Abstract
Piwi-interacting RNAs (piRNAs) are a distinct class of small non-coding RNAs specifically expressed in the germline of many species. They are most notably required for transposon silencing. Loss of piRNAs results in defects in germ cell development, and thus, infertility. Most studies of piRNAs have been done in Drosophila, but much progress has also been made on piRNAs in the germline of mammals and other species in the past few years. This review provides a summary of our current knowledge of the biogenesis and functions of piRNAs during mouse spermatogenesis and discusses challenges in the mammalian piRNA field.
Keywords: piRNA, Piwi, transposon silencing, meiosis, spermatogenesis, male fertility
Introduction
Small non-coding RNAs play important roles in many aspects of development, including cell fate specification and pluripotency.1-4Three main classes of small non-coding RNAs are expressed during spermatogenesis: microRNAs (miRNAs), endogenous small interfering RNAs (endo-siRNAs), and piRNAs.5,6 Out of these three classes of small non-coding RNAs, piRNAs are the most highly expressed during spermatogenesis but the least understood.
The piRNAs are required during multiple stages of spermatogenesis including de novo DNA methylation, meiosis, and spermiogenesis (Fig. 1).7 Genome-wide reprogramming occurs in primordial germ cells. During reprogramming, all DNA methyl marks are erased in germ cells. This ensures that all germ cells are epigenetically equal before they differentiate into male and female germ cells and establish their respective parental imprints during de novo DNA methylation.8,9 However, loss of DNA methyl marks or lack of de novo methylation results in the activation of many genes that are normally silenced, including transposons, and leaves the genome highly susceptible to damage.10 The piRNAs are required to silence transposons during this critical developmental period. The piRNA pathway is also required for meiosis and spermiogenesis. During meiosis, each spermatocyte produces four spermatids, each with a unique mixture of maternal and paternal DNA. Because of meiotic recombination and random segregation of homologous chromosomes, each haploid spermatid is unique in its DNA content, underscoring the vast diversity among gametes in non-inbred species. Spermiogenesis follows meiosis and consists of dramatic molecular and morphogenetic changes such as histone replacement, nuclear elongation, formation of the acrosome, and development of flagellum.
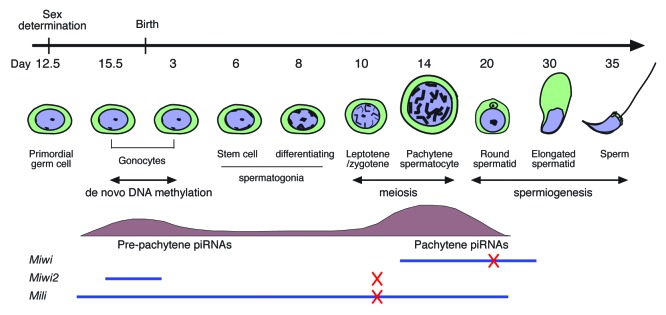
Figure 1. Developmental expression of piRNAs and PIWI proteins during mouse spermatogenesis. Pre-pachytene piRNAs are present prenatally in pro-spermatogonia and postnatally in spermatogonia. Pachytene piRNAs are present postnatally in pachytene spermatocytes and round spermatids. MIWI is expressed in pachytene spermatocytes and round spermatids. MIWI2 is expressed in pro-spermatogonia. MILI is expressed from pro-spermatogonia to round spermatids. Miwi-null mutants exhibit spermiogenic arrest. Mili- and Miwi2-null mutants exhibit meiotic arrest. The spermatogenic arrest points are indicated by Xs in red. This figure is modified from Figure 1 in the publication from Zheng and Wang.62
The piRNA pathway consists of many evolutionarily conserved protein factors (Table 1). This article provides a review on the current knowledge of the piRNA pathway by discussing mammalian protein factors and their roles in the biogenesis and function of piRNAs. While several recent reviews focus on the study of piRNAs in Drosophila,6,11-13 our review focuses on the mammalian piRNA pathway.
Table 1. Components of the piRNA pathway.
| Mouse protein | Knockout phenotype | Role in the piRNA pathway | Fly homolog | References |
|---|---|---|---|---|
| PIWIL1 (MIWI) | Spermiogenic arrest | Primary biogenesis | PIWI | 49, 51 |
| PIWIL2 (MILI) | Meiotic arrest | Primary and secondary biogenesis | PIWI | 16, 52 |
| PIWIL4 (MIWI2) | Meiotic arrest | Secondary biogenesis | PIWI | 50 |
| MOV10L1 | Meiotic arrest | Primary biogenesis | ARMITAGE | 71, 115 |
| DDX4 (MVH) | Meiotic arrest | Secondary biogenesis | VASA | 98, 116 |
| TDRD1 | Meiotic arrest | Primary biogenesis | TUDOR | 60, 105 |
| TDRD9 | Meiotic arrest | Secondary biogenesis | Spn-E | 75 |
| TDRD12 | Meiotic arrest | Secondary biogenesis | Yb | 92, 117 |
| TDRKH | Meiotic arrest | Primary biogenesis | PAPI | 82 |
| MitoPLD | Meiotic arrest | Primary biogenesis | ZUCCHINI | 97, 110 |
| GASZ | Meiotic arrest | Primary biogenesis | 73 | |
| MAEL | Meiotic arrest | Primary and secondary biogenesis | MAELSTROM | 74, 100 |
| GPAT2 | N/A | Primary biogenesis | 96 | |
| FKBP6 | Meiotic arrest | Secondary biogenesis | SHUTDOWN | 99 |
| GTSF1 | Meiotic arrest | Transcriptional transposon silencing | GTSF1 | 101–103 |
| A-MYB | Meiotic arrest | Transcription of piRNA precursors | 79 |
piRNAs
The piRNAs were initially discovered in the Drosophila germline as small RNAs transcribed from repetitive elements such as retrotransposons, DNA transposons, and the Su(Stellate) locus.14,15 Since then, piRNAs have also been found to be expressed in the germline of many other metazoan species, including mouse, rat, zebrafish, Xenopus, silkworm, and C. elegans.16-31 There are hundreds of thousands if not millions of distinct piRNA sequences within a species, and piRNA sequences are not conserved among different species.13 The piRNAs (~25–30 nt) are different in size from miRNAs and siRNAs (21–24 nt). In addition, the 5′ ends of piRNAs have a preference for a uridine. The 3′ ends of piRNAs are 2’-O-methylated.16,20,32,33 By definition, piRNAs are bound to PIWI proteins. Two classes of piRNAs are generated during mouse spermatogenesis: pre-pachytene and pachytene piRNAs (Fig. 1).
Pre-pachytene piRNAs
Pre-pachytene piRNAs are generated prenatally in pro-spermatogonia and are also present in postnatal male germ cells until the onset of meiosis. Pre-pachytene piRNAs make up about 5% of known piRNAs and can be further divided into two groups: fetal pre-pachytene piRNAs and postnatal pre-pachytene piRNAs.17,18,34 Fetal pre-pachytene piRNAs constitute the majority of pre-pachytene piRNAs, are expressed in pro-spermatogonia, and associate with the PIWI proteins PIWIL2 (MILI) and PIWIL4 (MIWI2). About half of fetal pre-pachytene piRNAs are derived from transposable elements. MILI predominantly binds to piRNAs that are sense to transposable elements, while MIWI2 preferentially binds to piRNAs that are antisense to transposable elements.18 A small percentage (3%) of fetal pre-pachytene piRNAs are derived from the exons of protein-coding genes.18 Postnatal pre-pachytene piRNAs only associate with MILI, which is the sole PIWI protein present at this stage (Fig. 1). A relatively similar amount of postnatal pre-pachytene piRNAs are derived from transposable elements; however, the profile of the transposable elements from which postnatal pre-pachytene piRNAs originate is different from that of transposable elements from which fetal pre-pachytene piRNAs are derived.18 A greater percentage (20%) of postnatal pre-pachytene piRNAs are also derived from the exons of protein-coding genes.18
Pachytene piRNAs
The second class of piRNAs, termed pachytene piRNAs, consists of the remaining 95% of known piRNAs.16,17,20,34,35 They are present postnatally in pachytene spermatocytes and round spermatids (Fig. 1). Pachytene piRNAs associate with the PIWI proteins MILI and PIWIL1 (MIWI). Unlike pre-pachytene piRNAs, most pachytene piRNAs are derived from non-repetitive, intergenic regions, called pachytene piRNA clusters.20,34 These piRNAs have no known targets and are hypothesized to be the degradation products of larger, non-coding RNAs that are selectively targeted for processing by the piRNA pathway.36 A small percentage (~20%) of pachytene piRNAs are derived from transposable elements.16,20 Pachytene piRNA clusters are on average larger than pre-pachytene piRNA clusters. In addition, pachytene piRNA and pre-pachytene piRNA clusters show little overlap.
PIWI Proteins
PIWI proteins are a subfamily of the Argonaute protein family. PIWI proteins are predominantly expressed in the gonad, whereas Ago proteins are ubiquitously expressed.37 Members of the Argonaute protein family bind various classes of small non-coding RNAs and are involved in RNA-induced silencing.38 There are four main structural domains in Argonaute proteins: N-terminal, MID, PAZ, and PIWI domains.39 Much of the work on the functions of these domains has been done using Ago proteins. The N-terminal domain is required for RNA duplex unwinding and regulates the catalytic activity of Ago proteins.40-42 The MID domain incorporates and stabilizes the 5′ end of the small RNA.43-45 The PAZ domain incorporates the 3′ end of the small RNA and determines the length of small RNAs that are incorporated.39,46-48 Finally, the PIWI domain contains an RNase H-like fold with a conserved aspartate-aspartate-glutamate catalytic motif that allows Argonaute proteins to silence their targets through the endonuclease/slicer activity.39
There are three PIWI proteins present in mice: MILI, MIWI, and MIWI2 (Table 1). They are expressed during different stages of spermatogenesis (Fig. 1). Mili is expressed from the gonocyte stage to the round spermatid stage.49 Miwi2 expression is restricted to the gonocyte stage.50 Miwi is expressed from the late pachytene stage to the round spermatid stage.51 Disruption of either Mili or Miwi2 results in meiotic arrest, and inactivation of Miwi results in spermiogenic arrest.50-52 MIWI, MIWI2, and MILI associate with piRNAs of ~30 nt, ~28 nt, and ~26 nt, respectively.16-18,20,21,31,53 MILI and MIWI exhibit slicer activity for their targets.54,55 Small RNA-induced transcriptional silencing is present in plants, yeast, and even human cells with siRNAs and Ago proteins.56-58 piRNA-mediated transcriptional silencing is also present in Drosophila where piRNAs guide PIWI to its target sequences in the nucleus and recruit HP1a.59 Like PIWI, MIWI2 localizes to the nucleus in a piRNA-dependent manner.18,55,60,61 These studies suggest that MIWI2 may repress the expression of its targets through piRNA-mediated transcriptional silencing.
piRNA Functions
piRNAs bind to the PIWI proteins to form piRNA-induced silencing complexes (piRISC). Both pre-pachytene and pachytene piRNAs are important for transposon silencing. Genetically, pre-pachytene piRNAs are essential for meiosis, whereas pachytene piRNAs are required for spermiogenesis (Fig. 1).17,62
Transposon silencing
The most important and well-conserved role of piRNAs is to silence transposable elements during germ cell development. Transposable elements are mobile genetic elements that move and replicate by inserting themselves into the genome.63 They constitute a significant portion of the genome of many species, including humans.64 Maintaining genome integrity is of paramount importance during germ cell development because genetic information is passed on to future generations. Transposon activity alters the genome and causes DNA damage in a variety of ways.65 Therefore, transposable elements are usually silenced. There are two types of transposable elements: DNA transposons and retrotransposons. DNA transposons are not active in mammalian genomes due to accumulated mutations and truncations. Retrotransposons make up the majority of transposable elements in the genome.66 Retrotransposons are first transcribed into RNA intermediates. The RNA intermediate is reverse transcribed into DNA and the new retrotransposon then inserts itself into a new location in the genome. Retrotransposons can be further divided into long-terminal repeat (LTR) and non-LTR retrotransposons. Long interspersed (LINE) and short interspersed (SINE) elements are non-LTR retrotransposons, and they constitute a large portion of active transposable elements in the genome.65 Intracisternal A-particle (IAP), a LTR retrotransposon, also constitutes a significant portion of active transposable elements in the genome but is expressed at a much lower level compared with LINE and SINE elements.65
Transposable elements in mammals are primarily silenced through DNA methylation, and therefore, become active during genome-wide reprogramming when DNA methyl marks are erased. Fetal pre-pachytene piRNAs are required to silence transposable elements through de novo methylation of transposon promoters.17,50,61MILI associates with pre-pachytene piRNAs that are sense to transposable elements in prospermatogonia, suggesting that the transcripts of active transposable elements are processed by the piRNA pathway, and subsequently, incorporated into MILI. A loss of piRNAs leads to decreased DNA methylation of transposable elements. It is hypothesized that MIWI2 recruits DNA methylation machinery to re-establish DNA methyl marks on the promoters of active transposable elements during de novo methylation. Indeed, inactivation of MIWI2 or DNMT3L, a protein that interacts with DNMT3A and DNMT3B during de novo methylation, results in a similar phenotype.18,67,68Interestingly, postnatal pre-pachytene piRNAs and DNA methylation of LINE1 promoters are not required to maintain silencing of LINE1 elements in spermatogonia.69 Instead, H3K9 dimethylation is sufficient to silence LINE1 at this stage.69
Pachytene piRNAs are also involved in silencing transposable elements. Miwi slicer mutant mice display an increased level of LINE1 transcripts with high complementarity to pachytene piRNAs.54 Upregulation of the pachytene piRNA cluster 1082B results in decreased expression of LINE1 and IAP elements.70 However, LINE1 upregulation in the absence of pachytene piRNAs is minimal compared with LINE1 upregulation in the absence of pre-pachytene piRNA function (> 10-fold), suggesting that transposon silencing may be a minor role for pachytene piRNAs.62,71
Silencing of other genes
While piRNAs are known to silence transposons, piRNA-mediated silencing of other genes is relatively unexplored. Recently, it was shown that piRNAs are required to silence the paternally imprinted gene, Rasgfr1, during genome-wide de novo methylation. piRNAs target a retrotransposon sequence within a non-coding RNA that spans the differentially methylated region (DMR) of Rasgfr1, and a reduction in piRNAs results in a decreased amount of methylation at the Rasgfr1 DMR.72
Meiosis
Many mouse mutants defective in the piRNA pathway exhibit meiotic arrest at the zygotene stage, including Mili, Miwi2, Ddx4, Mov10l1, GasZ, Mael, Tdrd9, etc. (Table 1).49,50,52,71,73-75 Spermatocytes in these mutants exhibit massive DNA damage. It is hypothesized that the de-repression of transposable elements in germ cells from these piRNA-defective mutants causes massive DNA damage, and thus, leads to meiotic arrest. However, a causative relationship between de-repression of transposable elements and meiotic arrest has not been established.
Spermiogenesis
Originally, pachytene piRNAs were believed to target complementary sequences required for the maturation of round spermatids. Postnatal disruption of Mov10l1 results in a lack of pachytene piRNA biogenesis and spermiogenic arrest at the round spermatid stage.62 In addition, recent findings point toward a role for pachytene piRNAs during later stages of spermiogenesis. The loading of pachytene piRNAs onto MIWI is required for the ubiquitin-mediated degradation of MIWI through the APC proteasome pathway in elongating and elongated spermatids.76 MIWI degradation results in the removal of the PIWI/piRNA pathway in late spermiogenesis and is required for the formation of sperm. This finding suggests that pachytene piRNAs are by-products of their precursors and play a passive role in marking MIWI for degradation in late spermatids rather than a role in targeting complementary sequences.
piRNA-Independent Functions of PIWI Proteins
A growing body of studies has shown that, in addition to piRNA-mediated silencing of transposable elements, PIWI proteins have piRNA-independent functions. In Drosophila, PIWI functions in the renewal of germline stem cells in a piRNA-independent manner.77 Mili and Miwi2 mutant mice exhibit a progressive depletion of male germ cells with age, suggesting that they may be required for spermatogonial stem cell renewal.50,78 However, it is currently unknown whether the function of MILI and MIWI2 in spermatogonial stem cell renewal is independent of piRNAs.
MILI and MIWI are implicated in translational control of mRNAs. Both MILI and MIWI associate with polysomes in an RNA-dependent manner, but only MILI regulates global translation levels.35,78 Therefore, MILI may affect germline stem cell renewal and differentiation through its regulation of global translation levels. While MIWI does not appear to regulate global translation levels, it binds to and regulates the expression of a subset of miRNAs and spermiogenic mRNAs that are not targets of pachytene piRNAs.35,36 Many of the MIWI-bound mRNAs are involved in the formation of repressive mRNA ribonucleoproteins (mRNPs) and their abundance is dramatically reduced in round spermatids from Miwi-null mice.36 Therefore, it is hypothesized that MIWI binding may stabilize its target RNAs for their translation in elongating spermatids.
piRNA Biogenesis
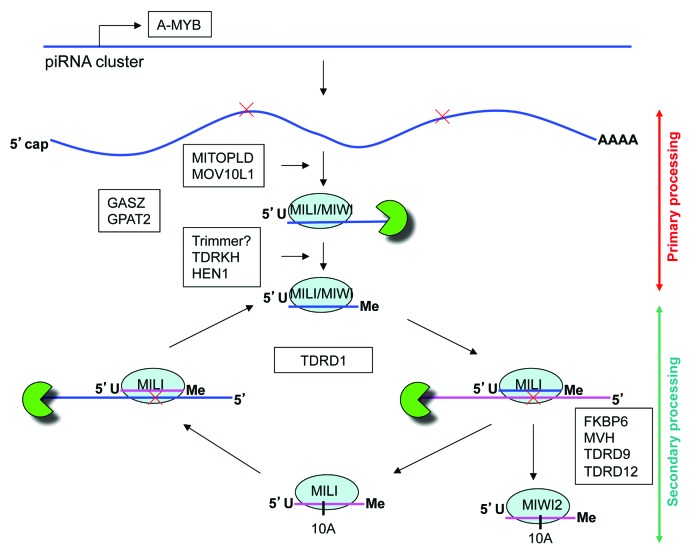
piRNAs are generated through two pathways: primary processing and the ping-pong amplification loop. Primary piRNAs are first generated through primary processing and then certain primary piRNAs enter the ping-pong amplification loop to generate secondary piRNAs (Fig. 2). The ping-pong amplification loop is also called secondary piRNA processing.
Figure 2. The mammalian piRNA biogenesis pathway. Genomic loci encoding piRNAs (piRNA clusters) are transcribed. The piRNA pathway recognizes these RNAs as piRNA precursors and processes them into mature piRNA through primary processing. The piRNA precursors are cleaved by an endonuclease into piRNA intermediates. The piRNA intermediates are incorporated into MILI or MIWI protein complex and are then trimmed and modified at their 3′ ends into mature piRNAs. Mature piRNA are amplified through ping-pong amplification loop. Mature piRNA guide MILI protein complex to complementary RNAs. MILI cleaves complementary RNAs to generate secondary piRNA intermediates that are loaded onto another MILI protein complex or MIWI2 protein complex. Secondary piRNA intermediates are trimmed and modified at their 3′ ends to generate secondary piRNAs. Representative protein factors involved in each step are listed.
Primary processing
During primary processing, the genomic loci that encode piRNAs are initially transcribed as long RNAs, termed piRNA precursors, that can be tens to hundreds of kilobases long.13 Transcription of pachytene piRNA precursors is initiated by the A-MYB transcription factor.79 A-MYB is involved in a positive feedback loop with itself and also regulates the transcription of many other genes involved in the piRNA pathway such as Tdrd1, Miwi, and MitoPLD.79 The piRNA precursors are 5′ capped and 3′ polyadenylated.79 One major difference between piRNA biogenesis and microRNA/siRNA biogenesis is that piRNA biogenesis is DICER-independent.53 Furthermore, computational analysis of regions in the piRNA precursors immediately surrounding pachytene piRNAs shows a lack of stem loops.16
The piRNA precursors are first processed into shorter RNAs, termed piRNA intermediates, by an endonuclease that recent studies suggests is MitoPLD/Zucchini (Fig. 2).80,81The piRNA intermediates associate with MILI and MIWI.36,82The 5′ ends of the piRNA intermediates correspond to the 5′ ends of mature piRNAs, and thus, contain a preference for uridine.36,82However, it is unclear whether piRNA precursors are preferentially cleaved into RNAs with 5′ uridines, or whether MILI and MIWI preferentially stabilize RNAs with 5′ uridines. Ago proteins recognize the identity of the 5′ terminal nucleotide through the MID domain and tend to incorporate miRNAs with a 5′ uridine.43-45,83 This 5′U bias is conserved in PIWI proteins. The exact lengths of piRNA intermediates are likely variable. MILI-bound piRNA intermediates of 32–40 nt have recently been reported.82
The piRNA intermediates are trimmed into mature-length piRNAs presumably through Mg2+-dependent 3′-5′ exonucleolytic activity, termed Trimmer activity. Trimmer activity is present in the cellular lysate from BmN4 silkworm ovarian cells.84TDRKH promotes the 3′ trimming of piRNA intermediates.82After trimming, the mature piRNA is 2’-O-methylated at its 3′ end by HEN1 and incorporated into the PAZ domain of the PIWI protein.32,33,85,862’-O-methylation of piRNAs is closely coupled with trimming of piRNA intermediates.84 In further support of this, MILI-bound piRNA intermediates in Tdrkh-null mice are 2’-O-methylated.82
Secondary processing (Ping-pong amplification loop)
While primary processing generates the initial pool of piRNAs, the ping-pong amplification loop generates secondary piRNAs and amplifies the pool of both primary and secondary piRNAs. During the ping-pong amplification, primary piRNAs guide the PIWI proteins they associate with to complementary RNA targets.87,88PIWI proteins cleave their RNA targets between the 10th and 11th nucleotides relative to the primary piRNA, generating the 5′ end of the secondary piRNA.87,88Thus, a signature of secondary piRNAs is a preference for an adenosine at the 10th nucleotide.18,87,88Secondary piRNA intermediates are loaded onto other PIWI proteins and their 3′ ends are also processed by Trimmer activity. Secondary piRNAs in turn guide PIWI proteins to RNA targets to slice them and produce primary piRNAs at the same time.
The ping-pong amplification loop is only observed during fetal pre-pachytene piRNA biogenesis in mice, where primary piRNAs are loaded onto MILI and secondary piRNAs are loaded onto both MILI and MIWI2.18,55While MIWI2 is involved in the linear generation of secondary piRNAs, MILI forms an intra-amplification loop with itself to amplify piRNAs.55 Because MILI predominantly associates with fetal pre-pachytene piRNAs that are sense to transposable elements, it is believed that transcripts of transposable elements are fed into the piRNA pathway to generate the initial pool of primary piRNAs. Secondary piRNAs are antisense to transposable elements and guide MIWI2 to silence transposable elements in the nucleus. This mechanism allows germ cells to mount an adaptive defense against endogenous transposable elements through the piRNA pathway. Interestingly, secondary piRNAs are required to silence LINE1 but not IAP elements.55 This could be due to the significantly lower expression of IAP elements in the genome so that primary processing alone is sufficient to silence active IAP elements. In support of this notion, mouse mutants with defects in primary piRNA biogenesis such as Mili and Mov10l1 exhibit de-repression of both LINE1 and IAP, whereas mouse mutants with defects in secondary piRNA production such as Miwi2 and Tdrd9 appear to display de-repression of only LINE1.50,61,71,75
PIWI-Associated Proteins
The piRNA pathway consists of many proteins in addition to PIWI proteins and piRNAs (Table 1). Many of these proteins are associated with PIWI proteins (Fig. 3). Studies of these additional proteins have provided significant mechanistic insights into the piRNA pathway.
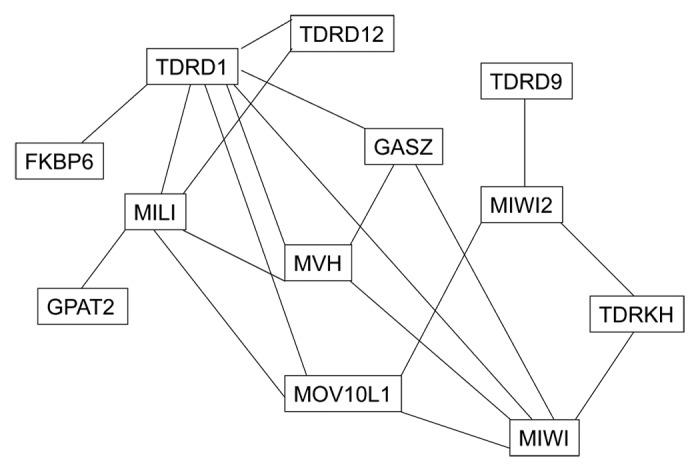
Figure 3. Interaction map of protein factors in the mammalian piRNA pathway. Solid lines represent associations that have been confirmed through co-immunoprecipitation.
Tudor domain-containing proteins (TDRDs)
TDRDs are members of the Tudor protein family, characterized by the Tudor domain.89 TDRDs contain one or more extended Tudor domains. Tudor domains bind to symmetrically dimethylated arginine sites (sDMA), which are found in many PIWI and PIWI-associated proteins.27,60,90,91Many TDRDs associate with PIWI proteins, including TDRD1, TDRKH, TDRD6, TDRD7, TDRD8, TDRD9, and TDRD12 (Fig. 3).75,82,91-94With the exception of TDRD8, all the TDRDs that associate with PIWI proteins are required for spermatogenesis (Table 1).95 TDRD1, TDRKH, TDRD9, and TDRD12 play critical roles in the piRNA pathway.60,75,82,92TDRDs are believed to function as scaffolds for the interaction of various proteins in the piRNA pathway.
TDRKH is a mitochondrial protein.82 It associates with MIWI and MIWI2 and is required for the processing of most MILI-bound piRNA intermediates into mature piRNAs.82 The presence of the remaining mature piRNAs in Tdrkh-null mice suggests that there may be more than one mechanism through which piRNA intermediates are processed. TDRKH may function as a scaffold for mouse PIWI proteins and the Trimmer during the trimming process. TDRKH could also function to recruit Trimmer to the nuage. TDRD1 associates with MILI and disruption of Tdrd1 alters the MILI-bound piRNA profile.60,91 TDRD12 is a putative RNA helicase and associates with MILI and TDRD1.92 TDRD12 is required for the generation of MIWI2-bound secondary piRNAs.92 TDRD9 associates with MIWI2 and localizes to the nucleus.75 TDRD9 may act as a scaffold for MIWI2 and the DNA methylation machinery during de novo methylation of active transposable elements.
Other factors
In addition to TDRDs, a number of other protein factors function in the piRNA pathway (Table 1 and Fig. 3). GASZ and GPAT2 associate with MILI and are required for primary piRNA biogenesis.73,96 GPAT2 is a mitochondrial outer membrane protein. MitoPLD is another mitochondrial membrane protein and exhibits endonuclease activity for single-stranded RNAs in vitro.80,81,97 The endonuclease activity of Zuc, the Drosophila homolog of MitoPLD, is required for piRNA biogenesis.80,81 MitoPLD is a candidate for the endonuclease that cleaves piRNA precursors into piRNA intermediates. However, in the MitoPLD mouse mutants, piRNAs are still present, even though their production is not normal, suggesting that MitoPLD may not be the only endonuclease responsible for cleavage in mice.97 MOV10L1 is a putative RNA helicase required for the processing of piRNA precursors into mature piRNAs.62 MOV10L1 associates with all three mouse PIWI proteins.71 MOV10L1 is a master regulator of the piRNA pathway and is required for the biogenesis of both prepachytene and pachytene piRNAs.62,71 DDX4 (MVH) and FKBP6 are components of the TDRD1 protein complex and are required for the loading of MIWI2-bound secondary piRNAs.98,99MVH is an RNA helicase, and like TDRD12, may be required for ribonucleotide protein (RNP) remodeling during the loading of secondary piRNA intermediates onto MIWI2. FKBP6 associates with HSP90, which is required for the loading of plant and fly miRNAs into the RNA-induced silencing complex (RISC).99 FKBP6 may recruit HSP90 in the loading of secondary piRNA intermediates onto MIWI2. MAEL co-localizes with MIWI2 and TDRD9 in prospermatogonia and associates with MILI and MIWI in spermatocytes.74,100MAEL promotes the nuclear localization of MIWI2 and the onset of piRNA biogenesis in gonocytes.100 Finally, GTSF1 associates with PIWI in the nucleus and is required for PIWI-mediated transcriptional silencing of transposable elements, but does not affect piRNA biogenesis in Drosophila.101,102GTSF1 is also essential for transposon silencing in mice.103 Identification of these additional protein factors underscores the complexity of the piRNA pathway.
Compartmentalization of the piRNA Pathway
Proteins in the piRNA pathway are regulated spatially within germ cells. During spermatogenesis, various electron-dense subcellular bodies, termed nuages, form in the cytoplasm of germ cells. Nuages are believed to be RNA processing centers and are prominent in the piRNA pathway.
The intermitochondrial cement (IMC) is made up of granules, termed pi-bodies.100 The IMC is present in prospermatogonia, spermatogonia, and mid-late pachytene spermatocytes. The IMC is located between mitochondria, and its association with mitochondria is required for its formation. Many components of the piRNA pathway such as MILI, MOV10L1, TDRD1, TDRKH, GASZ, and MVH localize to the IMC.71,73,82,100 MIWI and MAEL also localize to the IMC along with the previously listed components in mid-late pachytene spermatocytes.104 Some of these components such as TDRD1 are required for formation of the IMC.105
The piP-bodies are nuages that are present in prospermatogonia adjacent to the IMC and have a sponge-like appearance.100 TDRKH, MIWI2, TDRD9, MVH, and MAEL are localized to piP-bodies.82,100 MAEL is required for the proper structure of piP-bodies and localization of piRNA pathway components to piP-bodies.100 In addition to components of the piRNA pathway, piP-bodies also contain components of P-bodies that are involved in the storage and degradation of mRNAs.100 The localization of MILI and MIWI2 to the IMC and piP-bodies, respectively, suggests that the IMC is a processing center for primary piRNAs and that piP-bodies are processing centers for secondary piRNAs. It is possible that crosstalk exists between these two nuages.106
Chromatoid body (CB) is the most well-studied nuage in the germline, but still remains mysterious. It was discovered more than half a century ago.107 It first forms in late pachytene spermatocytes where it is associated with the nuclear envelope but not mitochondria.104 It disappears in the diplotene stage of meiosis I but reappears in spermatocytes during meiosis II as large electron-dense bodies. The electron-dense bodies aggregate into a single nuage in round spermatids. The CB moves toward the base of the flagellum and is discarded along with the cytoplasm in residual bodies. The piRNA pathway is the most prominent functional pathway in chromatoid body. Many factors in the piRNA pathway such as MILI, MIWI, MVH, TDRD1, and MAEL are localized to the CB.104 Factors such as MIWI and TDRD1 are required for the formation and architecture of the CB.51,108 TDRD6 and TDRD7 play important roles in the assembly of chromatoid bodies.94,109 In addition to the piRNA pathway, the chromatoid body also contains components of P-bodies and factors involved in the microRNA pathway.104,110 Therefore, the chromatoid body is a major RNA processing center in round spermatids.
piRNAs Outside the Germline
piRNA-like small RNAs (termed pilRNAs) have been found in somatic tissues outside testes including brain, hippocampus, kidney, liver, lung, and spleen.111,112 The pilRNAs map to intergenic regions and the 3′ UTRs of sense strands.111,112 pilRNAs in the mouse hippocampus bind to MIWI.111 Human PIWI proteins HILI and HIWI are found to be expressed in many cancer cell types including pancreatic, breast, and colon cancers.113 Nevertheless, the biological significance of piRNA-like small RNA species and PIWI proteins in non-gonadal tissues remains unknown. Given the large number of mouse mutants with defects in the piRNA pathway (Table 1), it would be very informative to examine these mouse mutants for any somatic phenotypes in future studies.
Future Challenges
In this review, we have detailed the current knowledge on the function and biogenesis of piRNAs during mouse spermatogenesis. piRNAs are the most abundant class of small RNAs in the germline, and much progress has been made on their biogenesis and functions within the past few years. The piRNA field is advancing rapidly, but many outstanding questions remain to be answered.
A conserved role of piRNAs is to silence transposable elements. However, many piRNAs do not target transposable elements, including piRNAs derived from intergenic regions and coding mRNAs.114 Future studies should concentrate on elucidating the molecular functions of these non-repeat-derived sense piRNAs. Can these non-repeat-derived piRNAs target partially complementary RNAs? Do piRNAs have a seed sequence like microRNAs? Initial analysis of sequences of piRNAs associated with MILI and MIWI do not support the existence of a seed sequence in piRNAs, but more functional studies will be needed to address this question.36
The molecular causes of meiotic arrest in piRNA-defective mutants remain to be elucidated. It is believed that de-repression of transposable elements in germ cells of piRNA-defective mutants causes massive DNA damage, which in turn, results in meiotic arrest. However, it has not been shown that de-repression of transposable elements actually leads to DNA damage in the germ cells. This is complicated by the fact that early meiotic germ cells naturally generate DNA double strand breaks for meiotic recombination, which triggers the DNA damage response. A related question is whether de-repression of transposable elements necessarily results in integration of transposable elements into the genome in the germ cells. Most mouse piRNA mutants exhibit meiotic arrest at the zygotene stage, suggesting that common defects among these mutants might cause failures in chromosomal synapsis and meiotic recombination. To date, the exact cause of meiotic arrest in mouse piRNA mutants remains an open question.
IMC and piP-bodies are located adjacent to mitochondria. Protein factors required for piRNA biogenesis such as TDRKH, GPAT2, and MitoPLD are localized to the outer mitochondrial membrane, suggesting a possible link between mitochondria and piRNA biogenesis.96,97,110 Do these protein factors have independent functions in mitochondrial biology and the piRNA pathway? Or are mitochondria directly involved in piRNA biogenesis?
Biogenesis of piRNAs is independent of DICER, but the mechanisms underlying piRNA biogenesis, in particular primary biogenesis, remain mysterious. Biogenesis of pachytene piRNAs only involves primary processing and thus constitutes an ideal system for dissecting the primary processing pathway. A few outstanding questions remain. How are piRNA precursors chosen to be fed into the piRNA pathway? Why are piRNAs produced from some mRNA transcripts but not others? MitoPLD is a strong candidate for the enzyme that initially cleaves piRNA precursor transcripts. Are there other endonucleases involved in the initial cleavage? What is the identity of the 3′-5′ trimmer for piRNA intermediates? While many of the protein factors involved in piRNA biogenesis have been defined, the mechanisms through which these factors regulate piRNA biogenesis are largely unknown. Development of biochemical assays will aid in defining their enzymatic activities and thus in elucidating their roles in mammalian piRNA biogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Anastasios Vourekas for critical reading of the manuscript. This work was supported in part by NIH grant R01HD069592.
Glossary
Abbreviations:
- piRNA
piwi-interacting RNA
- miRNA
microRNA
- endo-siRNA
endogenous small interfering RNA
- siRNA
small-interfering RNA
- RISC
RNA-induced silencing complex
- piRISC
piRNA-induced silencing complex
- LTR
long terminal repeat
- LINE
long interspersed nucleotide element
- SINE
short interspersed nucleotide element
- IAP
intracisternal A-particle
- DMR
differentially methylated region
- mRNP
mRNA ribonucleoprotein
- TDRD
tudor-domain containing
- RNP
ribonucleoprotein
- IMC
intermitochondrial cement
- CB
chromatoid body
- pilRNAs
piRNA-like RNAs
References
- 1.Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–68. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–88. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olovnikov I, Aravin AA, Fejes Toth K. Small RNA in the nucleus: the RNA-chromatin ping-pong. Curr Opin Genet Dev. 2012;22:164–71. doi: 10.1016/j.gde.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook MS, Blelloch R. Small RNAs in germline development. Curr Top Dev Biol. 2013;102:159–205. doi: 10.1016/B978-0-12-416024-8.00006-4. [DOI] [PubMed] [Google Scholar]
- 6.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuma S, Nakano T. piRNA and spermatogenesis in mice. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110338. doi: 10.1098/rstb.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer CB, Ooi SK, Bestor TH, Bourc’his D. Epigenetic decisions in mammalian germ cells. Science. 2007;316:398–9. doi: 10.1126/science.1137544. [DOI] [PubMed] [Google Scholar]
- 9.Trasler JM. Epigenetics in spermatogenesis. Mol Cell Endocrinol. 2009;306:33–6. doi: 10.1016/j.mce.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–9. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- 11.Pek JW, Patil VS, Kai T. piRNA pathway and the potential processing site, the nuage, in the Drosophila germline. Dev Growth Differ. 2012;54:66–77. doi: 10.1111/j.1440-169X.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- 12.Saito K. The epigenetic regulation of transposable elements by PIWI-interacting RNAs in Drosophila. Genes Genet Syst. 2013;88:9–17. doi: 10.1266/ggs.88.9. [DOI] [PubMed] [Google Scholar]
- 13.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–58. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 14.Aravin AA, Naumova NM, Tulin AV, Vagin VV, Rozovsky YM, Gvozdev VA. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr Biol. 2001;11:1017–27. doi: 10.1016/S0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 15.Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–50. doi: 10.1016/S1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 16.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 17.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–7. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 18.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–99. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, Sasaki H, Minami N, Imai H. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006;20:1732–43. doi: 10.1101/gad.1425706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 21.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–14. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaoka S, Hayashi N, Katsuma S, Kishino H, Kohara Y, Mita K, Shimada T. Bombyx small RNAs: genomic defense system against transposons in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2008;38:1058–65. doi: 10.1016/j.ibmb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Armisen J, Gilchrist MJ, Wilczynska A, Standart N, Miska EA. Abundant and dynamically expressed miRNAs, piRNAs, and other small RNAs in the vertebrate Xenopus tropicalis. Genome Res. 2009;19:1766–75. doi: 10.1101/gr.093054.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilczynska A, Minshall N, Armisen J, Miska EA, Standart N. Two Piwi proteins, Xiwi and Xili, are expressed in the Xenopus female germline. RNA. 2009;15:337–45. doi: 10.1261/rna.1422509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol. 2009;11:652–8. doi: 10.1038/ncb1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houwing S, Berezikov E, Ketting RF. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 2008;27:2702–11. doi: 10.1038/emboj.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Chen PY, Manninga H, Slanchev K, Chien M, Russo JJ, Ju J, Sheridan R, John B, Marks DS, Gaidatzis D, et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 2005;19:1288–93. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–7. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 32.Kirino Y, Mourelatos Z. 2′-O-methyl modification in mouse piRNAs and its methylase. Nucleic Acids Symp Ser (Oxf) 2007:417–8. doi: 10.1093/nass/nrm209. [DOI] [PubMed] [Google Scholar]
- 33.Saito K, Sakaguchi Y, Suzuki T, Suzuki T, Siomi H, Siomi MC. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3′ ends. Genes Dev. 2007;21:1603–8. doi: 10.1101/gad.1563607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan H, Lin X, Zhang Z, Zhang W, Liao S, Wang L, Han C. piRNA profiling during specific stages of mouse spermatogenesis. RNA. 2011;17:1191–203. doi: 10.1261/rna.2648411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A. 2006;103:13415–20. doi: 10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vourekas A, Zheng Q, Alexiou P, Maragkakis M, Kirino Y, Gregory BD, Mourelatos Z. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat Struct Mol Biol. 2012;19:773–81. doi: 10.1038/nsmb.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Höck J, Meister G. The Argonaute protein family. Genome Biol. 2008;9:210. doi: 10.1186/gb-2008-9-2-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 39.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–7. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 40.Kwak PB, Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat Struct Mol Biol. 2012;19:145–51. doi: 10.1038/nsmb.2232. [DOI] [PubMed] [Google Scholar]
- 41.Schürmann N, Trabuco LG, Bender C, Russell RB, Grimm D. Molecular dissection of human Argonaute proteins by DNA shuffling. Nat Struct Mol Biol. 2013;20:818–26. doi: 10.1038/nsmb.2607. [DOI] [PubMed] [Google Scholar]
- 42.Hauptmann J, Dueck A, Harlander S, Pfaff J, Merkl R, Meister G. Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nat Struct Mol Biol. 2013;20:814–7. doi: 10.1038/nsmb.2577. [DOI] [PubMed] [Google Scholar]
- 43.Parker JS, Roe SM, Barford D. Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature. 2005;434:663–6. doi: 10.1038/nature03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:666–70. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank F, Sonenberg N, Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–22. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- 46.Lingel A, Simon B, Izaurralde E, Sattler M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat Struct Mol Biol. 2004;11:576–7. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- 47.Ma JB, Ye K, Patel DJ. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature. 2004;429:318–22. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan KS, Yan S, Farooq A, Han A, Zeng L, Zhou MM. Structure and conserved RNA binding of the PAZ domain. Nature. 2003;426:468–74. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- 49.Kuramochi-Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y, Nakano T. Two mouse piwi-related genes: miwi and mili. Mech Dev. 2001;108:121–33. doi: 10.1016/S0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 50.Carmell MA, Girard A, van de Kant HJ, Bourc’his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12:503–14. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–30. doi: 10.1016/S1534-5807(02)00165-X. [DOI] [PubMed] [Google Scholar]
- 52.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–49. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 53.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–4. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 54.Reuter M, Berninger P, Chuma S, Shah H, Hosokawa M, Funaya C, Antony C, Sachidanandam R, Pillai RS. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature. 2011;480:264–7. doi: 10.1038/nature10672. [DOI] [PubMed] [Google Scholar]
- 55.De Fazio S, Bartonicek N, Di Giacomo M, Abreu-Goodger C, Sankar A, Funaya C, Antony C, Moreira PN, Enright AJ, O’Carroll D. The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature. 2011;480:259–63. doi: 10.1038/nature10547. [DOI] [PubMed] [Google Scholar]
- 56.Mette MF, Aufsatz W, van der Winden J, Matzke MA, Matzke AJ. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 2000;19:5194–201. doi: 10.1093/emboj/19.19.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schramke V, Allshire R. Hairpin RNAs and retrotransposon LTRs effect RNAi and chromatin-based gene silencing. Science. 2003;301:1069–74. doi: 10.1126/science.1086870. [DOI] [PubMed] [Google Scholar]
- 58.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–92. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 59.Brower-Toland B, Findley SD, Jiang L, Liu L, Yin H, Dus M, Zhou P, Elgin SC, Lin H. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007;21:2300–11. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol. 2009;16:639–46. doi: 10.1038/nsmb.1615. [DOI] [PubMed] [Google Scholar]
- 61.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–17. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng K, Wang PJ. Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet. 2012;8:e1003038. doi: 10.1371/journal.pgen.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A. 1950;36:344–55. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 65.Kazazian HH., Jr. Mobile elements: drivers of genome evolution. Science. 2004;303:1626–32. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 66.Jurka J, Kapitonov VV, Kohany O, Jurka MV. Repetitive sequences in complex genomes: structure and evolution. Annu Rev Genomics Hum Genet. 2007;8:241–59. doi: 10.1146/annurev.genom.8.080706.092416. [DOI] [PubMed] [Google Scholar]
- 67.Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–9. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 68.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129:1983–93. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 69.Di Giacomo M, Comazzetto S, Saini H, De Fazio S, Carrieri C, Morgan M, Vasiliauskaite L, Benes V, Enright AJ, O’Carroll D. Multiple epigenetic mechanisms and the piRNA pathway enforce LINE1 silencing during adult spermatogenesis. Mol Cell. 2013;50:601–8. doi: 10.1016/j.molcel.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 70.Nordstrand LM, Furu K, Paulsen J, Rognes T, Klungland A. Alkbh1 and Tzfp repress a non-repeat piRNA cluster in pachytene spermatocytes. Nucleic Acids Res. 2012;40:10950–63. doi: 10.1093/nar/gks839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A. 2010;107:11841–6. doi: 10.1073/pnas.1003953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe T, Tomizawa S, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, Iida N, Hoki Y, Murphy PJ, Toyoda A, et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science. 2011;332:848–52. doi: 10.1126/science.1203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, et al. GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet. 2009;5:e1000635. doi: 10.1371/journal.pgen.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell. 2008;15:285–97. doi: 10.1016/j.devcel.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, et al. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell. 2009;17:775–87. doi: 10.1016/j.devcel.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 76.Zhao S, Gou LT, Zhang M, Zu LD, Hua MM, Hua Y, Shi HJ, Li Y, Li J, Li D, et al. piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis. Dev Cell. 2013;24:13–25. doi: 10.1016/j.devcel.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 77.Klenov MS, Sokolova OA, Yakushev EY, Stolyarenko AD, Mikhaleva EA, Lavrov SA, Gvozdev VA. Separation of stem cell maintenance and transposon silencing functions of Piwi protein. Proc Natl Acad Sci U S A. 2011;108:18760–5. doi: 10.1073/pnas.1106676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, Nakano T, Lin H. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem. 2009;284:6507–19. doi: 10.1074/jbc.M809104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–83. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–7. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- 82.Saxe JP, Chen M, Zhao H, Lin H. Tdrkh is essential for spermatogenesis and participates in primary piRNA biogenesis in the germline. EMBO J. 2013;32:1869–85. doi: 10.1038/emboj.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seitz H, Tushir JS, Zamore PD. A 5′-uridine amplifies miRNA/miRNA* asymmetry in Drosophila by promoting RNA-induced silencing complex formation. Silence. 2011;2:4. doi: 10.1186/1758-907X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawaoka S, Izumi N, Katsuma S, Tomari Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell. 2011;43:1015–22. doi: 10.1016/j.molcel.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 85.Simon B, Kirkpatrick JP, Eckhardt S, Reuter M, Rocha EA, Andrade-Navarro MA, Sehr P, Pillai RS, Carlomagno T. Recognition of 2′-O-methylated 3′-end of piRNA by the PAZ domain of a Piwi protein. Structure. 2011;19:172–80. doi: 10.1016/j.str.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Tian Y, Simanshu DK, Ma JB, Patel DJ. Structural basis for piRNA 2′-O-methylated 3′-end recognition by Piwi PAZ (Piwi/Argonaute/Zwille) domains. Proc Natl Acad Sci U S A. 2011;108:903–10. doi: 10.1073/pnas.1017762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–90. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 88.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 89.Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP. The tudor domain 'royal family': Tudor, plant agenet, chromo, PWWP and MBT domains. Trends Biochem Sci. 2003;28:69–74. doi: 10.1016/S0968-0004(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 90.Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–62. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Saxe JP, Tanaka T, Chuma S, Lin H. Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr Biol. 2009;19:640–4. doi: 10.1016/j.cub.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pandey RR, Tokuzawa Y, Yang Z, Hayashi E, Ichisaka T, Kajita S, Asano Y, Kunieda T, Sachidanandam R, Chuma S, et al. Tudor domain containing 12 (TDRD12) is essential for secondary PIWI interacting RNA biogenesis in mice. Proc Natl Acad Sci U S A. 2013;110:16492–7. doi: 10.1073/pnas.1316316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, et al. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A. 2009;106:20336–41. doi: 10.1073/pnas.0911640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasileva A, Tiedau D, Firooznia A, Müller-Reichert T, Jessberger R. Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr Biol. 2009;19:630–9. doi: 10.1016/j.cub.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bao J, Wang L, Lei J, Hu Y, Liu Y, Shen H, Yan W, Xu C. STK31(TDRD8) is dynamically regulated throughout mouse spermatogenesis and interacts with MIWI protein. Histochem Cell Biol. 2012;137:377–89. doi: 10.1007/s00418-011-0897-9. [DOI] [PubMed] [Google Scholar]
- 96.Shiromoto Y, Kuramochi-Miyagawa S, Daiba A, Chuma S, Katanaya A, Katsumata A, Nishimura K, Ohtaka M, Nakanishi M, Nakamura T, et al. GPAT2, a mitochondrial outer membrane protein, in piRNA biogenesis in germline stem cells. RNA. 2013;19:803–10. doi: 10.1261/rna.038521.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watanabe T, Chuma S, Yamamoto Y, Kuramochi-Miyagawa S, Totoki Y, Toyoda A, Hoki Y, Fujiyama A, Shibata T, Sado T, et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell. 2011;20:364–75. doi: 10.1016/j.devcel.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Takamatsu K, Chuma S, Kojima-Kita K, Shiromoto Y, Asada N, Toyoda A, Fujiyama A, et al. MVH in piRNA processing and gene silencing of retrotransposons. Genes Dev. 2010;24:887–92. doi: 10.1101/gad.1902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiol J, Cora E, Koglgruber R, Chuma S, Subramanian S, Hosokawa M, Reuter M, Yang Z, Berninger P, Palencia A, et al. A role for Fkbp6 and the chaperone machinery in piRNA amplification and transposon silencing. Mol Cell. 2012;47:970–9. doi: 10.1016/j.molcel.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 100.Aravin AA, van der Heijden GW, Castañeda J, Vagin VV, Hannon GJ, Bortvin A. Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet. 2009;5:e1000764. doi: 10.1371/journal.pgen.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dönertas D, Sienski G, Brennecke J. Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. Genes Dev. 2013;27:1693–705. doi: 10.1101/gad.221150.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohtani H, Iwasaki YW, Shibuya A, Siomi H, Siomi MC, Saito K. DmGTSF1 is necessary for Piwi-piRISC-mediated transcriptional transposon silencing in the Drosophila ovary. Genes Dev. 2013;27:1656–61. doi: 10.1101/gad.221515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshimura T, Toyoda S, Kuramochi-Miyagawa S, Miyazaki T, Miyazaki S, Tashiro F, Yamato E, Nakano T, Miyazaki J. Gtsf1/Cue110, a gene encoding a protein with two copies of a CHHC Zn-finger motif, is involved in spermatogenesis and retrotransposon suppression in murine testes. Dev Biol. 2009;335:216–27. doi: 10.1016/j.ydbio.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Meikar O, Da Ros M, Korhonen H, Kotaja N. Chromatoid body and small RNAs in male germ cells. Reproduction. 2011;142:195–209. doi: 10.1530/REP-11-0057. [DOI] [PubMed] [Google Scholar]
- 105.Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci U S A. 2006;103:15894–9. doi: 10.1073/pnas.0601878103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chuma S, Pillai RS. Retrotransposon silencing by piRNAs: ping-pong players mark their sub-cellular boundaries. PLoS Genet. 2009;5:e1000770. doi: 10.1371/journal.pgen.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eddy EM. Cytochemical observations on the chromatoid body of the male germ cells. Biol Reprod. 1970;2:114–28. doi: 10.1095/biolreprod2.1.114. [DOI] [PubMed] [Google Scholar]
- 108.Kojima K, Kuramochi-Miyagawa S, Chuma S, Tanaka T, Nakatsuji N, Kimura T, Nakano T. Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells. Genes Cells. 2009;14:1155–65. doi: 10.1111/j.1365-2443.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 109.Tanaka T, Hosokawa M, Vagin VV, Reuter M, Hayashi E, Mochizuki AL, Kitamura K, Yamanaka H, Kondoh G, Okawa K, et al. Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc Natl Acad Sci U S A. 2011;108:10579–84. doi: 10.1073/pnas.1015447108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell. 2011;20:376–87. doi: 10.1016/j.devcel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, Kosik KS. Identification of piRNAs in the central nervous system. RNA. 2011;17:1090–9. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan Z, Hu HY, Jiang X, Maierhofer V, Neb E, He L, Hu Y, Hu H, Li N, Chen W, et al. Widespread expression of piRNA-like molecules in somatic tissues. Nucleic Acids Res. 2011;39:6596–607. doi: 10.1093/nar/gkr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki R, Honda S, Kirino Y. PIWI expression and function in cancer. Front Genet. 2012;3:204. doi: 10.3389/fgene.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robine N, Lau NC, Balla S, Jin Z, Okamura K, Kuramochi-Miyagawa S, Blower MD, Lai EC. A broadly conserved pathway generates 3’UTR-directed primary piRNAs. Curr Biol. 2009;19:2066–76. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A. 2010;107:11847–52. doi: 10.1073/pnas.1007158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A. 1994;91:12258–62. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Handler D, Olivieri D, Novatchkova M, Gruber FS, Meixner K, Mechtler K, Stark A, Sachidanandam R, Brennecke J. A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 2011;30:3977–93. doi: 10.1038/emboj.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]