Abstract
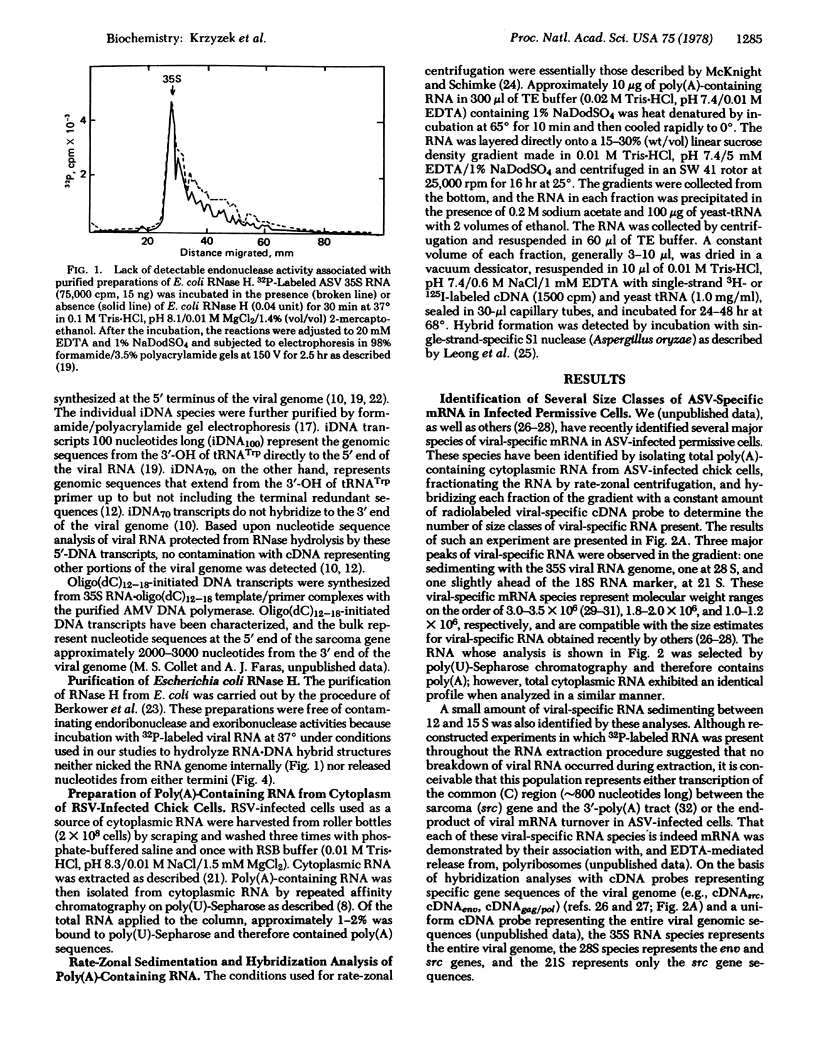
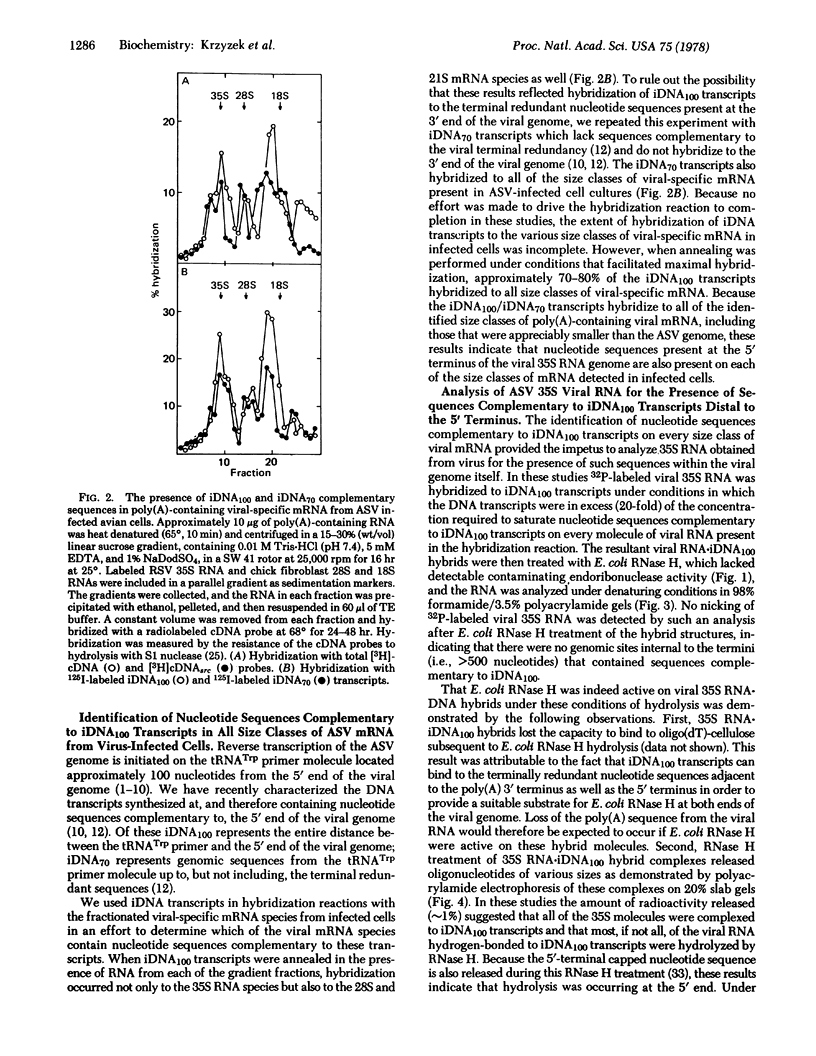
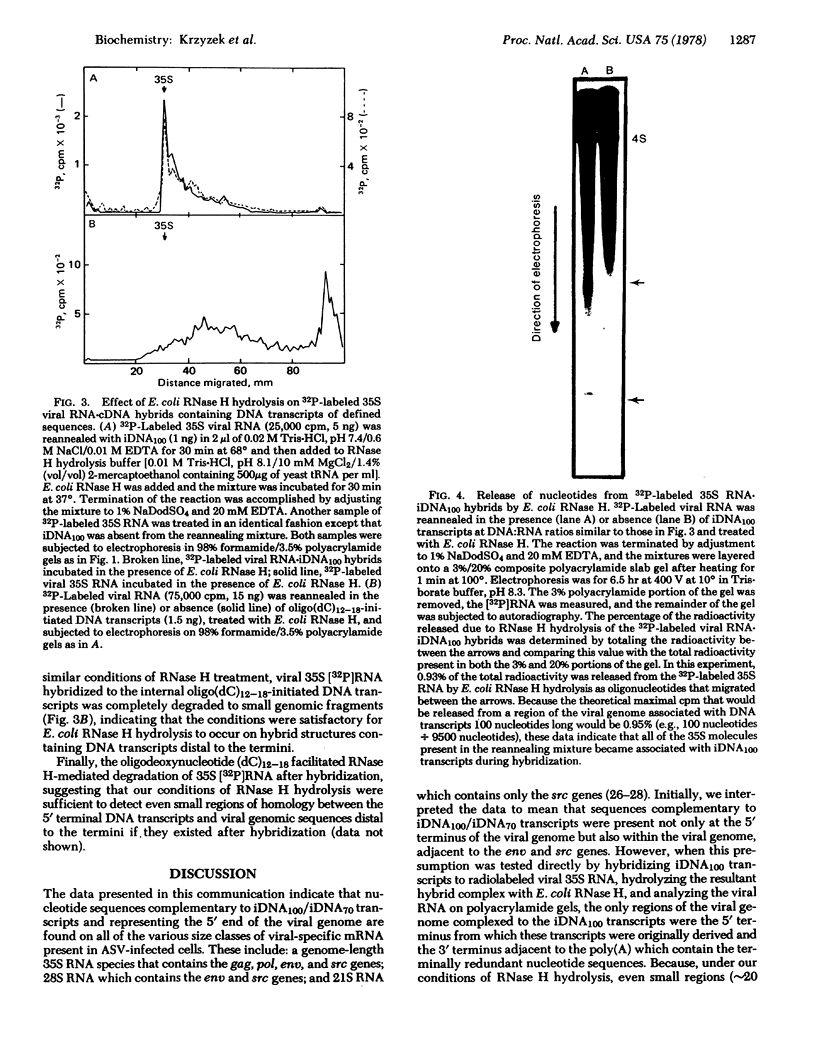
The 5′-terminal nucleotide sequences of the avian sarcoma virus (ASV) genome are transcribed by the reverse transcriptase in vitro into a DNA transcript that represents the entire distance (∼100 nucleotides) between the tRNATrp primer molecule and the 5′ terminus. We have used these DNA100 transcripts in hybridization reactions with ASV-specific RNA from infected avian cells and find nucleotide sequences complementary to these transcripts on all of the various size classes of viral mRNA identified. Similar hybridization results were obtained with a specific DNA transcript complementary to viral genomic nucleotide sequences between the tRNATrp primer molecule and up to, but not including, the terminal redundant sequences (DNA70), indicating that the observed hybridization of DNA100 to all size classes of viral RNA in infected cells did not reflect hybridization of DNA100 to the terminal redundant sequences at the 3′ end of the viral genome. Escherichia coli RNase H hydrolysis of RNA·DNA hybrids consisting of genomic 35S RNA obtained from virus and DNA100 transcripts indicated that viral genomic sequences complementary to these DNA transcripts were not present at sites distal to the ends of the RNA genome and therefore not adjacent to the corresponding gene sequences representing the various species of viral mRNA from infected cells. These studies suggest that the 5′-terminal genomic nucleotide sequences, or a portion thereof, are somehow added or “spliced” onto each ASV-specific mRNA species in infected cells either during or after transcription of proviral DNA for some as yet undetermined purpose.
Keywords: gene splicing, retrovirus RNA, hybridization, RNase H hydrolysis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Terminal redundancy and the origin of replication of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1908–1912. doi: 10.1073/pnas.74.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Cahill J. F., Faras A. J., Parsons J. T. Terminally repeated sequences in the avian sarcoma virus RNA genome. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2389–2393. doi: 10.1073/pnas.74.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of theavian oncornavirus genome by the RNA-directed DNA polymerase: analysis of DNA transcripts synthesized in reconstructed enzymatic reactions. J Virol. 1977 Apr;22(1):86–96. doi: 10.1128/jvi.22.1.86-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Parsons J. T. Integration of avian sarcoma virus DNA sequences in transformed mammalian cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4301–4305. doi: 10.1073/pnas.74.10.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: tryptophan tRNA as the major species of primer RNA. J Virol. 1976 Mar;17(3):1049–1051. doi: 10.1128/jvi.17.3.1049-1051.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Vogt P. K., Nicolson M. O., McAllister R. M. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. doi: 10.1101/sqb.1974.039.01.096. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Adenovirus amazes at Cold Spring Harbor. Nature. 1977 Jul 14;268(5616):101–104. doi: 10.1038/268101a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]




