Abstract
Small GTPase Rab35 is a key regulator of neurite outgrowth, and its activation dramatically enhances nerve growth factor (NGF)-induced neurite outgrowth. We recently reported finding that Rab35 and its effector molecules recruit EHD1, a facilitator of vesicle formation, to Arf6-positive perinuclear recycling endosomes (hereafter simply referred to as recycling endosomes) in response to NGF stimulation. Although Rab35 is likely to promote the formation of transport vesicles from recycling endosomes that contributes to neurite outgrowth, the destination of the vesicles during neurite outgrowth remains unknown. Here we report finding that Rab35 is translocated from recycling endosomes to neurite tips in a late phase of NGF stimulation. We found that Rab35 immunofluorescence signals accumulated at recycling endosomes during the first 6 h, i.e., the early phase of NGF stimulation and then translocated to neurite tips during the late phase of NGF stimulation (i.e., > 6 h to < 36 h after NGF stimulation). These findings suggest that Rab35 regulates membrane trafficking from recycling endosomes to neurite tips during neurite outgrowth.
Keywords: Rab35, Arf6, recycling endosome, neurite outgrowth
Rab family small GTPases are conserved intracellular molecular switches that regulate membrane trafficking, in which proteins and lipids are transported from one organelle to another organelle by vesicles.1-4 It is widely known that Rabs impact a variety of cellular processes, including cell division, signal transduction, and neuronal functions, by regulating membrane trafficking.5,6 In mammals, the Rab family consists of approximately 60 members, and each member is thought to localize at certain organelles in an isoform-specific manner.7 Rabs cycle between a GTP-bound active form and a GDP-bound inactive form, and active Rabs promote membrane trafficking by recruiting isoform-specific effector molecules to organelles,8,9 where the active Rabs are present. Because of the large number of Rab isoforms, however, the precise properties of each Rab, e.g., its effector molecules and membrane trafficking route (point of departure and destination) that it governs, are not fully understood.
We and others have previously demonstrated finding that one of the Rab family proteins, Rab35, affects neurite outgrowth of neuronal cells; activation of Rab35 dramatically promotes nerve growth factor (NGF)-induced neurite outgrowth of PC12 cells, while inactivation of Rab35 inhibits it.9-11 Rab35 accumulates at Arf6-positive recycling endosomes in the early phase of NGF stimulation (i.e., 1–6 h after NGF stimulation).12,13 After arriving at the recycling endosomes Rab35 simultaneously recruits two distinct effector molecules, centaurin-β2/ACAP2 and MICAL-L1, and they induce association of EHD1, a dynamin-like protein that facilitates vesicle formation, with the same compartment.14-16 These previous findings strongly suggested that Rab35 mediates membrane trafficking that starts at Arf6-positive recycling endosomes and promotes neurite outgrowth, but the destination of the Rab35-dependent membrane trafficking during neurite outgrowth remained unknown. In this study we analyzed the intracellular localization of Rab35 during NGF-induced neurite outgrowth more precisely in an attempt to identify the route of Rab35-dependent membrane trafficking.
First, we performed immunofluorescence analyses of Rab35 during neurite outgrowth. Consistent with the findings in our previous report, when PC12 cells were stimulated with NGF, Rab35 accumulated in the perinuclear compartment (i.e., at Arf6-positive perinuclear recycling endosomes) during the first 6 h of stimulation (Fig. 1B, arrows in bottom panels). Interestingly, however, after NGF stimulation for 36 h Rab35 immunofluorescence signals were also observed in neurite tips (Fig. 1B, arrowheads in the upper far right panel; almost all of the cells [92.0 ± 2.0%] exhibited the neurite tip localization of Rab35). By contrast, the immunofluorescence signals of Arf6, a perinuclear recycling endosomal protein, remained in the perinuclear area even after NGF stimulation for 36 h, and, in contrast to Rab35, no Arf6-positive signals were observed in the neurite tips, indicating that recycling endosomes themselves are not transported to neurites (Fig. 2; only a few cells [2.0 ± 3.5%] exhibited the weak neurite tip localization of Arf6). Therefore, the change in the distribution of the Rab35 immunofluorescence signals is likely to be attributable to translocation of the Rab35-positive vesicles that had been generated from recycling endosomes.
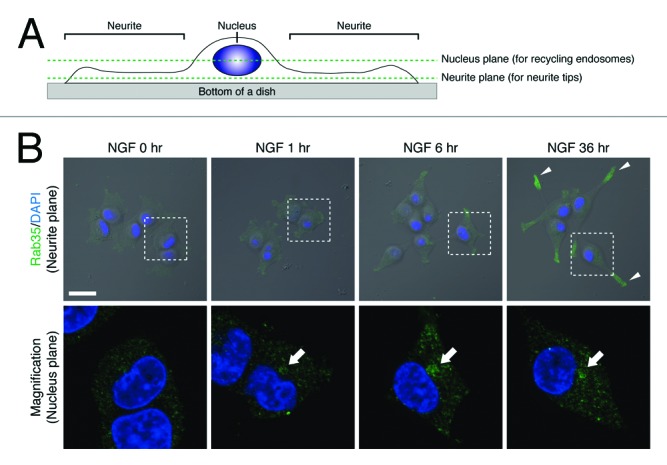
Figure 1. Rab35 is translocated to neurite tips in the late phase of NGF stimulation. (A) A schematic representation of the two different focal planes that were used to capture recycling endosomes and neurite tips. The nucleus plane generally contains recycling endosomes, and the neurite plane generally contains neurite tips. (B) After NGF stimulation for 0 h (no NGF stimulation), 1 h, 6 h, and 36 h, PC12 cells were fixed and stained with anti-Rab35 antibody and DAPI. Fluorescence images of Rab35 (neurite planes) superimposed on bright-field images are shown at the top. The panels at the bottom are magnified views of the corresponding boxed areas of the nucleus plane in the panels directly above. Scale bars, 20 μm.
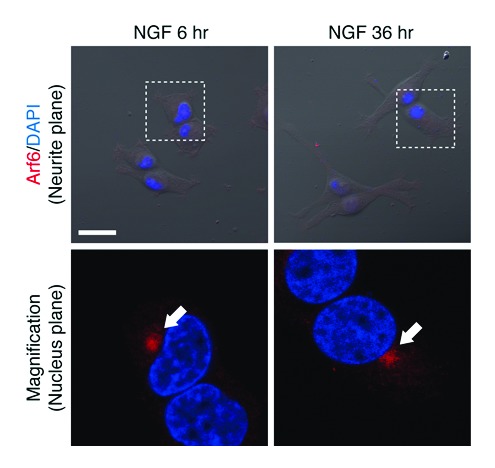
Figure 2. Arf6 is localized at perinuclear recycling endosomes even in the late phase of NGF stimulation. After NGF stimulation for 6 h and 36 h, PC12 cells were fixed and stained with anti-Arf6 antibody and DAPI. Fluorescence images of Arf6 (neurite planes) superimposed on bright-field images are shown at the top. The panels at the bottom are magnified views of the corresponding boxed areas of the nucleus plane in the panels directly above. Scale bars, 20 μm.
To investigate whether Rab35-positive vesicles are actually translocated to neurite tips, we performed live cell imaging of Rab35 in PC12 cells under a confocal laser-scanning microscope by using an EGFP-tagged constitutively active mutant of Rab35 (EGFP–Rab35-Q67L) that dramatically promotes NGF-induced neurite outgrowth. The images showed that many Rab35-positive vesicles moved from the perinuclear area to the neurite (Fig. 3 and Movie S1). We therefore concluded that Rab35 is translocated from Arf6-positive recycling endosomes to neurite tips during neurite outgrowth (Fig. 4).
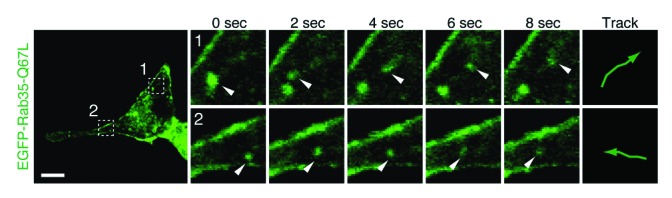
Figure 3. Rab35-positive vesicles move toward or into neurites. Living PC12 cells expressing EGFP–Rab35-Q67L were transferred to a live cell imaging chamber, and fluorescence images were captured. The fluorescence image of EGFP–Rab35-Q67L at 0 s is shown on the left. The time-lapse images of the boxed areas captured at 2 s intervals are shown on the right. Scale bars, 10 μm.
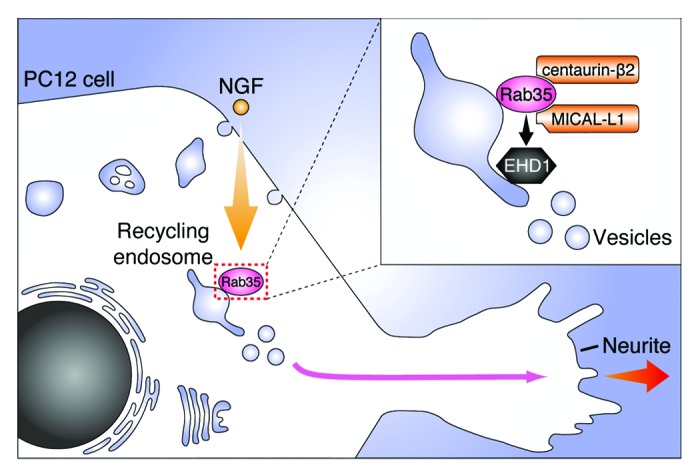
Figure 4. A model of the role of Rab35 during NGF-induced neurite outgrowth of PC12 cells. In response to NGF stimulation, Rab35 accumulates at Arf6-positive recycling endosomes.12,13 Rab35 then recruits two distinct effector molecules, centaurin-β2 and MICAL-L1, to the same compartment, and they recruit EHD1, a facilitator of vesicle formation during the early phase of NGF stimulation (i.e., 1–6 h after NGF stimulation).16 The Rab35-positive vesicles are subsequently translocated to the neurite tips during the late phase of NGF stimulation (i.e., > 6 h to < 36 h after NGF stimulation), and presumably supply the proteins and lipids to neurite tips that enable neurite outgrowth.
In addition to regulating neurite outgrowth, Rab35 has been reported to regulate various other cellular and intracellular processes, including cytokinesis, cell migration, adherens junction formation, GLUT4 trafficking, and exosome secretion.17-28 Because of the increasing evidence of its significance, considerable attention has recently been directed toward Rab35,29 but the precise properties of Rab35-dependent membrane trafficking, including where it starts and where it ends, are not fully understood. In the present study, we discovered that Rab35 is dramatically translocated from recycling endosomes to neurite tips during neurite outgrowth, suggesting that neurite tips are the final destination of Rab35-dependent membrane trafficking. Since it has been proposed that supplying proteins and lipids to neurite tips is required for neurite outgrowth,30,31 Rab35 presumably functions as the molecular switch that regulates the supply process. Future investigation of the involvement of Rab35 in the trafficking of specific proteins and lipids (e.g., neuronal cell adhesion molecules) to neurites will be necessary to determine whether it actually does so.
The findings in our study give rise to another important question. How are Rab35-positive vesicles specifically targeted to neurites instead of the entire plasma membrane? The same question might be asked in regard to various other Rab35-dependent cellular processes, i.e., cytokinesis, cell migration, and adherens junction formation, because they often accompany polarized trafficking. Although the molecular mechanism that connects Rab35 and polarized trafficking is currently unknown, elucidation of the nexus downstream of Rab35, including the discovery of a missing link(s) between Rab35 and polarized trafficking, will be necessary to answer this question.
Materials and Methods
Antibodies and plasmids
Anti-Rab35 antibody was prepared as described previously.12 Anti-Arf6 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was obtained commercially. The Alexa 488/594-conjugated secondary antibodies were from Invitrogen Corp. (Carlsbad, CA, USA). The pEGFP-C1 vector (Clontech-Takara Bio Inc., Shiga, Japan) harboring mouse Rab35-Q67L (a constitutively active mutant of Rab35) cDNA was prepared as described previously.12
Cell culture and transfection
PC12 cell culture and plasmid transfection were performed essentially as described previously.12
Immunofluorescence analyses and live cell imaging
Immunofluorescence analyses were performed essentially as described previously.12 Because of the difference between the spatial distribution of the recycling endosomes and neurite tips within NGF-stimulated PC12 cells (Fig. 1A), it is virtually impossible to detect both structures in a single focal plane by confocal microscopy. To overcome this problem, we captured two different focal planes, a “nucleus plane” that passed through the middle of the nucleus and a “neurite plane” that passed through an entire neurite, and displayed fluorescence images of recycling endosomes in the nucleus plane and fluorescence images of neurite tips in the neurite plane separately (Fig. 1, Fig. 2). The number of cells that exhibited neurite tip localization of Rab35 (or Arf6) was manually counted under a confocal fluorescence microscope (FV1000, Olympus, Tokyo, Japan), and the results are reported as means ± SD from three independent experiments (n = 50 for each experiment). To perform the live cell imaging, PC12 cells were seeded in 35 mm glass-bottom dishes (MatTek Corp., Ashland, MA, USA) coated with 100 μg/mL poly-l-lysine (Sigma-Aldrich Corp., St. Louis, MO, USA) in 2 mL of Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% fetal bovine serum (Sigma-Aldrich Corp.) and 10% horse serum (GIBCO, Carlsbad, CA, USA). At 60 h after transfection with pEGFP-C1–Rab35-Q67L, the glass-bottom dishes were transferred to a live cell imaging chamber attached to a confocal time-lapse microscope (FV500, Olympus), which maintains the temperature at 37 °C and the CO2 concentration at 5%. The fluorescence images of EGFP–Rab35-Q67L in living PC12 cells were captured at 2 s intervals over a period of 1 min.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Morié Ishida and Megumi Aizawa for technical assistance, and members of the Fukuda Laboratory for valuable discussions. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, and Technology (MEXT) of Japan and by the Daiichi-Sankyo Foundation of Life Science (to M. F.). H. K. was supported by the Japan Society for the Promotion of Science (JSPS) and by the International Advanced Research and Education Organization of Tohoku University (IAREO).
Glossary
Abbreviation:
- NGF
nerve growth factor
References
- 1.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 2.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 5.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 6.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–13. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics. 2008;7:1031–42. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Kanno E, Ishibashi K, Kobayashi H, Matsui T, Ohbayashi N, Fukuda M. Comprehensive screening for novel Rab-binding proteins by GST pull-down assay using 60 different mammalian Rabs. Traffic. 2010;11:491–507. doi: 10.1111/j.1600-0854.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 10.Chevallier J, Koop C, Srivastava A, Petrie RJ, Lamarche-Vane N, Presley JF. Rab35 regulates neurite outgrowth and cell shape. FEBS Lett. 2009;583:1096–101. doi: 10.1016/j.febslet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda M, Kobayashi H, Ishibashi K, Ohbayashi N. Genome-wide investigation of the Rab binding activity of RUN domains: development of a novel tool that specifically traps GTP-Rab35. Cell Struct Funct. 2011;36:155–70. doi: 10.1247/csf.11001. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J Cell Sci. 2012;125:2235–43. doi: 10.1242/jcs.098657. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Fukuda M. Arf6, Rab11 and transferrin receptor define distinct populations of recycling endosomes. Commun Integr Biol. 2013;6:e25036. doi: 10.4161/cib.25036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–72. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 15.Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122–31. doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi H, Fukuda M. Rab35 establishes the EHD1-association site by coordinating two distinct effectors during neurite outgrowth. J Cell Sci. 2013;126:2424–35. doi: 10.1242/jcs.117846. [DOI] [PubMed] [Google Scholar]
- 17.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–25. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Sato K, Liou W, Pant S, Harada A, Grant BD. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008;27:1183–96. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, Kruhlak MJ, Samelson LE, Shaw S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–30. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Fonovic M, Suyama K, Bogyo M, Scott MP. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250–4. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 21.Shim J, Lee S-M, Lee MS, Yoon J, Kweon H-S, Kim Y-J. Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol Cell Biol. 2010;30:1421–33. doi: 10.1128/MCB.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–32. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the Ca2+-activated K+ channel, KCa2.3, is dependent upon RME-1, Rab35/EPI64C, and an N-terminal domain. J Biol Chem. 2010;285:17938–53. doi: 10.1074/jbc.M109.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell. 2011;145:117–32. doi: 10.1016/j.cell.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Schottenfeld-Roames J, Ghabrial AS. Whacked and Rab35 polarize dynein-motor-complex-dependent seamless tube growth. Nat Cell Biol. 2012;14:386–93. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davey JR, Humphrey SJ, Junutula JR, Mishra AK, Lambright DG, James DE, Stöckli J. TBC1D13 is a RAB35 specific GAP that plays an important role in GLUT4 trafficking in adipocytes. Traffic. 2012;13:1429–41. doi: 10.1111/j.1600-0854.2012.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allaire PD, Seyed Sadr M, Chaineau M, Seyed Sadr E, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126:722–31. doi: 10.1242/jcs.112375. [DOI] [PubMed] [Google Scholar]
- 28.Charrasse S, Comunale F, De Rossi S, Echard A, Gauthier-Rouvière C. Rab35 regulates cadherin-mediated adherens junction formation and myoblast fusion. Mol Biol Cell. 2013;24:234–45. doi: 10.1091/mbc.E12-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaineau M, Ioannou MS, McPherson PS. Rab35: GEFs, GAPs and effectors. Traffic. 2013;14:1109–17. doi: 10.1111/tra.12096. [DOI] [PubMed] [Google Scholar]
- 30.Pfenninger KH. Plasma membrane expansion: a neuron’s Herculean task. Nat Rev Neurosci. 2009;10:251–61. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 31.Sann S, Wang Z, Brown H, Jin Y. Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 2009;19:317–24. doi: 10.1016/j.tcb.2009.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


