the stomach has been adequately mobilized, the wrap will remain in place when the grasper is released from the stomach (Point A). If the wrap is pulled back through the retroesophageal window, the wrap is being tethered (usually by retrogastric attachments) and further mobilization is necessary. When pulling the stomach through the retroesophageal window, it is vital that the stomach maintains correct anteroposterior orientation. In other words, the anterior aspect of the stomach is brought through so that it is flat against the posterior aspect of the (neo-) esophagus. The arrows indicate the staple line of the Collis gastroplasty. If correct orientation of the wrap is maintained, the staple line will be positioned on the inferior edge of the wrap and the greater curvature of the stomach will be sewn to itself with two stitches at the short gastric remnants. (See inset: A to D; B to C) The location of point C is determined using a ‘shoe shine’ maneuver after the wrap is pulled through the window. A grasper is placed at point B and another grasper secures the stomach along the line of the short gastric arteries at point C. Point B and point C are brought together around the esophagus, and the tightness of the wrap around the esophagus is assessed. We prefer a ‘floppy’ fundoplication, with enough space between the wrap and esophagus to allow passage of an atraumatic instrument. If the wrap is deemed to be too tight with the ‘shoe shine’ test, point C is moved further distal on the greater curve (toward point D) and the ‘shoe shine’ maneuver is repeated. Once the wrap is deemed appropriate, a suture is placed from point C to the esophagus to point B to secure the wrap. A second suture is placed from point D to the esophagus (just above the GEJ or at the base of the neo-esophagus) and then to point A. When completed, the external, visible aspects of the wrap will be the posterior wall of the stomach. If the stomach is brought through without maintaining this orientation, the posterior aspect of the stomach will be incorrectly approximated to the back of the esophagus. This results in a folding of the stomach around the esophagus rather than a wrapping of the esophagus and likely contributes to gas bloat symptoms postoperatively. Furthermore, the folded stomach fails to create an adequate antireflux barrier, as evidenced by the absence of the endoscopic ‘stack of coils’ appearance of the newly created antireflux valve. (Video 4)
Repairing the Hiatus
We repair the hiatus in all patients regardless of the decision for fundoplication. If the crura are being tethered by phrenogastric or phrenosplenic attachments, additional dissection is performed to completely mobilize the crura. It is important to avoid direct application of the graspers to the crural muscles as much as possible, as this trauma will disrupt the integrity of the muscle and reduce the likelihood of a primary repair. Maintaining the integrity of the crura requires careful identification of the peritoneal reflection and the pleural reflection within the mediastinum. The pleural reflection, particularly on the left, can often be identified crossing the midline over the esophagus. Identification of the lining early in the procedure will minimize injury to the crural lining and reduce the incidence of intraoperative pneumothorax.
We have found that, with meticulous attention to crural dissection and complete separation of the phrenosplenic attachments, the crura can be sufficiently mobilized to perform a tension-free, primary suture closure of the hiatus in the majority of patients (~85%). We then reapproximate the fully mobilized crura, without tension, using heavy suture (0-gauge). (Figure 8) If the crura were denuded of overlying peritoneum, are sufficiently attenuated such that the ability to hold suture is compromised or are unable to be re-approximated without tension, we reinforce the crura with bioprosthetic mesh.
Figure 8.
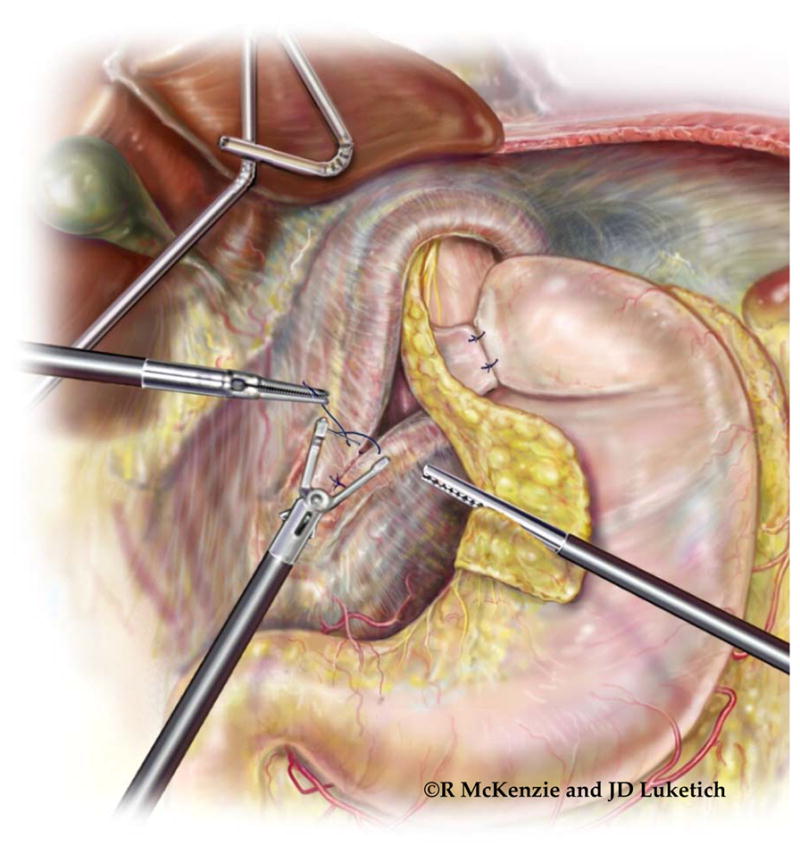
Tension-free hiatal closure. After completion of the fundoplication wrap, the bougie is removed and the crura assessed for tissue integrity and mobility. The crura are re-approximated with heavy (0-gauge) braided, permanent suture. The final, tension-free closure is illustrated in the inset. (Video 4)
Postoperative Course
Following completion of the operation, the patient is typically extubated and transferred to the recovery room. The decision to admit the patient to the intensive care unit (ICU) for postoperative observation is patient-specific and depends on the patient’s comorbid diseases, the urgency of the operation (elective versus non-elective), intraoperative concerns, and the length of the operation. In our series of more than 650 patients, 32% were admitted to the ICU postoperatively with a median ICU stay of 2 days (interquartile range 1–3 days). The median postoperative length of hospital stay was 3 days (interquartile range 2–5 days), reflecting the increased complexity of paraesophageal hernia repair when compared with standard antireflux procedures for small hiatal hernias and gastroesophageal reflux disease. [9] We routinely perform barium swallow prior to discharge to document subdiaphragmatic positioning of the fundoplication wrap and look for unrecognized esophageal or gastric injury or staple-line leak (in patients who received an esophageal lengthening (Collis) procedure. We have instituted clinical pathways for routine follow-up, including barium esophagram and symptom assessment with validated measures for gastroesophageal reflux disease health-related quality of life (GERD-HRQL) [18] and overall quality of life (SF-36). [19] All patients are seen 2 weeks after surgery and again 1 year after surgery. Barium esophagram is performed 1 year after surgery and then at 2-year intervals. We ask all of our patients to return at least every two years to ensure that recurrent symptoms or recurrent hernia are recognized early and managed appropriately. Common postoperative complaints include dysphagia, heartburn, gas bloat and diarrhea. By providing routine follow-up, we ensure that symptoms are addressed appropriately, either with medical therapy, endoscopy and dilation, or reoperation, as needed. With this approach, in both intermediate (median 30 months) [9] and long-term (median 44 months) [11] follow-up, good-to-excellent results are reported in up to 90% of patients, with radiographic recurrence rates of ~16% and reoperation rates of less than 5%. [9,11]
Comment
In 2011, foregut surgeons continue to debate whether the optimal approach to repair of paraesophageal hernias should be via an open or minimally invasive approach. With increasing experience in advanced laparoscopic techniques, the laparoscopic repair has become the standard approach to paraesophageal hernia at our center. With careful attention to the details described herein, such as complete reduction of the mediastinal hernia sac, extensive mediastinal mobilization of the esophagus followed by precise identification of the GEJ through mobilization of the gastric fat pad, and assiduous maintenance of crural integrity, long-term radiographic durability and symptom relief is possible. [9,11]
Supplementary Material
References
- 1.Collis JL. An operation for hiatus hernia with short esophagus. J Thorac Surg. 1957 Dec;34(6):768–773. discussion 774-768. [PubMed] [Google Scholar]
- 2.Hakanson BS, Thor KB, Thorell A, Ljungqvist O. Open vs laparoscopic partial posterior fundoplication. A prospective randomized trial. Surg Endosc. 2007 Feb;21(2):289–298. doi: 10.1007/s00464-006-0013-8. [DOI] [PubMed] [Google Scholar]
- 3.Schauer PR, Ikramuddin S, McLaughlin RH, Graham TO, Slivka A, Lee KK, Schraut WH, Luketich JD. Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg. 1998 Dec;176(6):659–665. doi: 10.1016/s0002-9610(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 4.Altorki NK, Yankelevitz D, Skinner DB. Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg. 1998 Apr;115(4):828–835. doi: 10.1016/S0022-5223(98)70363-0. [DOI] [PubMed] [Google Scholar]
- 5.Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg. 1998 Jan;115(1):53–60. doi: 10.1016/s0022-5223(98)70442-8. discussion 61-52. [DOI] [PubMed] [Google Scholar]
- 6.Luketich JD, Raja S, Fernando HC, Campbell W, Christie NA, Buenaventura PO, Weigel TL, Keenan RJ, Schauer PR. Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg. 2000 Oct;232(4):608–618. doi: 10.1097/00000658-200010000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierre AF, Luketich JD, Fernando HC, Christie NA, Buenaventura PO, Litle VR, Schauer PR. Results of laparoscopic repair of giant paraesophageal hernias: 200 consecutive patients. Ann Thorac Surg. 2002 Dec;74(6):1909–1915. doi: 10.1016/s0003-4975(02)04088-2. discussion 1915-1906. [DOI] [PubMed] [Google Scholar]
- 8.Pitcher DE, Curet MJ, Martin DT, Vogt DM, Mason J, Zucker KA. Successful laparoscopic repair of paraesophageal hernia. Arch Surg. 1995 Jun;130(6):590–596. doi: 10.1001/archsurg.1995.01430060028006. [DOI] [PubMed] [Google Scholar]
- 9.Luketich JD, Nason KS, Christie NA, Pennathur A, Jobe BA, Landreneau RJ, Schuchert MJ. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010 Feb;139(2):395–404. 404 e391. doi: 10.1016/j.jtcvs.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferri LE, Feldman LS, Stanbridge D, Mayrand S, Stein L, Fried GM. Should laparoscopic paraesophageal hernia repair be abandoned in favor of the open approach? Surg Endosc. 2005 Jan;19(1):4–8. doi: 10.1007/s00464-004-8903-0. [DOI] [PubMed] [Google Scholar]
- 11.Nason KS, Luketich JD, Qureshi I, Keeley S, Trainor S, Awais O, Shende M, Landreneau RJ, Jobe BA, Pennathur A. Laparoscopic repair of giant paraesophageal hernia results in long-term patient satisfaction and a durable repair. J Gastrointest Surg. 2008 Dec;12(12):2066–2075. doi: 10.1007/s11605-008-0712-7. discussion 2075-2067. [DOI] [PubMed] [Google Scholar]
- 12.Zurawska U, Parasuraman S, Goldhaber SZ. Prevention of pulmonary embolism in general surgery patients. Circulation. 2007 Mar 6;115(9):e302–307. doi: 10.1161/CIRCULATIONAHA.106.674663. [DOI] [PubMed] [Google Scholar]
- 13.Zollinger RJ, Zollinger RS. Cholesystectomy, Hasson Open Technique, Laparoscopic. In: ZR Jr, ZR Sr, editors. Zollinger’s Atlas of Surgical Operations. 8. The McGraw-Hill Companies, Inc; 2003. [Google Scholar]
- 14.Whitson BA, Hoang CD, Boettcher AK, Dahlberg PS, Andrade RS, Maddaus MA. Wedge gastroplasty and reinforced crural repair: important components of laparoscopic giant or recurrent hiatal hernia repair. J Thorac Cardiovasc Surg. 2006 Nov;132(5):1196–1202. e1193. doi: 10.1016/j.jtcvs.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Davis RE, Awad ZT, Filipi CJ. Technical factors in the creation of a “floppy” Nissen fundoplication. Am J Surg. 2004 Jun;187(6):724–727. doi: 10.1016/j.amjsurg.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly MJ, Mullins SG, Saye WB, Pinto SE, Falkner PT. Laparoscopic posterior partial fundoplication: analysis of 100 consecutive cases. J Laparoendosc Surg. 1996 Jun;6(3):141–150. doi: 10.1089/lps.1996.6.141. [DOI] [PubMed] [Google Scholar]
- 17.el-Sherif AE, Adusumilli PS, Pettiford BL, d’Amato TA, Schuchert MJ, Clark A, DiRenzo C, Landreneau JP, Luketich JD, Landreneau RJ. Laparoscopic clam shell partial fundoplication achieves effective reflux control with reduced postoperative dysphagia and gas bloating. Ann Thorac Surg. 2007 Nov;84(5):1704–1709. doi: 10.1016/j.athoracsur.2007.05.085. [DOI] [PubMed] [Google Scholar]
- 18.Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996 Sep;183(3):217–224. [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.