Summary
The adhesion of infected red blood cells (iRBCs) to human endothelium is considered a key event in the pathogenesis of cerebral malaria and other life‐threatening complications caused by the most prevalent malaria parasite Plasmodium falciparum. In the past 30 years, 14 endothelial receptors for iRBCs have been identified. Exposing 10 additional surface proteins of endothelial cells to a mixture of P. falciparum isolates from three Ghanaian malaria patients, we identified seven new iRBC receptors, all expressed in brain vessels. This finding strongly suggests that endothelial binding of P. falciparum iRBCs is promiscuous and may use a combination of endothelial surface moieties.
Introduction
Severe malaria caused by infection with Plasmodium falciparum is a complex clinical syndrome comprising a number of life‐threatening conditions including cerebral malaria (CM). Several lines of evidence indicate that a major mechanism underlying the pathology of CM is the ‘cytoadherence’ of infected red blood cells (iRBCs) to the endothelium lining the microvasculature of the brain, which may cause disturbances of the cerebral blood flow and metabolism as well as local inflammatory responses (Berendt et al., 1994; van der Heyde et al., 2006).
Cytoadherence is mediated by the binding of Plasmodium‐derived proteins exposed on the surface of iRBCs to defined structures on endothelial cells. One of the major adhesion molecules of the parasite is the P. falciparum erythrocyte membrane protein 1 (PfEMP1) (Leech et al., 1984). PfEMP1 comprises a great variety of proteins encoded by 60 var genes in the individual parasite genome and numerous variants in the parasite population at large. By varying and switching var gene expression the parasites show different and changing binding phenotypes, accompanied by antigenic variation (Miller et al., 2002).
Until now, 14 endothelial surface structures have been identified to serve as receptors for P. falciparum iRBCs, including CD36, intercellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), platelet/endothelial cell adhesion molecule (PECAM‐1), neural cell adhesion molecule (NCAM) and endothelial protein C receptor (EPCR). Additionally, chondroitin sulfate A (CSA) expressed on syncytiotrophoblast cells can serve as attachment points for iRBCs in the placenta. Only CD36, ICAM‐1, PECAM‐1, EPCR and CSA have been studied in detail (Serghides et al., 2003; Chakravorty and Craig, 2005; Rowe et al., 2009; Berger et al., 2013; Moxon et al., 2013; Turner et al., 2013), revealing a unique association of binding to CSA with placental malaria (Duffy and Fried, 2005). For CM it remains unclear whether any single one of the known endothelial structures has a pivotal role. ICAM‐1 was implicated by immunopathological studies of post‐mortem tissues showing colocalization of sequestration with ICAM‐1 expression in the brain (Turner et al., 1994) and by an in vitro analysis of isolates from CM patients preferentially binding to recombinant ICAM‐1 (Newbold et al., 1997). In studies with field isolates from Asia, however, ICAM‐1 binding was not associated with severe malaria (Ockenhouse et al., 1991; Udomsangpetch et al., 1996). CD36 is the quantitatively most important receptor as almost all non‐placental P. falciparum isolates derived from patients bind to it, but a major role of CD36 in cerebral malaria is uncertain because (i) the degree of binding to CD36 by iRBCs isolated from malaria patients did not correlate with severity of the disease (Newbold et al., 1997) and (ii) CD36 expression levels on brain endothelial cells was found to be low in CM patients (Turner et al., 1994). EPCR is the most recently identified endothelial receptor, which has been implicated in the pathology of severe malaria. While Turner et al. showed the direct interaction of EPCR with domain cassettes 8 and 13 of PfEMP1 (Turner et al., 2013), Moxon and colleagues revealed a role of EPCR in CM using an entirely different approach (Moxon et al., 2013). In post‐mortem studies of children who died of CM, they observed a colocalization of endothelial sites of adherent iRBCs and a loss of EPCR antigens.
For a better understanding of the pathology of CM but also for strategies to develop a vaccine against CM, it is of relevance whether the repertoire of endothelial receptors for iRBC is rather limited or broader than presently recognized. Therefore, we studied 10 surface moieties of the cerebral endothelium for their ability to serve as iRBC receptors. Seven of these showed iRBC binding when a mixture of patient isolates of P. falciparum from Ghana, West Africa, was used. This finding strongly suggests that, in contrast to our present understanding, endothelial binding of P. falciparum iRBC is highly promiscuous.
Results and discussion
The tetraspanins CD9 and CD151, multidrug‐resistance protein 1 (MDR1), multidrug resistance‐associated protein 2 (MRP2), the histamine H1 receptor (HRH1), oxidized low‐density lipoprotein receptor 1 (LOX1) and vascular adhesion protein 1 (VAP‐1) as well as truncated forms of tumour necrosis factor receptors 1 (TNFR1) and 2 (TNFR2) and the erythropoietin receptor (EPOR) were expressed in glycosaminoglycan‐deficient Chinese hamster ovary (CHO‐745) cells. All have been shown to be expressed on human brain endothelial cells (Cordon‐Cardo et al., 1989; Rossler et al., 1992; Salmi et al., 1993; Purkiss et al., 1994; Sawamura et al., 1997; Sincock et al., 1997; Dombrowski et al., 2001; Kadhim et al., 2006; Wosik et al., 2007; Medana et al., 2009). Tagged with green fluorescent protein (GFP) at their intracellular domains, the proteins were localized to the cell membrane by microscopy, and extracellular exposure was confirmed using non‐permeabilized cells probed with specific antibodies; exceptions were LOX1, which was not GFP labelled but shown to be surface expressed by antibody staining, and MRP2, for which no antibodies or antisera are available directed to extracellular domains but which showed a characteristic GFP fluorescence at the outer cell membrane (Fig. S1). Binding of P. falciparum iRBCs was studied using trophozoite stages of the laboratory strain FCR3 and of isolates from three Ghanaian patients with severe falciparum malaria. The patients' samples were pooled, expanded, frozen in aliquots and used as such in order not to introduce additional artefacts by cloning and prolonged in vitro cultivation. Cells transfected with CD36, which has been found to bind iRBC from almost all P. falciparum isolates (Newbold et al., 1997; Rowe et al., 2009), were used as positive controls for adhesion.
Significant binding of the pool of field isolates to CHO‐745 cells transfected with CD9, CD151, MDR1, MRP2, or truncated forms of EPOR (EPORsh), TNFR1 (TNFR1sh) and TNFR2 (TNFR2sh) was detected (Fig. 1A) whereas the laboratory strain FCR3 showed marginal binding, if any (Fig. 1B). Neither FCR3 nor the field isolates caused iRBC binding to cells transfected with HRH1, LOX1 or VAP‐1 (Fig. 1A and B) while both bound most strongly to the positive control CD36. Inhibition assays were performed with purified immunoglobulin G from rat or rabbit antisera (αCD9, αCD151, αEPOR, αTNFR1) and mouse monoclonal antibodies (αMDR1, αTNFR2) respectively. All showed statistically significant inhibition in a dose‐dependent manner, confirming the specificity of the binding reactions of MDR1, EPOR, TNFR1 and TNFR2 (Fig. 2). This interpretation is less straightforward for the tetraspanins CD9 and CD151 because their major function is to assemble multi‐component complexes on cellular surfaces, so‐called tetraspanin‐enriched microdomains, which may enhance the binding capacity of other cellular receptors (Barreiro et al., 2005). These might include NCAM and other, as yet uncharacterized iRBC receptors which may be present on non‐transfected CHO‐745 cells (Andrews et al., 2005; Pouvelle et al., 2007).
Figure 1.
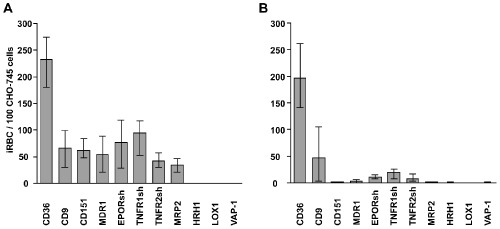
Binding of P. falciparum iRBCs to endothelial surface proteins expressed on CHO cells. Binding of RBCs infected with a pool of P. falciparum isolates from Ghanaian patients (A) or P. falciparum laboratory strain FCR3 (B) to CHO‐745 cells expressing CD36, CD9, CD151, MDR1, EPORsh, TNFR1sh, TNFR2sh, MRP2, HRH1, LOX1 and VAP‐1 respectively. Bars indicate median iRBC numbers specifically bound to 100 CHO cells as determined by microscopic inspection of 500 CHO cells in three independent experiments, each performed in triplicate.
Figure 2.
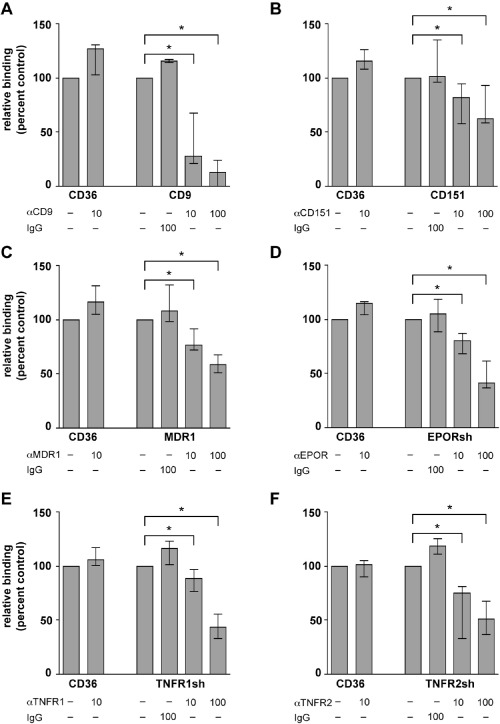
Inhibition of P. falciparum iRBC binding by antibodies to endothelial surface proteins. Inhibition of iRBC binding infected with a mixture of P. falciparum patient isolates to CHO cells expressing the endothelial surface proteins CD9 (A), CD151 (B), MDR1 (C), EPORsh (D), TNFR1sh (E) and TNFR2sh (F) by antibodies to endothelial surface proteins as indicated. Binding to cells expressing CD36 (CD36) and binding inhibition by non‐specific mouse IgG2a (IgG) were used for control. Binding in the presence of 10 μg ml−1 (10) and 100 μg ml−1 (100) antibodies is compared to the binding in the absence of antibodies (−). Median percentages with ranges of three independent experiments are shown, each performed in triplicate. Asterisks mark significant differences with P < 0.05.
We conclude from our data that the binding behaviour of P. falciparum iRBCs is highly promiscuous. If our findings obtained with a field isolate pool can be extrapolated to the plethora of parasite clones in the field, it would suggest that P. falciparum may take advantage of many endothelial surface moieties for the adhesion of iRBCs. As indicated by the binding to MDR1 and MRP2, this may include structures which physiologically do not function as cellular receptors, as has first been noted for chondroitin sulfate A (Rogerson et al., 1995). Possibly the knob‐like protrusions formed by the parasite at the iRBC surface allow binding to surface structures that are less prominent than genuine receptors.
Certain lines of evidence have indicated that ICAM‐1 and EPCR may play a pivotal role in the adhesion of iRBC in cerebral vessels and, therefore, in the pathology of CM (Turner et al., 1994; 2013; Newbold et al., 1997; Moxon et al., 2013). Our data indicate, however, that multiple ‘receptors’ may participate in the binding of iRBCs to cerebral endothelia as all seven iRBC receptors identified here have been shown to be expressed on endothelial cells of the human brain (Cordon‐Cardo et al., 1989; Rossler et al., 1992; Sincock et al., 1997; Dombrowski et al., 2001; Kadhim et al., 2006; Wosik et al., 2007; Medana et al., 2009). In fact, it is conceivable that the brain may be a common site of iRBC adhesion and sequestration because, constituting the blood–brain barrier, the cerebral endothelium is extraordinarily rich in receptor and transporter molecules offering a broad variety of binding sites for iRBCs, which all in all are armed with an even broader variety of adhesion ligands (Miller et al., 2002). Thus, our findings could question present concepts of developing anti‐disease vaccines in malaria based on the inhibition of cerebral iRBC adhesion (Smith et al., 2000).
Except for the adhesion itself, functional implications of the newly discovered binding reactions have not been the objective of our study and, to this end, any considerations must remain highly speculative. Nevertheless, some comments appear justified.
Several mechanisms have been proposed of how endothelial adhesion of P. falciparum iRBC might contribute to organ failure and, in particular, to CM. One is the concept of a mechanical occlusion of blood flow by a ‘sludge’ of adherent iRBCs, which bind additional iRBCs as well as uninfected RBCs and platelets. More recently, it has been discussed that microcirculatory disturbances could also be initiated by pro‐inflammatory cytokines, which are released systemically or locally through adherent iRBCs (Clark et al., 2006). These and additional mechanisms might involve the deregulation of endothelial receptor signalling and the damaging of endothelial barrier functions by iRBCs (Miller et al., 2002). Therefore, an elucidation of the role of the adhesion process in the development of severe malaria requires further studies.
CD9 and CD151 belong to the protein family of tetraspanins, which have been found to be involved in a variety of cellular functions including proliferation, adhesion, migration, cell fusion as well as binding and processing of pathogens. They may contribute to the cellular attachment of certain bacteria (Green et al., 2011) and have been shown to be utilized by P. falciparum sporozoites (Silvie et al., 2003) and several viruses for host cell entry (Pileri et al., 1998; Yoshida et al., 2008). A physical association of CD9 and CD151 with ICAM‐1 and VCAM‐1 has been reported to play a crucial role in the firm adhesion of leucocytes during extravasation (Barreiro et al., 2005). Both ICAM‐1 and VCAM‐1 are well‐known iRBC receptors (Rowe et al., 2009). Therefore, direct binding of iRBCs to the tetraspanins may further contribute to a deregulation of leucocyte trafficking, which may play an important role in the pathogenesis of CM (Miller et al., 2002). Furthermore, CD9 is expressed on platelets (Miao et al., 2001), and clumping of iRBCs and platelets has been found associated with CM (Pain et al., 2001). Thus, similar to CD36, CD9 may contribute to the clumping of iRBCs and platelets and to the binding of such aggregates to the vessel wall.
Cerebral sequestration of iRBCs has been found to be positively correlated with the expression of EPOR in brain vessels (Medana et al., 2009), which is in good agreement with our finding of iRBC binding to EPOR. While the function of EPOR on brain endothelial cells is not yet fully understood, it has been reported that EPOR acts as an active transporter of erythropoietin (EPO) across the blood–brain barrier (Brines et al., 2000) and that EPO inhibits the permeability of the blood–brain barrier (Casals‐Pascual et al., 2008). In addition, elevated EPO concentrations in the plasma of children with CM have been found associated with a reduced risk of neurological sequelae (Martinez‐Estrada et al., 2003). Therefore, EPO is being discussed as a neuroprotective agent and an adjuvant treatment for severe malaria. Thus, binding of iRBCs to EPOR might contribute to the pathology of CM not only by disturbing the cerebral microcirculation but also by affecting the integrity of the blood brain barrier through blocking of EPO binding sites.
Several lines of evidence indicate that TNF plays an important role in the development of CM (Grau et al., 1989; Kwiatkowski et al., 1990), and its two receptors TNFR1 and TNFR2 are most likely to mediate this effect. It is reasonable to assume that direct binding of iRBCs to these receptors could in one way or another modify TNF activities, including the induction and upregulation of ICAM‐1 and other endothelial iRBC receptors (Mackay et al., 1993; Lucas et al., 1997; Stoelcker et al., 2002).
MDR1 and MRP2 are ATP‐Binding‐Cassette (ABC) transporters that may play a critical role in neuroprotection by eliminating a wide range of toxic compounds from the brain (Begley, 2004). Both proteins span the cell membrane multiple times with their functional ATP‐binding cassettes located at the cytosolic side. Neither transporter has so far been recognized to serve as receptors nor have they been implicated in the attachment or invasion of pathogens. Therefore, our data on MDR1 and MRP2 show that P. falciparum iRBC may use an extraordinarily broad variety of endothelial surface structures for adherence.
In conclusion, it is conceivable that the additional endothelial receptors for iRBC described here may contribute to the pathology of severe malaria and, in particular, of CM through a number of mechanisms, which, besides the mechanical obstruction of blood flow, possibly include interference with important functions of brain endothelial cells.
Experimental procedures
Ethics statement
Patient isolates were collected from three children diagnosed with severe falciparum malaria of a case–control study conducted in Kumasi, Ghana (May et al., 2007). The study had been approved by the Committee for Research, Publications and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (reference number CHRPE/01/11). All procedures were explained to parents or guardians of the participating children in the local language, and written or thumb‐printed informed consent was obtained (May et al., 2007).
Animal use for antisera generation was carried out in strict accordance with the recommendations of the European Union guidelines for the handling of laboratory animals (Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes). The procedures were approved by the Behörde für Gesundheit und Verbraucherschutz der Stadt Hamburg according to §10a TierSchG (German Protection of Animals Act).
P. falciparum isolates
Plasmodium falciparum strain FCR3 was obtained from Dr Mo‐Quen Klinkert. In vitro cultivation of P. falciparum was carried out in human Rh‐positive RBCs of blood group 0 according to standard protocols (Trager and Jensen, 1976). Patient isolates were cryopreserved, adapted to in vitro cultivation and pooled. Parasite growth was synchronized using 5% sorbitol (Lambros and Vanderberg, 1979).
Protein expression by CHO cells
Chinese hamster ovary cells defective in glycosaminoglycan biosynthesis (CHO‐745) were obtained from American Type Culture Collection (Esko et al., 1985) and grown in Ham's F‐12 media (PAA Laboratories) supplemented with 10% fetal calf serum, penicillin and streptomycin. Expression constructs were generated according to established protocols. cDNA of CD9 and CD151 were cloned into pEGFP‐N1 (Clontech Laboratories), MDR1, MRP2 and HRH1 (Missouri S&T cDNA Resource Center) into pAcGFP‐N1 (Clontech Laboratories), and cDNA of VAP‐1 and LOX1 (ImaGenes GmbH) into pAcGFP‐C1 (Clontech Laboratories) and pcDNA3.1(+) (Invitrogen) respectively. For EPOR, TNFR1 and TNFR2 (ImaGenes GmbH) intracellularly truncated forms (EPORsh, TNFR1sh and TNFR2sh respectively) were generated by cloning amino acids 1–336 of EPOR (using primers 1 and 2), amino acids 1–252 of TNFR1 (using primers 3 and 4) and amino acids 1–307 of TNFR2 (using primers 5 and 6) into pAcGFP‐N1.
Primer 1: 5′‐AAT AAG CTT ACC ACC ATG GAC CAC CTC GGG GCG‐3′
Primer 2: 5′‐ATT CCG CGG TGA CTC TGA GAG GAC TTC CAG‐3′
Primer 3: 5′‐AAT CTC GAG ACC ACC ATG GGC CTC TCC ACC GTG‐3′
Primer 4: 5′‐CAA TCC GCG GTG TCG ATT TCC CAC A‐3′
Primer 5: 5′‐AAT CTC GAG ACC ACC ATG GCG CCC GTC GCC GTC‐3′
Primer 6: 5′‐CAA TCC GCG GCT TAT CGG CAG GCA A‐3′
CHO‐745 cells were transfected using Lipofectamine 2000 (Invitrogen) complexed with pAcGFP1‐N1, pEGFP‐CD9, pEGFP‐CD151, pAcGFP‐MDR1, pAcGFP‐EPORsh, pAcGFP‐TNFR1sh, pAcGFP‐TNFR2sh, pAcGFP‐MRP2, pAcGFP‐HRH1, pcDNA3.1‐LOX1 and pAcGFP‐VAP‐1, respectively, according to the protocol of the manufacturer. Two days after transfection, 0.7 mg ml−1 G418 (Biochrom AG) was added to the cultures. G418‐resistant cells were harvested and subjected to FACS sorting using a BD FACSAria cell sorter (BD Biosciences). Single‐cell clones were maintained in 96‐well plates and screened for plasma membrane expression of recombinant proteins.
Antibodies
The following antibodies were used for immunofluorescence and inhibition studies: Mouse αMDR1 (UIC2, Millipore), αTNFR2 (MAB226, R&D Systems), αHRH1 (MAB4726, R&D Systems), αVAP‐1 (TK8‐14, Santa‐Cruz) and purified mouse IgG2a (MOPC‐173, Biozol) were purchased. Immune sera against human CD9, CD151, EPOR, TNFR1 and LOX1 were generated by cDNA immunization. Full‐length cDNA fragments of CD9, CD151 and LOX1 and truncated forms of EPOR (EPORsh) and TNFR1 (TNFR1sh) were cloned into pcDNA3.1(+) vector (Invitrogen) and coated onto 1 μm‐gold particles (Bio‐Rad). cDNA of CD9, EPORsh and TNFR1sh was ballistically injected into rats and cDNA of CD151 and LOX1 into rabbits at a pressure setting of 400 psi. Gene gun immunization was repeated three times at 6‐week intervals (Koch‐Nolte et al., 2005). The immune sera were tested by immunofluorescence staining of CHO cells transfected with CD9, CD151, EPORsh, TNFR1sh and LOX1 respectively. IgG‐antibodies were purified from sera by Protein G Sepharose 4 Fast Flow according to the protocol of the manufacturer (GE Healthcare) and referred to as αCD9, αCD151, αEPOR, αTNFR1 and αLOX1.
Immunofluorescence studies
CHO‐745 cells were grown on coverslips and fixed with 4% para‐formaldehyde. Surface‐exposed CD9, CD151, MDR1, EPORsh, TNFR1sh, TNFR2sh, HRH1, LOX1 and VAP‐1 were labelled using the respective antibodies and secondary antibodies conjugated to Alexa‐Fluor‐594 (Invitrogen). Nuclei were stained with DAPI at 0.1 μg ml−1 (Sigma). Fluorescence‐labelled cells were inspected using a Zeiss Axioskop2 plus microscope (Carl Zeiss AG). Mock‐transfected cells did not react with any of the specific antisera, and CHO cells transfected with endothelial receptors incubated with pre‐immune sera or with secondary antibodies alone did not show fluorescence in any experiment.
Static iRBC adhesion assays
Stably transfected CHO‐745 cells were grown on 13 mm coverslips and maintained in 24‐well plates. CHO‐745 cells were seeded at a density of 15 000 cells per well and grown for 48 h before assays. For inhibition assays, CHO‐745 transfectants were pre‐incubated with antibodies at 37°C for 30 min. Trophozoite stage parasite cultures at 1% haematocrit and 4% parasitaemia in binding medium (RMPI medium 1640 supplemented with 2% glucose, pH 7.2) were applied to CHO‐745 transfectants and incubated at 37°C for 1 h with gentle mixing at 10 min intervals. Afterwards, unbound erythrocytes were gently removed by washing with binding medium, and the cells were fixed with 1% glutaraldehyde in PBS at room temperature for 1 h. Cells on coverslips were stained with Giemsa, and the numbers of adherent iRBCs per 500 CHO‐745 cells were counted using a light microscope. Assays were performed in triplicate in three independent experiments, in each case determining the binding of iRBCs to mock‐transfected CHO‐745 and CHO‐745‐CD36 for reference. The numbers of iRBCs bound to mock transfectants were subtracted from the numbers of iRBCs bound to CHO‐745 cells expressing recombinant proteins. If this resulted in a negative value, the number of specifically bound iRBCs was set to zero. On average, 25.9 ± 34.9 iRBCs bound to 100 mock‐transfected CHO‐745 cells, which could not be inhibited by adding any of the antibodies to the endothelial surface proteins studied. Data are presented as median with range. For inhibition assays, binding in the presence of antibodies was expressed as percentage of binding in the absence of antibodies. For statistical evaluations the non‐parametric Mann–Whitney U test was used, whereby two‐sided P‐values of < 0.05 were considered significant (n = 6).
Supplementary Material
Fig. S1. Antibody staining of recombinant proteins expressed on the surface of CHO‐745 cells. Immunofluorescence analyses (IFA) with non‐permeabilized CHO‐745 cells confirm surface localization of the overexpressed endothelial proteins. GFP‐tagged CD9, CD151, MDR1, EPORsh, TNFR1sh, TNFR2sh, HRH1, VAP‐1 (green) as well as untagged LOX1 expressed in CHO‐745 cells were labelled with respective antibodies (red). No antibodies were available which are directed against any of the nine extracellular domains of MRP2. Nuclei were stained with DAPI (blue). n.d., not determined.
Acknowledgements
We thank Sandra Nyenhuis, Fabienne Seyfried, Gudrun Dubberke, Heidrun von Thien and the antibody core unit of the University Medical Center Hamburg‐Eppendorf for technical assistance. Furthermore, we are grateful to Mo‐Quen Klinkert for providing the FCR3 laboratory strain as well as to Jennifer Evans from the Kumasi Centre for Collaborative Research in Tropical Medicine and Tsiri Agbenyega of Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, for contributing patient isolates.
The authors declare no competing financial interests.
References
- Andrews, K.T., Adams, Y., Viebig, N.K., Lanzer, M., and Schwartz‐Albiez, R. (2005) Adherence of Plasmodium falciparum infected erythrocytes to CHO‐745 cells and inhibition of binding by protein A in the presence of human serum. Int J Parasitol 35: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Barreiro, O., Yanez‐Mo, M., Sala‐Valdes, M., Gutierrez‐Lopez, M.D., Ovalle, S., Higginbottom, A., et al (2005) Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 105: 2852–2861 [DOI] [PubMed] [Google Scholar]
- Begley, D.J. (2004) ABC transporters and the blood–brain barrier. Curr Pharm Des 10: 1295–1312 [DOI] [PubMed] [Google Scholar]
- Berendt, A.R., Tumer, G.D., and Newbold, C.I. (1994) Cerebral malaria: the sequestration hypothesis. Parasitol Today 10: 412–414 [DOI] [PubMed] [Google Scholar]
- Berger, S.S., Turner, L., Wang, C.W., Petersen, J.E., Kraft, M., Lusingu, J.P., et al (2013) Plasmodium falciparum expressing domain cassette 5 type PfEMP1 (DC5‐PfEMP1) bind PECAM1. PLoS ONE 8: e69117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines, M.L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N.C., Cerami, C., et al (2000) Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97: 10526–10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals‐Pascual, C., Idro, R., Gicheru, N., Gwer, S., Kitsao, B., Gitau, E., et al (2008) High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci USA 105: 2634–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty, S.J., and Craig, A. (2005) The role of ICAM‐1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol 84: 15–27 [DOI] [PubMed] [Google Scholar]
- Clark, I.A., Budd, A.C., Alleva, L.M., and Cowden, W.B. (2006) Human malarial disease: a consequence of inflammatory cytokine release. Malar J 5: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon‐Cardo, C., O'Brien, J.P., Casals, D., Rittman‐Grauer, L., Biedler, J.L., Melamed, M.R., and Bertino, J.R. (1989) Multidrug‐resistance gene (P‐glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc Natl Acad Sci USA 86: 695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski, S.M., Desai, S.Y., Marroni, M., Cucullo, L., Goodrich, K., Bingaman, W., et al (2001) Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 42: 1501–1506 [DOI] [PubMed] [Google Scholar]
- Duffy, P.E., and Fried, M. (2005) Malaria in the pregnant woman. Curr Top Microbiol Immunol 295: 169–200 [DOI] [PubMed] [Google Scholar]
- Esko, J.D., Stewart, T.E., and Taylor, W.H. (1985) Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA 82: 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau, G.E., Taylor, T.E., Molyneux, M.E., Wirima, J.J., Vassalli, P., Hommel, M., and Lambert, P.H. (1989) Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Green, L.R., Monk, P.N., Partridge, L.J., Morris, P., Gorringe, A.R., and Read, R.C. (2011) Cooperative role for tetraspanins in adhesin‐mediated attachment of bacterial species to human epithelial cells. Infect Immun 79: 2241–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyde, H.C., Nolan, J., Combes, V., Gramaglia, I., and Grau, G.E. (2006) A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol 22: 503–508 [DOI] [PubMed] [Google Scholar]
- Kadhim, H., Khalifa, M., Deltenre, P., Casimir, G., and Sebire, G. (2006) Molecular mechanisms of cell death in periventricular leukomalacia. Neurology 67: 293–299 [DOI] [PubMed] [Google Scholar]
- Koch‐Nolte, F., Glowacki, G., Bannas, P., Braasch, F., Dubberke, G., Ortolan, E., et al (2005) Use of genetic immunization to raise antibodies recognizing toxin‐related cell surface ADP‐ribosyltransferases in native conformation. Cell Immunol 236: 66–71 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski, D., Hill, A.V., Sambou, I., Twumasi, P., Castracane, J., Manogue, K.R., et al (1990) TNF concentration in fatal cerebral, non‐fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336: 1201–1204 [DOI] [PubMed] [Google Scholar]
- Lambros, C., and Vanderberg, J.P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65: 418–420 [PubMed] [Google Scholar]
- Leech, J.H., Barnwell, J.W., Miller, L.H., and Howard, R.J. (1984) Identification of a strain‐specific malarial antigen exposed on the surface of Plasmodium falciparum‐infected erythrocytes. J Exp Med 159: 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, R., Lou, J.N., Juillard, P., Moore, M., Bluethmann, H., and Grau, G.E. (1997) Respective role of TNF receptors in the development of experimental cerebral malaria. J Neuroimmunol 72: 143–148 [DOI] [PubMed] [Google Scholar]
- Mackay, F., Loetscher, H., Stueber, D., Gehr, G., and Lesslauer, W. (1993) Tumor necrosis factor alpha (TNF‐alpha)‐induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF‐R55. J Exp Med 177: 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Estrada, O.M., Rodriguez‐Millan, E., Gonzalez‐De Vicente, E., Reina, M., Vilaro, S., and Fabre, M. (2003) Erythropoietin protects the in vitro blood–brain barrier against VEGF‐induced permeability. Eur J Neurosci 18: 2538–2544 [DOI] [PubMed] [Google Scholar]
- May, J., Evans, J.A., Timmann, C., Ehmen, C., Busch, W., Thye, T., et al (2007) Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA 297: 2220–2226 [DOI] [PubMed] [Google Scholar]
- Medana, I.M., Day, N.P., Hien, T.T., White, N.J., and Turner, G.D. (2009) Erythropoietin and its receptors in the brainstem of adults with fatal falciparum malaria. Malar J 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, W.M., Vasile, E., Lane, W.S., and Lawler, J. (2001) CD36 associates with CD9 and integrins on human blood platelets. Blood 97: 1689–1696 [DOI] [PubMed] [Google Scholar]
- Miller, L.H., Baruch, D.I., Marsh, K., and Doumbo, O.K. (2002) The pathogenic basis of malaria. Nature 415: 673–679 [DOI] [PubMed] [Google Scholar]
- Moxon, C.A., Wassmer, S.C., Milner, D.A., Jr, Chisala, N.V., Taylor, T.E., Seydel, K.B., et al (2013) Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122: 842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold, C., Warn, P., Black, G., Berendt, A., Craig, A., Snow, B., et al (1997) Receptor‐specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg 57: 389–398 [DOI] [PubMed] [Google Scholar]
- Ockenhouse, C.F., Ho, M., Tandon, N.N., Van Seventer, G.A., Shaw, S., White, N.J., et al (1991) Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM‐1. J Infect Dis 164: 163–169 [DOI] [PubMed] [Google Scholar]
- Pain, A., Ferguson, D.J., Kai, O., Urban, B.C., Lowe, B., Marsh, K., and Roberts, D.J. (2001) Platelet‐mediated clumping of Plasmodium falciparum‐infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci USA 98: 1805–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri, P., Uematsu, Y., Campagnoli, S., Galli, G., Falugi, F., Petracca, R., et al (1998) Binding of hepatitis C virus to CD81. Science 282: 938–941 [DOI] [PubMed] [Google Scholar]
- Pouvelle, B., Matarazzo, V., Jurzynski, C., Nemeth, J., Ramharter, M., Rougon, G., and Gysin, J. (2007) Neural cell adhesion molecule, a new cytoadhesion receptor for Plasmodium falciparum‐infected erythrocytes capable of aggregation. Infect Immun 75: 3516–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkiss, J.R., West, D., Wilkes, L.C., Scott, C., Yarrow, P., Wilkinson, G.F., and Boarder, M.R. (1994) Stimulation of phospholipase C in cultured microvascular endothelial cells from human frontal lobe by histamine, endothelin and purinoceptor agonists. Br J Pharmacol 111: 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson, S.J., Chaiyaroj, S.C., Ng, K., Reeder, J.C., and Brown, G.V. (1995) Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum‐infected erythrocytes. J Exp Med 182: 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler, K., Neuchrist, C., Kitz, K., Scheiner, O., Kraft, D., and Lassmann, H. (1992) Expression of leucocyte adhesion molecules at the human blood–brain barrier (BBB). J Neurosci Res 31: 365–374 [DOI] [PubMed] [Google Scholar]
- Rowe, J.A., Claessens, A., Corrigan, R.A., and Arman, M. (2009) Adhesion of Plasmodium falciparum‐infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med 11: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi, M., Kalimo, K., and Jalkanen, S. (1993) Induction and function of vascular adhesion protein‐1 at sites of inflammation. J Exp Med 178: 2255–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura, T., Kume, N., Aoyama, T., Moriwaki, H., Hoshikawa, H., Aiba, Y., et al (1997) An endothelial receptor for oxidized low‐density lipoprotein. Nature 386: 73–77 [DOI] [PubMed] [Google Scholar]
- Serghides, L., Smith, T.G., Patel, S.N., and Kain, K.C. (2003) CD36 and malaria: friends or foes? Trends Parasitol 19: 461–469 [DOI] [PubMed] [Google Scholar]
- Silvie, O., Rubinstein, E., Franetich, J.F., Prenant, M., Belnoue, E., Renia, L., et al (2003) Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med 9: 93–96 [DOI] [PubMed] [Google Scholar]
- Sincock, P.M., Mayrhofer, G., and Ashman, L.K. (1997) Localization of the transmembrane 4 superfamily (TM4SF) member PETA‐3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin. J Histochem Cytochem 45: 515–525 [DOI] [PubMed] [Google Scholar]
- Smith, J.D., Craig, A.G., Kriek, N., Hudson‐Taylor, D., Kyes, S., Fagan, T., et al (2000) Identification of a Plasmodium falciparum intercellular adhesion molecule‐1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci USA 97: 1766–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelcker, B., Hehlgans, T., Weigl, K., Bluethmann, H., Grau, G.E., and Mannel, D.N. (2002) Requirement for tumor necrosis factor receptor 2 expression on vascular cells to induce experimental cerebral malaria. Infect Immun 70: 5857–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager, W., and Jensen, J.B. (1976) Human malaria parasites in continuous culture. Science 193: 673–675 [DOI] [PubMed] [Google Scholar]
- Turner, G.D., Morrison, H., Jones, M., Davis, T.M., Looareesuwan, S., Buley, I.D., et al (1994) An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule‐1 in cerebral sequestration. Am J Pathol 145: 1057–1069 [PMC free article] [PubMed] [Google Scholar]
- Turner, L., Lavstsen, T., Berger, S.S., Wang, C.W., Petersen, J.E., Avril, M., et al (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498: 502–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch, R., Taylor, B.J., Looareesuwan, S., White, N.J., Elliott, J.F., and Ho, M. (1996) Receptor specificity of clinical Plasmodium falciparum isolates: nonadherence to cell‐bound E‐selectin and vascular cell adhesion molecule‐1. Blood 88: 2754–2760 [PubMed] [Google Scholar]
- Wosik, K., Biernacki, K., Khouzam, M.P., and Prat, A. (2007) Death receptor expression and function at the human blood brain barrier. J Neurol Sci 259: 53–60 [DOI] [PubMed] [Google Scholar]
- Yoshida, T., Kawano, Y., Sato, K., Ando, Y., Aoki, J., Miura, Y., et al (2008) A CD63 mutant inhibits T‐cell tropic human immunodeficiency virus type 1 entry by disrupting CXCR4 trafficking to the plasma membrane. Traffic 9: 540–558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Antibody staining of recombinant proteins expressed on the surface of CHO‐745 cells. Immunofluorescence analyses (IFA) with non‐permeabilized CHO‐745 cells confirm surface localization of the overexpressed endothelial proteins. GFP‐tagged CD9, CD151, MDR1, EPORsh, TNFR1sh, TNFR2sh, HRH1, VAP‐1 (green) as well as untagged LOX1 expressed in CHO‐745 cells were labelled with respective antibodies (red). No antibodies were available which are directed against any of the nine extracellular domains of MRP2. Nuclei were stained with DAPI (blue). n.d., not determined.


