Abstract
Objectives
Swine have been regarded as intermediate hosts in the spread of influenza from birds to humans but studies of the sialylated glycans that comprise their respiratory tract have not been extensively studied in the past. This study analyzed the sialylated N‐glycan and O‐glycan profile of swine trachea and lung and correlated this with ex‐vivo infection of swine explants with avian influenza viruses.
Sample
Lungs and tracheal samples were obtained from normal farm and laboratory raised swine and used for ex vivo infection as well as mass spectrometric analysis. Infection of the ex vivo tissues used high pathogenic and low pathogenic avian viruses including the novel H7N9 virus that emerged in China in early 2013.
Main outcome measures
Assessment of successful replication was determined by TCID50 as well as virus immunohistochemistry. The N‐glycan and O‐glycan profiles were measured by MALDI‐TOF and sialylated linkages were determined by sialidase treatment. Lectin binding histochemistry was also performed on formalin fixed tissue samples with positive binding detected by chromogen staining.
Results
The swine respiratory tract glycans differed from the human respiratory tact glycans in two main areas. There was a greater abundance of Gal‐α‐Gal linkages resulting in a relative decrease in sialylated glycans. The swine respiratory tract also had a greater proportion of glycans containing Neu5Gc and Siaα2‐6 glycans than the human respiratory tract. Infection with avian viruses was confined primarily to lung bronchioles rather than trachea and parenchyma.
Conclusions
In contrast to previous studies we found that there was not as much expression of Siaα2‐3 glycans on the surface of the trachea. Infection of Siaα2‐3 binding avian viruses was restricted to the lower respiratory tract bronchioles. This finding may diminish the ability of the swine to act as an intermediary in the transmission of avian viruses to humans.
Keywords: Glycomics, influenza A virus, swine
Introduction
Influenza virus infection of the respiratory tract involves the recognition and interaction of the viral hemagglutinin with the Sia receptor present on the host cell. Since 1983, when Rogers and Paulson1, 2 delineated a difference in affinity between avian and human viruses, there have been detailed investigations on the distribution of different Sia linkages between different species and on the correlation between the receptor preference of influenza virus viz Siaα2‐6 and Siaα2‐3 and infection or transmission. The Siaα2‐6 is preferentially recognized by human and swine viruses, but not by equine or avian viruses. In contrast, avian viruses recognize glycoproteins with Siaα2‐3Gal‐GlcNAc and Siaα2‐3Gal‐GalNAc type of sialic acids as shown by carbohydrate binding array and more recently by glycan array.3, 4, 5, 6
Aquatic birds are the main reservoirs for all influenza viruses. Swine influenza viruses are derived from influenza viruses of avians or humans or reassortants between them. It has been proposed that swine could be intermediate hosts for genetic reassortment between avian and mammalian influenza viruses.7, 8 The concept of the pig as a “mixing vessel” for new viruses is further supported by the demonstration of Siaα2‐6 and Siaα2‐3 by lectin binding studies7, 9 and reports that swine are susceptible to a wide range of influenza viruses.10 However, more recent studies suggest that swine may not be as readily permissive to a wide range of avian influenza viruses; for example, there is low susceptibility of domestic pigs to highly pathogenic (HP) H5N1 infection under laboratory conditions.11 Even though surveillance of pigs in Indonesia showed a 7·4% isolation rate of this virus in a defined locality with little clinical symptoms,12 these reports are the exception than the rule. In addition, virus binding studies by van Riel et al.13 demonstrated no significant binding of H5N1 to the trachea or bronchi of pigs but to pneumocytes.
Previous research suggested that avian viruses would not transmit directly to humans as lectin binding studies indicated that the human upper respiratory tract was lined mainly by Siaα2‐6‐terminated glycans, as detected by Sambucus nigra agglutinin binding14 with only a limited detection of Siaα2‐3 glycan on goblet cells, as detected by the binding of Maackia amurensis agglutinin (MAA). However, the infection of humans with HP H5N1 virus in 1997 indicated that Siaα2‐3‐terminated glycans are present in human respiratory tract as the receptor for virus entry, and this was shown to be the case in the human alveolar epithelium.15, 16 Infection of H5N1 in humans results in severe disease but little evidence of direct human‐to‐human transmission. It has been suggested that the lack of Siaα2‐3 glycans in the human upper respiratory tract is responsible for the lack of human‐to‐human transmission.17, 18 Recent studies have demonstrated that mutations in the virus hemagglutinin that lead to a switch in the H5N1 virus receptor binding preference from Siaα2‐3 to Siaα2‐6 glycans contributed to the acquisition of airborne droplet transmission in ferrets, a well established correlate for transmission in humans.17, 19
In contrast to the Sia distribution in the human respiratory tract, recent lectin binding studies indicated that the presence of Siaα2‐3 in the porcine trachea was not as abundant as previously documented20, 21, 22 and this has been explained by the different isoforms of MAA used in these studies.23, 24 To clarify the distribution of influenza virus receptors in the porcine respiratory tract, Bateman et al.25 performed ultrasensitive mass spectrometric N‐ and O‐glycan profiles of cultured swine respiratory epithelial cells (SREC) and correlated this with infection by swine influenza viruses. They found that there was more Siaα2‐6 than Siaα2‐3, and Neu5Ac was more abundant than Neu5Gc, which would explain the relatively rarity of avian influenza virus infections becoming established in swine. However, since the SRECs were obtained from trachea and proximal bronchi,26 the role of the lung parenchyma in virus infection and replication still remained undecided.
To clarify the discrepancies between studies on the role of pigs in avian influenza virus infection, we explored the sialic acid (Sia) receptor distribution in the swine respiratory tract in order to provide insights into the permissiveness or lack of permissiveness of swine to avian influenza viruses. Furthermore, we performed mass spectrometric analysis on normal, healthy pig lung and trachea and correlated the glycan distribution data with virus tropism ascertained from pig respiratory organ explant infections. The viruses included in this study include the H7N9 avian virus, which has caused an outbreak in Mainland China in 2013, HP H5N1, and low pathogenic avian viruses of other subtypes on which glycan arrays had been previously performed. As miniature pigs have been used in experimental laboratory influenza infection or vaccination,27, 28, 29, 30 in this study, we also compared the mass spectrometric profile of miniature pigs with healthy farm pigs. This comparison is important to reveal the situation in the farm pigs which harbor the swine virus as well as being constantly exposed to the environment and therefore avian influenza viruses.
Materials and methods
Viruses used
A/Shanghai/2/2013 (Sh2/H7N9),A/Hong Kong/483/97 (H5N1), A/Hong Kong/486/97 (H5N1), A/Vietnam/3046/04 (H5N1), A/Vietnam/1203/04 (H5N1), A/Northern pintail/Hong Kong/MP5883/2004 (H5N8), A/Duck/Bavaria/1/1977 (H1N1), A/Quail/Hong Kong/G1/97 (H9N2), A/Chicken/Hong Kong/Y280/97 (H9N2). A/Hong Kong/415742/09 (H1N1pdm), A/swine/HK/4167/99 (H1N1), and A/Oklahoma/1992/05 (H3N2) were used as positive controls.
Swine respiratory organ explant culture
The intact respiratory organs of swine were obtained from pigs being slaughtered in a local abattoir. These tissues were taken from 6‐month‐old pigs (Sus Scrofa domestica) and delivered within 3 hours after slaughtering in a sterile plastic container at 4°C. Our previous experience with surveillance for swine influenza viruses at this abattoir in recent years has shown an influenza virus isolation rate of around 1%.31 To screen for evidence of current influenza infection in the animal, a tracheal swab was taken and tested for influenza M gene by real‐time qPCR; if a positive result was obtained, those particular set of tissues were discarded.
Tracheobronchial epithelium (TBE)
The tracheobronchial region was removed from the respiratory organ and moisturized by rinsing in transport medium. The cartilage of the tracheobronchial region was pinned on a dissection board while a pair of fine forceps was used together with a sharp scalpel to remove the TBE from the cartilage carefully. The TBE was cut using a disposable 5‐mm biopsy and cultured in MEM (Gibco, Carlsbad, CA, USA) with 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml kanamycin, 0·3 mg/ml glutamine, and 0·02 m HEPES at an air–liquid interface (ALI). The thin TBE explants were placed with the epithelial surface upward on a surgical sponge (Simport) and put inside a 12‐well plate. Culture medium was added into each well with the epithelial explants and the surgical sponge floating on the medium.
Lung parenchymal mucosa
We adopted the method of van Poucke et al.20 with slight modifications. The lung was perfused with cold transport medium using a clear tracheal tube 4·0 mm (Ruschelit, Research Triangle Park, NC, USA) through the bronchiole until the lobe was fully expanded. The transport medium was aspirated and replaced by 1% agarose in PBS (Sigma, St. Louis, MO, USA). Icepacks were then put below and above the lung lobe to solidify the agarose. This tissue was further embedded in 4% agarose and cut in slices, using a cryotome blade with a thickness <1 mm. The thin slices of explant were cut using disposable 5‐mm biopsy punch (Miltex, Plainsboro, NJ, USA) and cultured in DMEM (Gibco) with 2·5 μg/ml bovine insulin (Sigma), 0·5 μg/ml hydrocortisone (Sigma), 0·5 μg/ml vitamin A 0·5 (Sigma), 0·1 mg/ml gentamycin, and 50 μg/ml polymycin B.
The medium of these explants was changed every hour in the first four hours and incubated at 37°C in a humidified incubator with 5% CO2 supply and fixed in 10% saline‐buffered formalin at 0, 24, 48, and 72 hour after preparation, to evaluate their morphology, viability, and influenza receptor distribution.
Lectin binding and immunohistochemistry
Expression of Sialic acid by lectin histochemistry was performed using lectins SNA, MAA‐I, and MAA‐II as published previously and obtained from Vector Laboratories.23 PNA lectin preferentially binds Galß1‐3GalNAc. Thomsen‐Freidenreich (TF) antibody (A78‐G/A7) was purchased from LabVision Thermo Scientific (Waltham, MA, USA), and Neu5Gc antibody staining was performed using a chicken monoclonal antibody from Sialix (San Diego, CA, USA). Sections were pretreated with a sialidase (DAS181, Nex Bio, San Diego, CA, USA) for 2 hours at RT to remove both α2‐3‐ and α2‐6‐linked Sia.
Immunohistochemical staining and virus titration assay
Immunohistochemical staining of lung and tracheal tissue was carried out using the influenza nucleoprotein. The tissue sections were incubated with 0·1% pronase (Roche, Mannheim, Germany) in 0·1 m Tris (pH7·5) at 37°C for 1 minute and blocked with 3% H2O2 in TBS for 10 minute followed by treatment with an avidin/biotin blocking kit (Vector Lab). After blocking with 10% normal rabbit serum for 10 minute at RT, the sections were incubated with 1/25 (15 μg/ml) HB65 antibody for 1 hour at RT followed by biotinylated rabbit anti‐mouse (Dako Cytomation, Carpinteria, CA, USA) diluted 1/100 for 30 minute at RT. After incubation with Elite‐ABC kit (Vector Lab PK‐6100) diluted 1/50 for 30 minute at RT, the sections were developed with Vector NovaRed substrate kit (Vector Lab SK‐4800). To determine productive viral replication from the infected biopsies, the supernatant of the infected cultures was collected at 1, 24, 48, and 72 hour post‐infection and stored at −80°C for virus titration using TCID50 assay in MDCK cells as described previously.32 An increasing virus titer along the time course provided evidence of productive virus replication.
Mass spectrometry
Normal pig trachea and lung
Two 3‐month‐old pigs from a high health status farm in France that was negative for influenza A viruses were used. The animals were housed together in a HEPA‐filtered experimental unit with ad libitum access to water and food. At arrival, they were treated intramuscularly with ceftiofur (Naxcel®, Pfizer‐1 ml/20 kg body weight) to clear the respiratory tract from possible infections with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis. Two days later, they were euthanized by intravenous administration of thiopental (Penthotal®, Kela‐12·5 mg/kg body weight) and exsanguinated. Miniature pig lung was isolated from adult mini‐pigs (45–50 kg, 9–12 months old).33 All tissues were snap‐frozen in liquid nitrogen before glycan extraction. Additional blocks were placed in 10% neutral‐buffered formalin for tissue processing.
The lung and trachea N‐glycans were prepared for mass spectrometric analysis as previously described.34 Briefly, the tissues were homogenized and sonicated in a homogenization buffer of 1% CHAPS (v/v) in 25 mm Tris, 150 mm sodium chloride (NaCl), 5 mm EDTA in water, pH 7·4 and subsequently dialyzed in a 50 mm ammonium bicarbonate (Ambic) buffer. The samples were reduced, carboxymethylated, and digested with trypsin (EC 3.4.21.4; Sigma). The N‐glycans were released by digestion with PNGase F (EC 3.5.1.52; Roche Molecular Biochemicals) and purified by reverse‐phase C18 Sep‐Pak (Waters) chromatography. Sialidase cleavage was performed with Sialidase S (recombinant from Streptococcus pneumoniae expressed in E. coli, Glyco, 170 mU) and Sialidase A (recombinant from Arthrobacter ureafaciens expressed in E. coli, Glyco, 170 mU), in 50 mm Sodium acetate, pH 5·5. Sialidase S cleaves α2‐3 linkages and Sialidase A cleaves α2‐3 and α2‐6 linkages. Sialidase specificity and experimental conditions were established by experiments with sialylated glycan standards with known Sia linkages. The products of digestion were determined by mass spectrometry. The digest samples were lyophilized, permethylated, and purified by C18 Sep‐Pak (Waters). Matrix‐assisted laser desorbtion ionization–time of flight (MALDI‐TOF) data were acquired on a Voyager‐DE STR mass spectrometer (PerSeptive Biosystems, Framingham, MA, USA) in the reflectron positive mode with delayed extraction. Permethylated samples were dissolved in 10 μl methanol, and 1 μl of dissolved sample was premixed with 1 μl of matrix [20 mg/ml 2,5‐dihydroxybenzoic acid (DHB) in 70% (v/v) aqueous methanol before being loaded onto the sample plate. MALDI‐TOF/TOF experiments were performed on a 4800 Proteomics Analyzer (Applied Biosystems, Framingham, MA, USA) operated in the reflectron positive ion mode.
Statistical analysis
The differences of log10‐transformed viral titers from infected culture collected at different time points post‐infection and different viruses were compared using one‐way anova followed by Bonferroni multiple‐comparison test. Differences were considered as significant at P < 0·05. The statistical analysis was carried out using GraphPad Prism 5.
Results
Swine lung contains sialylated glycan receptors for influenza viruses
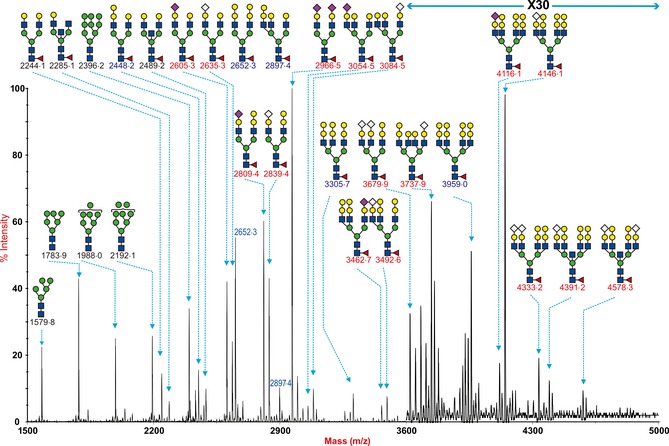
The MALDI‐TOF spectra derived from the permethylated N‐glycans of control pig lung (Figure 1) contained a diverse range of [M+Na]+ molecular ion signals. Expressed signals consistent with both high mannose type N‐glycans (m/z 1579·9, 1783·7, 1987·7, 2191·7 and 2395·8, Hex5‐9HexNAc2) and complex type bi‐, tri‐, tetra‐antennary and polylactosamine extended core fucosylated structures (m/z 2243·8‐ 5959·9, Hex3HexNAc4Fuc‐Hex13HexNAc8Fuc) were present. The complex glycans were capped with either the Galα1‐3Gal sequence (for example, m/z 2652·3, 3305·7, and 3959·0 highlighted in blue, Hex7HexNAc4Fuc‐Hex11HexNAc6Fuc consistent with bi‐, tri‐, and tetra‐antennary core fucosylated structures) or NeuAc (for example m/z 2605·1 and 3054·5 NeuAcHex5HexNAc4Fuc‐ NeuAcHex6HexNAc4Fuc consistent with mono‐sialylated bi‐ and tri‐antennary core fucosylated structures highlighted in red). Complex N‐glycans capped with the NeuGc form of Sia were also observed (for example m/z 2635·1, 2839·4, 3084·5, 3492·6 NeuGcHex6HexNAc4Fuc‐ NeuGcHex8HexNAc5Fuc also highlighted in red). A ratio of 3:2 Ac:Gc was identified comparing the peaks at m/z 2605,2635 and 2809,2839. This ratio appeared reversed for the larger tri‐ and tetra‐antennary structures. The second pig lung that was processed separately had a similar range of glycans present (Figure S1). The glycans detected and their relative abundance are listed in Table S1.
Figure 1.
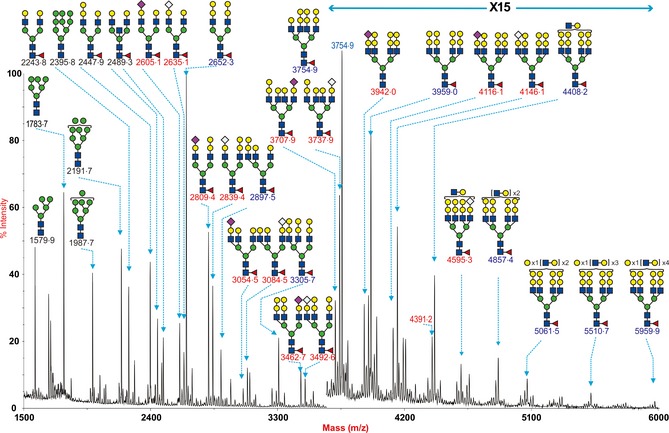
N‐glycan profile of swine lung. MALDI‐TOF mass spectra of permethylated N‐glycans from the intermedidate lobe of swine lung was obtained from the 50% MeCN fraction from a C18 Sep‐Pak column (“Materials and Methods”). Annotated structures are according to the Consortium for Functional Glycomics guidelines. All molecular ions are [M+Na]+. Putative structures are based on composition, tandem MS, and the biosynthetic knowledge. Structures that show sugars outside a bracket have not been unequivocally defined. Sialylated species are annotated in red and those with GalαGal are annotated in blue.
Siaα2‐3 and Siaα2‐6‐linked N‐glycans were found in pig lung
The proportion of α2‐3‐ versus α2‐6‐linked Sia present in the N‐glycans of porcine lung was further investigated by comparing sensitivity to digestion with linkage‐specific sialidases and control samples without sialidase treatment (Figure 2). Sialidase S was used for the specific release of α2‐3‐linked Sia, and Sialidase A for release of both α2‐3‐ and α2‐6‐linked Sia. The MALDI‐TOF spectra of porcine lung N‐glycans are shown after digestion with Sialidase S (Figure 2B), Sialidase A (Figure 2C), and control undigested (Figure 2A). Digestion of the pig lung N‐glycans with Sialidase A (Figure 2C, Table S1) caused a complete loss of all Sia‐containing glycans (e.g. m/z 2605·3, 2635·3, 2809·4, 2839·4, 2966·5, 3054·5, 3084·5, 3258·4, 3462·6, 3492·6, and 4146·1). Concurrent with these observations, the glycans at m/z 2244·0 (Hex5HexNAc4Fuc), 2448·1 (Hex6HexNAc4Fuc), 2693·2 (Hex6HexNAc5Fuc1), 3101·4 (Hex8HexNAc5Fuc), 3346·9 (Hex8HexNAc6Fuc), 3550·6 (Hex9HexNAc6Fuc), and 3754·6 (Hex10HexNAc6Fuc), which would be produced by de‐sialylating the above glycans, increased in abundance after Sialidase A treatment, confirming the presence of Sia‐containing N‐glycans in the pig lung. By contrast, digestion of the pig lung N‐glycans with Sialidase S (Figure 2B) caused a partial loss of Sia‐containing glycans with signals at m/z 2605·5, 2636·5, 2809·6, 2839·6, 3054·7, 3084·7, 3462·9, 3942·9, and 4146·3 still remaining. This confirmed that pig lung N‐glycans contained both α2‐3‐ and α2‐6‐linked Sia.
Figure 2.
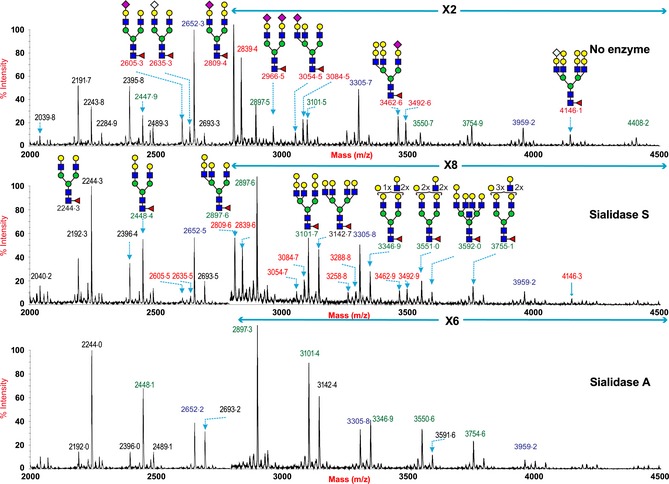
N‐glycan profile of swine lung following sialidase treatment. Partial MALDI‐TOF MS profiles of the permethylated N‐linked glycans derived from swine trachea after digestion with sialidase S (α2‐3 specific) or sialidase A (α2‐3 and α2‐6 specific). Data were obtained from the 50% acetonitrile fraction and all molecular ions are present in sodiated form ([M+Na]+). Sialylated species are annotated in red and those with GalαGal are annotated in blue.
Sialylated N‐linked glycans are also present in swine trachea
The MALDI‐TOF spectra of porcine trachea (Figure 3) showed fewer types of sialylated glycans compared with porcine lung. However, it should be noted that a more limited amount of tissue for glycomic analysis was available, which could inhibit the detection of higher molecular weight glycan species. The spectra derived from the control undigested sample indicated that similar to the lung, the pig trachea also expressed signals consistent with both high mannose type N‐glycans (m/z 1579·8 1783·9, 1988·0, 2192·1, and 2396·2, Hex5‐9HexNAc2) and complex type bi‐, tri‐, tetra‐antennary and polylactosamine extended core fucosylated N‐glycans (m/z 1836·2‐4245·5, Hex3HexNAc4Fuc‐Hex10HexNAc8Fuc). The complex glycans were also capped with either the Galα1‐3Gal sequence (for example m/z 2652·3, 3305·6 and 3959·0) or Sia, both NeuAc and NeuGc (for example, m/z 2809·4 and 2839·4). Digestion of the pig trachea N‐glycans with Sialidase A (Figure 4C) also caused a loss of most Sia‐containing glycans with a concurrent increase in the abundance of their de‐sialylated products (m/z 2244·2, 2448·4, 2693·6, and 3102·1), confirming the presence of Sia‐containing N‐glycans in the pig trachea. By contrast, digestion of the pig trachea N‐glycans with Sialidase S (Figure 4B) also caused a partial loss of Sia‐containing glycans with signals at m/z 2605·6, 2635·6, 2809·6, 2839·6, 3054·8, 3084·8, still remaining. Also of note is that the reduced complexity caused by the digestion with the sialidases affords the detection of higher MW polylac‐containing N‐glycans, for example, at m/z 4246 and 4492. This confirmed that pig trachea, such as the pig lung, contains N‐glycans with both α2‐3‐ and α2‐6‐linked Sia.
Figure 3.
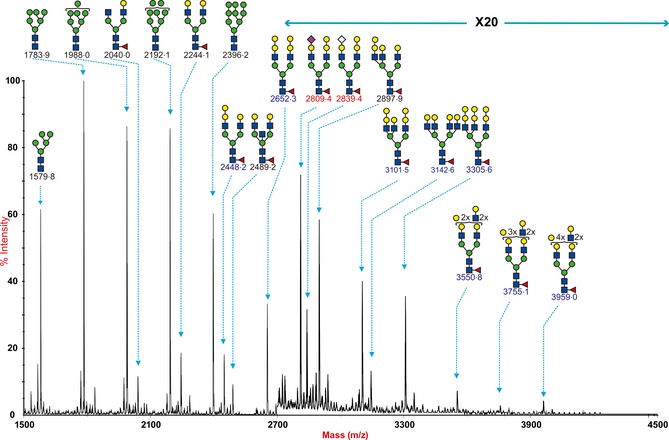
N‐glycan profile of swine trachea. MALDI‐TOF mass spectra of permethylated N‐glycans from the trachea of swine was obtained from the 50% MeCN fraction from a C18 Sep‐Pak column (“Materials and Methods”). Annotated structures are according to the Consortium for Functional Glycomics guidelines. All molecular ions are [M+Na]+. Putative structures are based on composition, tandem MS, and the biosynthetic knowledge. Structures that show sugars outside a bracket have not been unequivocally defined.
Figure 4.
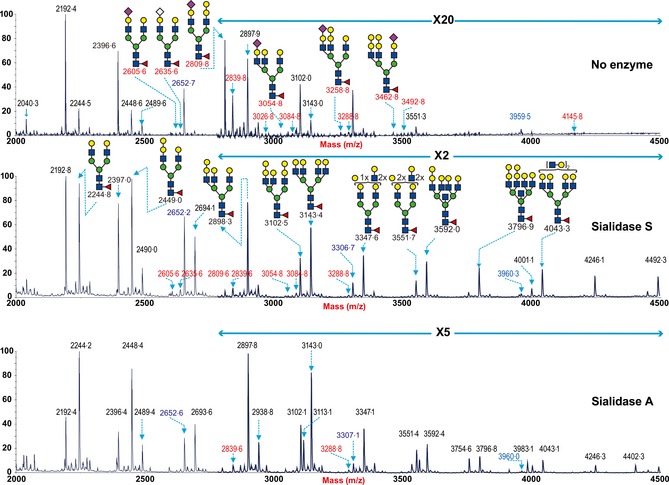
N‐glycan profile of swine trachea following sialidase treatment. Partial MALDI‐TOF MS profiles of the permethylated N‐linked glycans derived from swine trachea after digestion with sialidase S (α2‐3 specific) or sialidase A (α2‐3 and α2‐6 specific). Data were obtained from the 50% acetonitrile fraction and all molecular ions are present in sodiated form ([M+Na]+). Sialylated species are annotated in red and those with GalαGal are annotated in blue.
Siaα2‐6 dominancy of N‐linked glycans in pig trachea and lung
It has been demonstrated that relative quantitation based on signal intensities of permethylated glycans analyzed by MALDI‐TOF MS is a reliable method, especially when comparing signals over a small mass range within the same spectrum.35 We compared the changes in the intensities of glycans with a single galactose antenna, which will increase with the removal of Sia, with glycans that contained the Gal‐α Gal sequence, which will not change with sialidase treatment (e.g. m/z 2652·3 and 3305·8). This gives an indication of the relative activity of the two sialidases and therefore the relative abundance of α2‐3‐ and α2‐6‐linked Sia. For example (Figures 2 and 4), we compared the relative abundance of the peaks at m/z 2448/2652 and m/z 3102/3306, containing bi‐ and tri‐ antennary core fucosylated structures (Hex6HexNAc4Fuc/Hex7HexNAc4Fuc and Hex8HexNAc5Fuc/Hex9HexNAc5Fuc). In both the lung and trachea, there was a greater relative increase in the glycans at m/z 2448 and 3306 with Sialidase A digestion, indicating a higher expression of α2‐6‐linked Sia N‐glycans in the pig lung and trachea.
O‐Linked glycans
O‐Glycan data of the pig lung (Figure 5) showed the presence of both core 1 and core 2 structures. Low levels of mono‐sialylated core 2 structure at m/z 1344·7 were detected. In contrast, the main glycan present was the galactose‐terminated core 2 structure at m/z 1187·4
Figure 5.
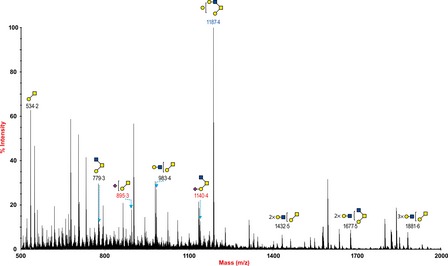
O‐glycan profile of swine lung. MALDI‐TOF mass spectra of permethylated O‐glycans of swine lung. O‐Glycomic profiles were obtained from the 35% MeCN fraction from a C18 Sep‐Pak column (“Materials and Methods”). Annotated structures are according to the Consortium for Functional Glycomics guidelines. All molecular ions are [M+Na]+. Putative structures are based on composition, tandem MS, and the biosynthetic knowledge.
Lung of minipig contained more Sia2‐3‐linked glycan than normal farm pigs
We compared the two lungs from normal pigs with lung tissue obtained from a minipig (Figure 6). Differences between the normal and minipig lungs were identified. In particular, the minipig lung tissues had a high abundance of monosialylated and disialylated biantennary core fucosylated structures (NeuAcHex5HexNAc4Fuc, NeuAc2Hex5HexNAc4Fuc) at m/z 2605·3 and 2966·5, which was relatively low in the normal pig lung. In contrast, the biantennary glycan with the terminal Galα1‐3Gal m/z 2652·3 (Hex7HexNAc4Fuc) was more abundant in the normal pigs than tissues from minipigs. The NeuAc to NeuGc ratio was similar between the normal and minipigs in the biantennary glycans at m/z 2605·3, 2635·3, 2809·4, 2839·4, 3054·5, and 3084·5, but again this ratio appeared reversed for the larger tri‐ and tetra‐antennary structures. In addition, the data from sialidase treatment indicated a greater abundance of α2‐3‐type linkages in the minipig lung compared with the normal pig lung.
Figure 6.
N‐glycan profile of mini‐pig lung. MALDI‐TOF mass spectra of permethylated N‐glycans from the intermedidate lobe of a mini‐pig lung was obtained from the 50% MeCN fraction from a C18 Sep‐Pak column (“Materials and Methods”). Annotated structures are according to the Consortium for Functional Glycomics guidelines. All molecular ions are [M+Na]+ Putative structures are based on composition, tandem MS, and the biosynthetic knowledge. Structures that show sugars outside a bracket have not been unequivocally defined. Sialylated species are annotated in red and those with GalαGal are annotated in blue.
Lectin histochemistry of trachea and lung samples
Lectin binding results showed that there was strong binding of SNA to the tracheal epithelium and bronchiolar epithelium with weak binding to pneumocytes. In contrast, there was no significant binding of MAA‐I or MAA‐II to the tracheal epithelium but binding to the submucosa (Figure 7). Binding of MAA‐I was seen to the bronchiolar epithelium with no significant binding of MAA‐II. This binding was removed with sialidase treatment. PNA binding was identified weakly in the tracheal and bronchiolar epithelium but increased marginally after sialidase treatment. There was a medium intense positive staining on the surface of the tracheal epithelium for NeuGc, and this was also present on the surface of alveolar epithelial cells (Figure S2).
Figure 7.
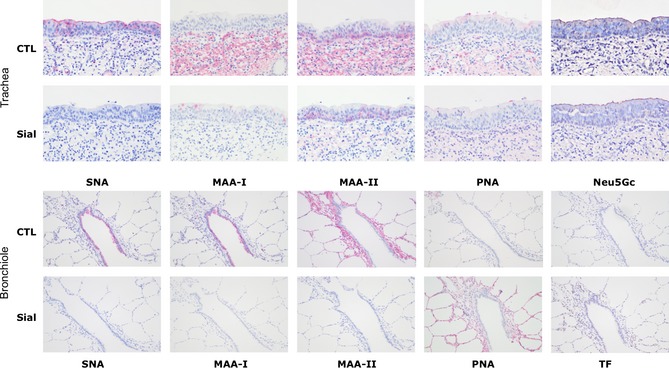
Lectin Histochemistry of swine trachea and lung. Trachea sections showing binding of SNA (α2‐6 glycans), MAA‐I (α2‐3 N glycans), MAA‐II (α2‐3 O‐glycans, PNA(Gals1‐3Gal/GalNAc) and Neu5Gc. CTL indicates control sections and Sial are sections pretreated with a sialidase that removes both α2‐3 and α2‐6 sialic acids. Lung and bronchiole sections are in the lower panel and TF indicates the Thomsen‐Freidenreich antibody which is more specific for Gals1‐3GalNAc. Magnification ×200.
Avian influenza A virus infection in pig lung and trachea
Virus replication kinetics of high and low pathogenic avian influenza viruses in tracheal and lung explants of normal pigs is shown (Figure 8). Unlike cell lines derived from a single tissue or tumor, pig explant cultures are more susceptible to donor‐to‐donor variations as they are from the respiratory organs of different pigs with individual variations. There was minimal replication of H5N1 in the swine trachea compared with the lung apart from one of four samples in which there was a 2 log increase with A/VN/3046/04 and A/VN/1203/04 infection and three of five cases where there was a 1 log increase for A/HK/483/97. In contrast, consistent replication in the swine lung was observed with most of the H5N1 viruses studied apart from A/VN/3046/04 where replication was only seen in two of five samples. Some of the low pathogenic avian viruses, A/Quail/HK/G1/97 (H9N2) and A/Northpintail/HK/MP5883/04 (H5N8), showed limited replication in the trachea, but most of them were able to replicate in pig lung. The recent H7N9 virus that has caused an outbreak in Mainland China in the early part of 2013 showed consistent replication in the bronchioles of the swine lung (Figure 9). The control viruses, A/swine/HK/4167 (H1N1), A/Oklahoma/1992/05 (H3N2), and A/Hong Kong/415742/09 (H1N1pdm), infected and replicated productively in all experiments with more than 2 log increase in trachea and at least 1 log increase in lung (Figure S3).
Figure 8.
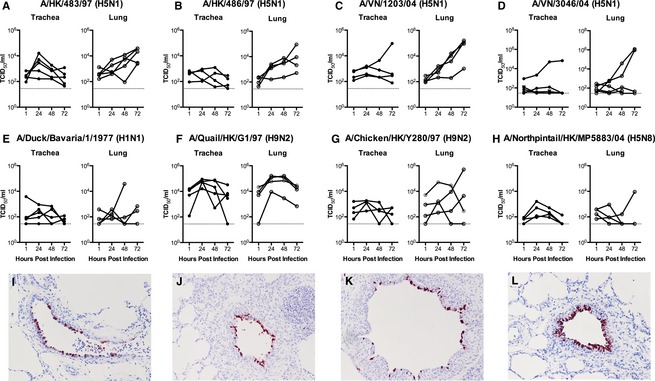
Virus replication kinetics of avian viruses in ex vivo trachea and lung. (A–H) Viral replication in ex vivo biopsies infected with 106TCID50/ml of influenza viruses as indicated by TCID50 assay. Each chart shows the virus titer from three independent experiments. (I–L) Immunohistochemistry for influenza nucleoprotein in the bronchioles of A/Duck/Bavaria/1/1977 9 (I), A/Quail/HK/G1/97 (J), A/Chicken/HK/Y280/97 (K) and A/Northpintail/HK/MP5883/04 (L).
Figure 9.
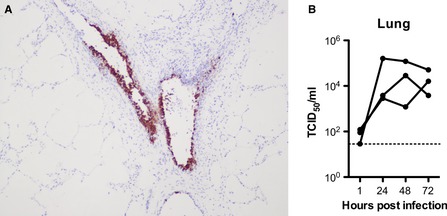
H7N9 replication in swine lung. (A) shows positive immunohistochemical staining in bronchioles, and (B) shows the virus titer from three independent experiments.
Immunohistochemistry of the ex vivo tissues was performed on all samples. This showed that at 24, 48, and 72 hours post‐infection, there was absent to minimal staining of the tracheal tissues with all viruses tested, even in the H5N1 cases where a 2 log increase in viral titer was seen (Figure S4). As these blocks were serially sectioned through this suggests that the infection is very focal. In contrast, positive antigen was detected in lung tissues infected with H5N1 avian viruses where infection was mainly localized to bronchioles (Figure 10). Pulmonary extension was mainly present in tissues infected with H5N1 in contrast to other avian viruses including H7N9 where infection was confined to bronchioles (Figure 8).
Figure 10.
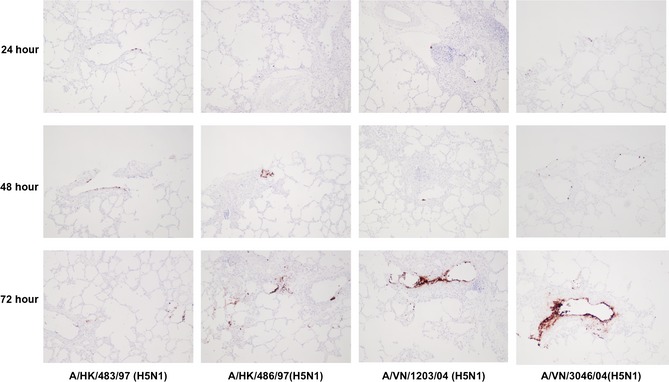
Immunohistochemistry of H5N1 replication in the swine lung. Ex vivo explants were infected with A/HK/483/97, A/HK/486/97, A/VN/1203/04 and A/VN/3046/04 and fixed at 24, 48 and 72 hours followed by tissue processing and staining for influenza nucleoprotein. Magnification ×200.
Discussion
We found minimal infection of ex vivo cultures of swine trachea with H5N1 and other avian Siaα2‐3 binding viruses, and this is thus in accord with the virus binding experiments of van Riel13 and with the lectin binding studies of the trachea where there was no significant binding of MAA‐I or MAA‐II to the tracheal epithelium. However, the presence of infection in bronchioles by H5N1 does appear to be in contrast to the previously published patterns of viral attachment. As the 2 isoforms of MAA and SNA do not have a strong binding affinity to NeuGc compared with NeuAc, an absence of lectin binding does not necessarily mean that Sia is not present. Indeed, the NeuGc antibody did stain the surface of the bronchi and bronchioles, indicating that NeuGc was present. Most of this staining was abolished with Sialidase A treatment, but not with Sialidase S treatment, indicating that the NeuGc is more 2‐6‐linked than 2‐3‐linked.
Using fresh explanted lung tissues from 6‐month‐old swine, we found that the main site of viral replication of all avian viruses was in the lung rather than the trachea (Figure 8), and immunohistochemistry localized this to bronchioles rather than pneumocytes. Our previous studies,20 and control H1N1 infection (Figure S1), show that the bronchioles appear to be a common site for replication of seasonal and avian viruses and a switch to an “avian” type of virus led to more lower respiratory tract tropism.36 In this area, there was binding with SNA and MAA‐1 (Figure 7). Binding with MAA‐II to the bronchioles was minimal, but there was staining with TF after sialidase treatment to pneumocytes, and PNA binding was also increased after sialidase treatment. These findings would indicate that α2‐3‐linked N‐glycans are the potential receptors for the avian viruses H5N1 and H5N8 in the bronchiolar epithelium. As the mass spectrometric data on the lung tissue demonstrated that there is a low quantity of Sia‐core 1 at m/z 895 and a very low level of di‐Sia‐core 1 at m/z 1256 (Figure 5), this would suggest that the main O‐glycan present in the pneumocytes is the sialylated TF glycan. The low abundance of the disialylated TF can be explained by the presence of the α1‐3‐galactose transferase enzyme in the pig lung which is absent in human tissues as these lack a functional GGTA1 (α1,3‐galactosyltransferase) gene.37 The presence of this enzyme leads to a competition of the terminal Gal in Sia‐core 1 for Sia and Gal, and the mass spectrometric findings suggest a greater preference for the Gal rather than the Sia, resulting in a very low level of Sia‐core 1. Therefore, this reduces the probability for H5N1 virus to infect the alveolar epithelium by utilizing Sia‐TF. Previous mass spectrometric studies have also shown that porcine submaxillary mucin has minimal di‐Sia‐core 1.38
The only previous publication that has addressed the glycan distribution in the porcine respiratory tract was by Bateman et al.25 who used tissues derived from the trachea.26 They found a greater abundance of α2‐6‐terminated glycans than that seen in our MS analysis where we found more α2‐3 glycans. This is explainable with the data in Figure 7 where there is no binding of MAA‐I or MAA‐II to the epithelium but positive binding of MAA‐I and ‐II to the submucosa. Similar findings were also reported by Nelli et al.21 As we are not currently able to separate the epithelium from the submucosa for our glycan analysis of tracheal tissues, the α2‐3 positive tissues most likely represent glycans present in the submucosa. When the MS findings were compared with human lung tissues,39 the two main differences present in the swine respiratory tract tissues were the high abundance of Galα1‐3Gal and relative lack of extended sialylated LacNAc repeats.
Our findings that H5N1 replicates in a similar anatomical site to where swine and human viruses replicate lend credence to evidence that the pig can still be a site for mixing of viruses and where H5N1 can acquire increased affinity to α2‐6‐terminated glycans.12 There was replication identified in the lung by H7N9 similar to other avian viruses (Figure 9), supporting data from a previous study that found replication in the respiratory tract by this recent H7N9 using PCR analysis.40 A recent study using an avian H4N6 has also shown scanty replication of avian influenza virus in alveolar tissues.22 Lectin binding studies and ex vivo cultures show that the trachea is not likely to be an ideal anatomical site for replication of predominantly α2‐3 binding viruses or virus growth but in contrast may be an anatomical region that favors a selection pressure for the emergence of avian viruses with a greater binding preference for α2‐6‐linked glycans. We found that there is limited evidence of infection of pneumocytes, and this may explain why influenza infection in swine produces few clinical symptoms and is not associated with great mortality. The high abundance of Galα1‐3Gal structures identified by our MS analysis leads to a relative decrease in sialylated glycans, especially those with an extended LAcNAc structure. If these extended structures are involved in influenza virus binding, this finding may explain the lack of replication in this anatomical site.41 The comparative lack of replication in pneumocytes can also be explained by the unique glycosylation features of porcine surfactant protein‐D which has greater inhibitory activities against a wide range of influenza A viruses compared with human surfactant proteins.42
The minipig has been often used as laboratory animal to study infection in a number of situations, including studies of swine influenza.28, 29 Our MS findings (Figure 6) show that the lung from these animals has a different glycan profile to that seen in normal pigs with more expression of higher molecular weight tetra‐antennary Neu5Gc‐containing N‐glycans and more α2‐3‐linked Sia glycans. This may give a bias toward susceptibility to different avian viruses, and so studies on virus tropism, infection, or transmission using these animals might need to be interpreted with caution, in the light of these findings. The pig tissues used for explants were obtained from local slaughter house which probably represents the true population in the farm and environment, as well as pre‐existing exposure to respiratory viruses; thus, we consider that these should continue to be utilized in the determination of tropism studies of different influenza viruses to demonstrate the potential public health risk.
In conclusion, our mass spectrometric analysis shows that compared with the human respiratory tract, the swine lung has fewer types of sialylated glycans present owing to the presence of the Galα1‐3Gal structures, and of the sialylated glycans present, there are more α2‐6 N‐glycans present than α2‐3 N‐glycans. This may account for the relative lack of replication of H5N1 in tracheal tissues. Our ex vivo evidence demonstrates that the lower respiratory tract of the domestic pig, in particular the bronchiolar region, is a site where α2‐3‐binding H5N1 and α2‐6‐binding H9N2 avian viruses can efficiently replicate. As this is also an anatomical site where swine and human viruses replicate, this demonstrates that the pig is still a likely candidate for the continuing emergence of novel reassorted viruses.
Supporting information
Figure S1. N‐glycan profile of two swine lungs for comparison.
Figure S2. Neu5Gc staining of swine bronchiole and lung parenchyma ×200.
Figure S3. Virus replication kinetics of 3 control swine (A) and human (B,C) viruses in ex vivo trachea and lung.
Figure S4. Immunohistochemistry for influenza nucleoprotein in the tracheas infected with A/Chicken/HK/Y280/97, A/Quail/HK/G1/97 (J), A/Northpintail/HK/MP5883/04 and A/Swine/HK/4167/99 and fixed at 48 hours.
Table S1. Swine lung N‐glycan profile.
Table S2. Swine lung sialidase treatment N‐glycan profile.
Table S3. Swine trachea N‐glycan profile.
Table S4. Swine trachea sialidase treatment N‐glycan profile.
Table S5. Swine lung O‐glycan profile.
Table S6. Mini‐pig lung N‐glycan profile.
Acknowledgements
This work was supported by Grant 082098 from the Wellcome Trust (to S. M. H., A. D. and J.N), Area of Excellence Scheme of the University Grants Committee (grant AoE/M‐12/96), Research Fund for Control of Infection Disease (to R.W.Y.C, #12110992), and additional funding was from a grant of the European Commission (FP7‐ GA258084). We thank Nan Jia for assistance with manuscript preparation.
Chan et al. (2013) Infection of swine ex vivo tissues with avian viruses including H7N9 and correlation with glycomic analysis. Influenza and Other Respiratory Viruses 7(6), 1269–1282.
References
- 1. Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983; 127:361–373. [DOI] [PubMed] [Google Scholar]
- 2. Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983; 304:76–78. [DOI] [PubMed] [Google Scholar]
- 3. Chen LM, Blixt O, Stevens J et al In vitro evolution of H5N1 avian influenza virus toward human‐type receptor specificity. Virology 2012; 422:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen LM, Rivailler P, Hossain J et al Receptor specificity of subtype H1 influenza A viruses isolated from swine and humans in the United States. Virology 2011; 412:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maines TR, Chen LM, Van Hoeven N et al Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology 2011; 413:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumari K, Gulati S, Smith DF, Gulati U, Cummings RD, Air GM. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J 2007; 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholtissek C, Burger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 1985; 147:287–294. [DOI] [PubMed] [Google Scholar]
- 8. Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res 2007; 38:243–260. [DOI] [PubMed] [Google Scholar]
- 9. Ito T, Couceiro JN, Kelm S et al Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 1998; 72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kida H, Ito T, Yasuda J et al Potential for transmission of avian influenza viruses to pigs. J Gen Virol 1994; 75(Pt 9):2183–2188. [DOI] [PubMed] [Google Scholar]
- 11. Lipatov AS, Kwon YK, Sarmento LV et al Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog 2008; 4:e1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nidom CA, Takano R, Yamada S et al Influenza A (H5N1) viruses from pigs, Indonesia. Emerg Infect Dis 2010; 16:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Riel D, Munster VJ, de Wit E et al Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol 2007; 171:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baum LG, Paulson JC. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem Suppl 1990; 40:35–38. [PubMed] [Google Scholar]
- 15. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature 2006; 440:435–436. [DOI] [PubMed] [Google Scholar]
- 16. Nicholls JM, Chan MC, Chan WY et al Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med 2007; 13:147–149. [DOI] [PubMed] [Google Scholar]
- 17. Imai M, Watanabe T, Hatta M et al Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russell CA, Fonville JM, Brown AE et al The potential for respiratory droplet‐transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 2012; 336:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herfst S, Schrauwen EJ, Linster M et al Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 2010; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res 2010; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trebbien R, Larsen LE, Viuff BM. Distribution of sialic acid receptors and influenza A virus of avian and swine origin in experimentally infected pigs. Virol J 2011; 8:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 2007; 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geisler C, Jarvis DL. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 2011; 21:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bateman AC, Karamanska R, Busch MG, Dell A, Olsen CW, Haslam SM. Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAc{alpha}2‐6 glycans. J Biol Chem 2010; 285:34016–34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Busch MG, Bateman AC, Landolt GA et al Identification of amino acids in the HA of H3 influenza viruses that determine infectivity levels in primary swine respiratory epithelial cells. Virus Res 2008; 133:269–279. [DOI] [PubMed] [Google Scholar]
- 27. Pascua PN, Song MS, Lee JH et al Evaluation of the efficacy and cross‐protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS ONE 2009; 4:e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Itoh Y, Shinya K, Kiso M et al In vitro and in vivo characterization of new swine‐origin H1N1 influenza viruses. Nature 2009; 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bai GR, Sakoda Y, Mweene AS et al Evaluation of the ESPLINE INFLUENZA A&B‐N Kit for the diagnosis of avian and swine influenza. Microbiol Immunol 2005; 49:1063–1067. [DOI] [PubMed] [Google Scholar]
- 30. Nishino M, Mizuno D, Kimoto T et al Influenza vaccine with Surfacten, a modified pulmonary surfactant, induces systemic and mucosal immune responses without side effects in minipigs. Vaccine 2009; 27:5620–5627. [DOI] [PubMed] [Google Scholar]
- 31. Vijaykrishna D, Smith GJ, Pybus OG et al Long‐term evolution and transmission dynamics of swine influenza A virus. Nature 2011; 473:519–522. [DOI] [PubMed] [Google Scholar]
- 32. Chan MC, Chan RW, Yu WC et al Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol 2010; 176:1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Chan CW, Nicholls JM, Liao S, Tse HF, Wu EX. MR study of the effect of infarct size and location on left ventricular functional and microstructural alterations in porcine models. J Magn Reson Imaging 2009; 29:305–312. [DOI] [PubMed] [Google Scholar]
- 34. Jang‐Lee J, North SJ, Sutton‐Smith M et al Glycomic profiling of cells and tissues by mass spectrometry: fingerprinting and sequencing methodologies. Methods Enzymol 2006; 415:59–86. [DOI] [PubMed] [Google Scholar]
- 35. Wada Y, Azadi P, Costello CE et al Comparison of the methods for profiling glycoprotein glycans–HUPO Human Disease Glycomics/Proteome Initiative multi‐institutional study. Glycobiology 2007; 17:411–422. [DOI] [PubMed] [Google Scholar]
- 36. Van Poucke S, Uhlendorff J, Wang Z et al Effect of receptor specificity of A/Hong Kong/1/68 (H3N2) influenza virus variants on replication and transmission in pigs. Influenza Other Respi Viruses 2013; 7:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larsen RD, Rivera‐Marrero CA, Ernst LK, Cummings RD, Lowe JB. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP‐Gal:beta‐D‐Gal(1,4)‐D‐GlcNAc alpha(1,3)‐galactosyltransferase cDNA. J Biol Chem 1990; 265:7055–7061. [PubMed] [Google Scholar]
- 38. Yamada K, Hyodo S, Matsuno YK et al Rapid and sensitive analysis of mucin‐type glycans using an in‐line flow glycan‐releasing apparatus. Anal Biochem 2007; 371:52–61. [DOI] [PubMed] [Google Scholar]
- 39. Chan RW, Chan MC, Wong AC et al DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother 2009; 53:3935–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu H, Wang D, Kelvin DJ et al Infectivity, Transmission, and Pathology of Human H7N9 Influenza in Ferrets and Pigs. Science 2013; 341:183–186. [DOI] [PubMed] [Google Scholar]
- 41. Nycholat CM, McBride R, Ekiert DC et al Recognition of sialylated poly‐N‐acetyllactosamine chains on N‐ and O‐linked glycans by human and avian influenza A virus hemagglutinins. Angew Chem Int Ed Engl 2012; 51:4860–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Eijk M, White MR, Batenburg JJ et al Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. Am J Respir Cell Mol Biol 2004; 30:871–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. N‐glycan profile of two swine lungs for comparison.
Figure S2. Neu5Gc staining of swine bronchiole and lung parenchyma ×200.
Figure S3. Virus replication kinetics of 3 control swine (A) and human (B,C) viruses in ex vivo trachea and lung.
Figure S4. Immunohistochemistry for influenza nucleoprotein in the tracheas infected with A/Chicken/HK/Y280/97, A/Quail/HK/G1/97 (J), A/Northpintail/HK/MP5883/04 and A/Swine/HK/4167/99 and fixed at 48 hours.
Table S1. Swine lung N‐glycan profile.
Table S2. Swine lung sialidase treatment N‐glycan profile.
Table S3. Swine trachea N‐glycan profile.
Table S4. Swine trachea sialidase treatment N‐glycan profile.
Table S5. Swine lung O‐glycan profile.
Table S6. Mini‐pig lung N‐glycan profile.


