Abstract
Rho GTPases play an essential role in regulating cell spreading, adhesion, and migration downstream of integrin engagement with the extracellular matrix. In this review, we focus on RhoA and Rac1—2 Rho GTPases that are required for efficient adhesion and migration—and describe how specific guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) regulate the extensive crosstalk that exists between them. In particular, we assess the role of GEFs and GAPs in light of recent, unexpected evidence concerning the spatiotemporal relationship between RhoA and Rac1 at the leading edge of migrating cells. Force is increasingly recognized as a key regulator of cell adhesion and we highlight the role of GEFs and GAPs in mechanotransduction, before debating the controversial role of tension in focal adhesion maturation.
Keywords: GAP, GEF, Rac1, Rho GTPase, RhoA, adhesion, integrin, mechanotransduction, migration
Introduction
The adhesion of cells to the extracellular matrix (ECM) is an important facet of mammalian physiology, and plays a critical role in regulating essential cellular functions such as migration, proliferation, and survival. Upon binding to the ECM, complex networks of intracellular signaling pathways are initiated, resulting in the spreading and adhesion of cells onto the ECM. The specific signaling molecules that become activated in response to attachment are dependent on a number of factors, including cell type and substrate composition. In addition, the rigidity of the ECM substrate is increasingly viewed as a key regulator of intracellular signaling cascades.
Integrins and Rho GTPases are essential in mediating cellular responses downstream of ECM engagement, and in this review we will discuss the role of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) in regulating these responses. We will begin by providing a brief introduction to these key molecular players, followed by a discussion of their intersecting roles in promoting cellular adhesion, spreading, and migration. Our focus will then turn to recent advances in our understanding of the role of mechanical tension in the development and maturation of cell adhesion and the crosstalk that exists between integrins and Rho GTPases in mediating these force-dependent responses.
The Extracellular Matrix
ECMs exist either as complex, 3-dimensional networks in which cells are embedded or as basement membranes which are laid down by many cells and which form a structural framework for tissue organization.1,2 The matrix provides biochemical and biomechanical signals to individual cells, thereby influencing many aspects of their behavior. The composition and physical properties of different ECMs are highly heterogeneous and vary both between and within certain tissues. As discussed later, tension plays a profound role in the development and maintenance of cellular adhesion, and changes in the compliance of the ECM (e.g., stiffening as a result of aging or tumor formation)2,3 can modulate adhesion signaling, thus contributing to the onset or progression of disease.4,5
The ECM is comprised of an interweaving mesh of fibrous proteins (e.g., collagen, fibronectin, elastin, and laminin) and various proteoglycans.2,6 These macromolecules combine to provide the ECM with structural integrity (e.g., collagen fibrils confer tensional strength and elastins allow the matrix to recoil in response to repetitive stretch)6-8 and form an adhesive substrate to which cells adhere. Experimentally, it has been difficult to examine cell interactions with the ECM within intact tissues but, by plating cells on surfaces coated with ECM components, this has been extensively explored in tissue culture. Although multiple ECM proteins have been investigated (e.g., collagen, fibronectin, laminin, and vitronectin), in this review we will mainly be focusing on signaling pathways initiated downstream of fibronectin engagement. Fibronectin is a large, dimeric glycoprotein containing repeating modules and an arginine-glycine-aspartic acid (RGD) cell adhesion motif, which is located within the FnIII10 module. Fibronectin also contains additional cell-binding domains, as well as cryptic sites that are exposed in response to force and are involved in matrix assembly.9-12 Although fibronectin can initiate adhesive responses via syndecan-4,13 it is best known for mediating cell attachment via integrins, which typically bind to the RGD motif.
Integrins
The integrins are a major family of cell adhesion molecules that interact either with components of the ECM or with other adhesion molecules on other cells.14,15 Twenty-four distinct integrins have been identified and each is heterodimer composed of an α and a β subunit. Both subunits span the membrane and typically have large extracellular but short intracellular domains. There are 18 α chains and 8 β chains, with several of the β subunits pairing with different α chains to generate integrins with unique binding properties. For example, the β1-integrin subunit can pair with 11 different α chains, and each has a distinct specificity. Similarly, some of the α chains can pair with more than one β subunit, as illustrated by αv, which can partner with 5 different β chains.
Integrins exhibit bidirectional signaling.14 Signals from within the cell can cause integrins to undergo conformational changes leading to integrin activation and an increased affinity for extracellular ligands.16 Conversely, the binding of integrins to their ligands and/or integrin clustering can initiate conformational changes to their cytoplasmic domains, altered binding interactions, and the activation of multiple signaling pathways. The cytoplasmic domains of α and β subunits associate with several scaffolding proteins that link to the cytoskeleton and participate in signaling. Many questions regarding how these short cytoplasmic domains mediate so many functions remain unanswered.
Most cells express multiple integrin types and, although different integrins can bind to the same components of the ECM, they presumably convey distinct information. An ECM protein such as fibronectin can engage several different integrins; however, the most prominent and widely-expressed fibronectin-binding integrins are α5β1 and αvβ3, both of which bind to the RGD sequence within fibronectin. Recent work from the Fässler group has demonstrated that these 2 integrins regulate Rho GTPase signaling differently,17 and this will be discussed later.
Rho GTPases
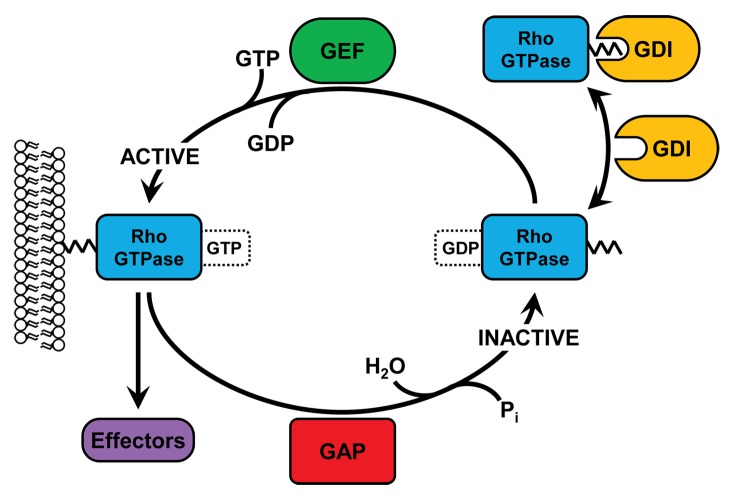
Rho (Ras homologous) family small GTPases are intracellular signaling molecules belonging to the Ras superfamily. There are 20 Rho family members, although the field is dominated by the study of the 3 ‘canonical’ members of this group: RhoA, Rac1, and Cdc42.18 When GTP-bound, Rho GTPases are active and capable of signaling to a diverse array of downstream effectors, through which they regulate a variety of cellular responses such as adhesion, spreading, migration, polarity, survival, and cell division. The activity of Rho family GTPases is determined by 3 different classes of regulatory molecules: GEFs, GAPs, and guanine nucleotide dissociation inhibitors (GDIs) (Fig. 1). GEFs activate Rho GTPases by catalyzing the exchange of a bound GDP molecule for GTP, whereas GAPs stimulate the intrinsic GTPase activity of Rho proteins, thus returning them to an inactive, GDP-bound state.19-21 GDIs are responsible for maintaining a cytosolic pool of stable, inactive Rho family GTPases which can readily translocate to membranes, where nucleotide exchange takes place (Fig. 1).22
Figure 1. Regulation of Rho GTPase activity by GEFs, GAPs, and GDIs. At the membrane, inactive, GDP-bound Rho GTPases can be activated by GEFs, which catalyze the exchange of GDP for GTP. Once GTP-bound, Rho GTPases can bind to a variety of downstream effectors and elicit diverse responses. GAPs catalyze the GTPase-dependent hydrolysis of GTP back into GDP, thus inactivating Rho proteins. In the cytosol, Rho GTPases are bound by GDIs which prevent nucleotide exchange and bury the prenylated C-terminus, thus preventing degradation.
Rho GTPases are essential in propagating integrin-mediated responses and, by tightly regulating the dynamics of the actin cytoskeleton, provide a key signaling link through which adhesion, spreading, and migration are controlled. These responses require coordinated regulation of Rho GTPase activity, which is provided as a consequence of GEF and GAP activation downstream of integrin ligation.
Rho and Rac Signal Downstream of Integrins to Regulate Cell Adhesion and Spreading
Upon ECM engagement, activated integrins initiate a cascade of intracellular signaling pathways ultimately leading to cellular spreading and adhesion onto the ECM substrate. The early stages of adhesion are characterized by the presence of small, nascent adhesions that form just behind the leading edge of a spreading or migrating cell.23,24 Their assembly requires actin but is independent of myosin II. Typically, nascent adhesions rapidly turn over and either disassemble (half-life < 60 s) or mature to become focal complexes, which are larger (~1 µm in diameter) and more stable adhesions that usually develop at the interface between the lamellipodium and lamella.24 Focal complexes are dependent on myosin II and are prominent in cells with high Rac1 activity and low RhoA activity. In turn, focal complexes can mature into focal adhesions, the largest and most stable matrix adhesion.25,26 Focal adhesions anchor stress fibers (large bundles of actin filaments) and are induced by active RhoA. Although these 3 types of adhesion are distinguishable, there is often a continuum between types and many of the same scaffolding and signaling proteins have been identified in each.25
Integrin-mediated spreading and focal adhesion maturation develop as a result of a biphasic reaction associated with the relative activities of RhoA and Rac1.27-32 Early adhesion, involving nascent adhesions, is dependent on Rac1 activation and a concomitant suppression of RhoA activity. In contrast, late adhesion, and mature focal adhesion formation, is reliant on elevated RhoA activity and Rac1 inhibition. The activation of Rho GTPases in adhesion signaling involves extensive crosstalk between integrins, Src family kinases, and between individual Rho GTPases themselves, as previously reviewed by Huveneers and Danen29 and by Guilluy et al.30 Here, we focus on the GEFs and GAPs that are involved in these responses, and the mechanisms through which they are regulated. The fine balance that exists between the opposing activities of RhoA and Rac1 is dictated by the spatiotemporal activation of specific GEFs and GAPs downstream of integrin engagement, and several of these GTPase regulators have been implicated in controlling specific phases of spreading and adhesion (Table 1).
Table 1. GEFs and GAPs involved in cell adhesion and migration.
| GEF/GAP | Rac/Rho specificity | Name | Common synonyms | Force-regulated | References |
| GEF | Rac | β-Pix | COOL-1, ArhGEF7 | Yes86 | 41,42,68,86,103 |
| DOCK180 | DOCK1 | - | 43,44,103 | ||
| Trio (N-term) | - | - | 48-50 | ||
| Vav2 | - | Yes132 | 53-55,132 | ||
| Tiam1 | - | - | 35,55,57,59,113 | ||
| α-Pix | COOL-2, ArhGEF6 | - | 65-67 | ||
| Asef2 | - | - | 114 | ||
| Rho | p190RhoGEF | Rgnef, ArhGEF28 | - | 76-78,99 | |
| p115RhoGEF | Lsc, ArhGEF1 | - | 79,80 | ||
| LARG | ArhGEF12 | Yes81 | 79,81,99 | ||
| GEF-H1 | Lfc, ArhGEF2 | Yes17,81 | 17,81,101 | ||
| Syx | Tech, PLEKHG5 | - | 94 | ||
| GAP | Rac | FilGAP | p73RhoGAP, ArhGAP24 | Yes83 | 82-84,104,106 |
| ArhGAP22 | - | Yes105 | 104,105 | ||
| RacGAP1 | MgcRacGAP, CYK4 | - | 85,107 | ||
| Rho | p190RhoGAP | GRLF1, p190A, ArhGAP35 | Yes131 | 27,28,69-72,99,131 |
GEFs and GAPs that are known to regulate RhoA or Rac1 during cell adhesion or migration are listed along with common synonyms. Those that have been shown to be regulated by tension are indicated.
Early Adhesion
Rac1 activation
Early adhesion is associated with Rac1-dependent lamellipodia formation and cell spreading. Rac1 activity stimulates the actin nucleating protein Arp2/3 via the WAVE complex,33,34 causing the polymerization of new actin filaments and resulting in the formation of a branched network of actin which is characteristic of lamellipodia. A number of GEFs have been implicated in activating Rac1 during the initial phase of cell adhesion, including β-Pix, DOCK180, Trio, Vav2, Tiam1, and α-Pix (Table 1). The activation of these GEFs downstream of integrin engagement is discussed below. To some extent, the involvement of specific GEFs appears to be dependent on cell type.35 Furthermore, the mechanisms through which these GEFs become activated downstream of integrins vary in each case, but typically involve interactions with components of nascent adhesions, which form in response to integrin engagement.
Integrins do not possess intrinsic catalytic activity, but instead mediate downstream signaling pathways by providing a scaffold onto which non-receptor tyrosine kinases are recruited and activated. Focal adhesion kinase (FAK) and Src are 2 such kinases, and localize to the intracellular tails of integrin β subunits upon integrin clustering. The integrin-FAK interaction stimulates autophosphorylation of the FAK residue tyrosine 397, which creates a binding site for the SH2 domain of Src, thus initiating the formation of a FAK-Src signaling complex at nascent adhesions.36 Src kinase activity increases upon interaction with FAK, and the kinase activity of FAK is stimulated by Src-dependent phosphorylation of additional tyrosine residues within the activation loop of FAK.37,38 The FAK-Src complex is in turn responsible for phosphorylating nascent adhesion-associated adaptor proteins, notably paxillin and p130Cas. These proteins can subsequently bind to other adaptor molecules; for example, both paxillin and p130Cas bind Crk, whereas paxillin associates with GIT2 (also known as PKL).39,40
GIT2 and Crk can recruit the GEFs β-Pix (also known as COOL-1 and ArhGEF7) and DOCK180 (also known as DOCK1), respectively, to nascent adhesions, and thereby promote Rac activation downstream of integrin engagement and FAK-Src complex formation. Upon binding of β-Pix to GIT2, Rac1 is recruited to adhesion sites and membrane ruffles by binding directly to the SH3 domain of β-Pix via its C-terminal tail.41 β-Pix also forms a complex with GIT1 and PAK, which can be localized to adhesions upon PAK-mediated phosphorylation of the paxillin residue serine 273.42 The activation of Rac1 by β-Pix has been shown to control cell spreading.41
p130Cas- and Crk-dependent DOCK180 activation has also been implicated in Rac1-dependent spreading.43,44 It was initially proposed that DOCK180 and its binding partner ELMO could mediate Rac1-dependent spreading downstream of integrin ligation via another Rho GTPase, RhoG.45,46 However, subsequent studies have indicated that RhoG is dispensable for integrin- and Rac1-dependent spreading.47
The N-terminal catalytic DH-PH domain of the GEF Trio, which has dual specificity for Rac1 and RhoG (the C-terminal DH-PH domain activates RhoA), has also recently been implicated in regulating cell spreading on fibronectin. Notably, spreading was mediated via Rac1 and was independent of RhoG activity.48 Trio has been shown to bind and be phosphorylated by FAK;49 however, the contribution of this interaction to Rac1 activity has not been directly demonstrated, and the mechanisms through which this GEF is regulated remain unclear.50
The Vav family GEFs Vav1 and Vav3 are crucial for the Rac-dependent spreading of hematopoietic cells51,52 and, in fibroblasts, this response can be regulated by Src-activated Vav2.53 Vav2 has recently been shown to interact with GIT2 and to drive recruitment of both GIT2 and β-Pix to adhesion sites in response to integrin engagement, although only upon the simultaneous activation of growth factor receptors.54 The Ras subfamily GTPase Rap1 has been implicated in regulating Rac1-dependent spreading on fibronectin by localizing Vav2 to sites of active lamellipodial extension.55 Rap1 associates with and can activate integrins via the focal adhesion protein talin and the adaptor molecule RIAM, although it is unclear if Vav2 localization is dependent on this interaction.56
The Rac GEF Tiam1 is also localized to membrane protrusions in a Rap1-dependent manner.55 Tiam1 can induce spreading via Rac1 upon interaction with 14-3-3ζ, an adaptor protein that associates with β1 and β3 integrins and p130Cas.57,58 Since Tiam1 is activated upon Src-dependent phosphorylation,59 14-3-3ζ may provide another link through which Rac can be activated downstream of Src signaling (though this has yet to be validated). Talin has also recently been identified to bind directly to Tiam1 and both components are required for spreading on fibronectin.35 Interestingly, talin recruitment to nascent adhesions has been shown to occur downstream of FAK,60 reinforcing the idea that FAK and Src can influence GEF activity in multiple ways.
In addition to the FAK-Src complex, integrin linked kinase (ILK) is capable of controlling Rac-dependent spreading downstream of integrin engagement. ILK associates with the cytoplasmic tails of β1 and β3 integrins and forms a heterotrimeric scaffolding complex with the adaptor proteins PINCH and α- or β-parvin.61-64 β-parvin is a binding partner for α-Pix and, in epithelial cells, this GEF is required for Rac-dependent spreading on fibronectin downstream of ILK.65-67 The related GEF β-Pix has also been linked to ILK-mediated spreading.68
RhoA inhibition
GEF-dependent Rac1 activity is a hallmark of early adhesion, but GAP-dependent RhoA inhibition is also characteristic of this adhesive phase. The suppression of RhoA activity in the early stages of adhesion is dependent on p190RhoGAP, a Rho-specific GAP which is activated downstream of integrin ligation in a Src-dependent manner.27,28 Upon fibronectin binding, tyrosine phosphorylation of p190RhoGAP is mediated via the integrin α5β1, whereas the syndecan-4 receptor drives PKCα-dependent p190RhoGAP localization.69 A complex of FAK and p120RasGAP has been shown to activate p190RhoGAP, although the role of Src in this response is unclear.70 Interestingly, Rac1 has been implicated as an upstream regulator of p190RhoGAP both as a result of direct interactions and indirectly, via reactive oxygen species production.71,72 Hence, Rac1 crosstalk to RhoA may be involved in regulating early adhesion events.
Late Adhesion
RhoA activation
After the initial phase of adhesion, the activity of RhoA gradually increases whereas Rac1 activity diminishes. Late adhesion is associated with RhoA-dependent stress fiber formation and focal adhesion maturation. RhoA is thought to mediate focal adhesion maturation via Rho kinase (ROCK)-induced myosin activity. ROCK is a RhoA effector which elevates the phosphorylation of the regulatory myosin light chain directly,73 as well as indirectly by phosphorylating and inhibiting the myosin phosphatase.74 By clustering integrins to focal adhesions and inducing the recruitment of other proteins to these sites, contractility is traditionally assumed to promote focal adhesion maturation and cytoskeletal reinforcement.18,75 However, the role of tension in focal adhesion maturation has recently been challenged (as discussed below). RhoA is nevertheless involved in the later stages of adhesion and its activation has been attributed to the activities of several GEFs, including p190RhoGEF, p115RhoGEF, LARG, and GEF-H1 (Table 1).
The Rho-specific GEF p190RhoGEF (also known as Rgnef) has been shown to stimulate RhoA activity and focal adhesion formation downstream of integrin engagement.76,77 Interestingly, a GEF-independent role for p190RhoGEF in regulating the localization, and subsequent activation, of FAK to early adhesion complexes has also been discovered recently.78
Evidence for the involvement of p115RhoGEF (also known as Lsc) and LARG arose from the finding that both of these GEFs could activate RhoA in fibroblasts plated on fibronectin, and that their depletion diminished stress fiber and focal adhesion formation.79 Upon G protein-coupled receptor stimulation, p115RhoGEF is inhibited by the Rac1 effector PAK1.80 Hence, it is possible that the decreased Rac activity associated with late-phase spreading may facilitate Rho activation by alleviating PAK1 inhibition of p115RhoGEF. It is currently unknown, however, if the same crosstalk exists downstream of integrin engagement. LARG has been shown to be recruited to integrin adhesions in response to mechanical tension, and its activation is dependent on the Src family kinase Fyn.81 In the same study, GEF-H1, a microtubule-associated GEF, was also identified to influence RhoA activity in a tension-dependent manner. In contrast to LARG, GEF-H1 is activated downstream of FAK, in a signaling cascade involving Ras and ERK.81
Rac1 inhibition
The suppression of Rac1 activity at later stages of adhesion appears to be dependent on crosstalk originating from elevated RhoA activity and the associated increase in actomyosin contractility. Indeed, Rac activity can be suppressed by FilGAP, a filamin A-associated GAP which is activated upon phosphorylation by the Rho effector ROCK.82 Filamin A links the cytoplasmic tails of β integrin subunits to actin and, in a reconstituted in vitro system, external shear or myosin II-dependent force production results in the dissociation of FilGAP from filamin A.83 In intact cells, this force-induced activity of FilGAP can suppress the formation of Rac-dependent lamellipodia.84
RacGAP1 has also recently been identified to inhibit Rac at the latter stages of adhesion.85 This Rac-specific GAP is recruited to sites of β1-integrin activation in the lamellipodia of spreading cells by a complex containing filamin A and the adaptor protein IQGAP185 (which does not have intrinsic GAP activity). The signaling events that trigger the spatiotemporal localization of these components await investigation, as does the potential for RacGAP1 to respond to force.
Further evidence for the role of intracellular tension in inhibiting Rac activity has arisen from a proteomic approach that examined changes in the composition of focal adhesions in response to myosin II activity. Notably, recruitment of the Rac GEF β-Pix to adhesion complexes was negatively regulated by myosin II-dependent contractility.86 Hence, in addition to mediating FilGAP-dependent suppression of Rac1, elevated RhoA activity and the associated increase in actomyosin contractility may also inhibit Rac activity by spatially restricting the interaction of Rac with one of its GEFs.
The findings described above have not only enhanced our understanding of the complex antagonism that exists between RhoA and Rac1 downstream of integrin ligation, but have also provided support for the notion that mechanical tension is a critical regulator of cellular adhesion and spreading. An increasing body of work has provided insight into how mechanical force contributes to the crosstalk between specific integrins and Rho GTPases, although the relationship between mechanical force and focal adhesion maturation is disputed. These issues will be discussed after first considering how these signaling components control cell migration.
Crosstalk between Rho GTPases is Critical for Integrin-Mediated Cell Migration
The directional migration of cells across the ECM is a dynamic process that is fundamentally linked to spatially-regulated Rho GTPase activity. The onset of migration is dependent on the formation of a polarized cell morphology, with protrusive actin structures forming at the leading edge of the cell in tandem with retraction and detachment of the cell rear. As described above, integrins form an important link between the ECM and the actin cytoskeleton, and the adhesions formed in response to integrin engagement stabilize actin protrusions and provide the traction that is required for cell motility. These sites of ECM-actin adhesion are also organized throughout the cell in a polarized fashion, with small nascent adhesions repeatedly assembling and disassembling at the leading edge, in a process known as adhesion turnover, whereas larger, more mature adhesions form at the cell rear. For an overview of the role of adhesion dynamics in migration, readers are referred to an excellent review by Parsons et al.25 Here, we will focus on the crosstalk that exists between specific Rho GTPases in the regulation of cell migration.
The polarized processes that occur in migrating cells are directly dependent on the localized activities of the Rho GTPases Rac1, Cdc42, and RhoA. Rac1 and Cdc42 are typically thought to act at the leading edge of migrating cells, where they induce actin protrusions. Rac1 activity at the leading edge gives rise to lamellipodia formation, whereas Cdc42 is associated with the extension of filopodia, which are thin bundles of actin filaments that protrude from the leading edge of migratory cells and act as probes for sensing the external environment.87,88 Actin polymerization is stimulated downstream of these 2 GTPases via effectors such as WAVE, WASP, and PAK. WAVE and WASP activate the actin nucleating complex Arp2/3, whereas PAK leads to LIM kinase activation, which inhibits cofilin-mediated breakdown of actin filaments.88
For efficient locomotion, leading edge advancement must be coupled to retraction at the rear of the cell. Given the role of RhoA in stimulating actomyosin contractility through its effector ROCK, it is not surprising that this GTPase has been implicated in regulating the retraction of cell tails.89,90 Hence, the established view of the role of Rho GTPases in polarized migration is that Rac1 and Cdc42 localize to the leading edge, whereas RhoA is found predominantly toward the rear of migrating cells. In recent years, however, this notion has been challenged as a result of data generated using Rho GTPase fluorescence resonance energy transfer (FRET) biosensors.91-93 Strikingly, RhoA activity is also observed to occur at the leading edge of mouse embryonic fibroblasts (MEFs) migrating on fibronectin, in advance of Rac1 and Cdc42 both spatially and temporally.92 Peak Rac1 activity is observed approximately 40 s after peak RhoA activity,92 suggesting that RhoA or RhoA-mediated events may be responsible for initiating Rac1 activity at the leading edge.
These results are paradoxical to the generally accepted dogma that Rac1 activity precedes that of RhoA in spreading cells (as discussed above), and a definitive explanation for this apparent discrepancy has yet to be provided. An important factor is likely to be that many of the signaling pathways affecting integrin-dependent Rho GTPase activity have been determined using bulk biochemical measurements. Indeed, the biphasic activities of RhoA and Rac1 in fibronectin-adhering cells were determined in this manner. It was anticipated that studies using FRET-based biosensors to visualize the spatiotemporal activities of GTPases in individual cells would confirm the earlier findings; hence, it was surprising that they instead revealed RhoA to be active in advance of Rac1 at the extreme edge of migrating cells. It is possible that the high activity of RhoA in this region represents only a small fraction of the total activity of RhoA in the cell, and is therefore not detected when less-sensitive, bulk measurements are made. Another factor contributing to the different observations may be that most of the biosensor data are generated from cells that have already fully spread and are migrating, and hence may have different signaling dynamics to those that are only beginning the adhesion process.
Given the role of RhoA in promoting myosin contractility via ROCK, it seems contradictory that this GTPase is found at the front of the lamellipodium, a region of membrane extension. It has been proposed, however, that RhoA may contribute to protrusion via its effector mDia,93 an actin-polymerizing formin protein. Intriguingly, the RhoA GEF Syx has recently been identified to selectively couple RhoA to mDia at the leading edge of migrating cells.94 Another potential mechanism through which RhoA may selectively activate mDia rather than ROCK at the leading edge is through adhesion-induced ROCK inhibition.95 As cells extend their leading edge, integrin engagement with the ECM may promote the tyrosine phosphorylation of ROCK, leading to the suppression of ROCK-mediated contraction. This pathway has also been implicated in promoting the turnover of focal complexes behind the leading edge of migrating cells.96
Much remains to be learned about the role of Rho GTPases and the crosstalk that exists between them in regulating cell migration. In particular, the GEFs and GAPs that are responsible for mediating the spatiotemporal activation of Rho and Rac in migrating cells are incompletely understood, as are the mechanisms that regulate the spatial distribution of these components. The subcellular localization of Rho GTPases is dependent on a number of factors including post-translational modifications to the hypervariable C-terminal regions of these proteins and their interactions with RhoGDIs.97 In migrating cells, spatially-restricted Rho GTPase activity could be speculated to be controlled by the interaction of GEFs and GAPs with specific scaffolding proteins, and the diverse composition of adhesion complexes at various stages of maturation may influence GEF and GAP localization. The development of biosensors for specific GEFs and GAPs would greatly enhance our understanding of how Rho GTPases are spatiotemporally regulated in migrating cells.
To some extent, the same pathways that are involved in regulating cell adhesion and spreading downstream of integrins (as described above) also appear to play a role in regulating migration. The sheer number of GEFs and GAPs that have been implicated in regulating adhesion and migration (Table 1) can appear bewildering, but it is important to note that variations between cell types are common. Although most of the GEFs, GAPs, and GTPases mentioned in this review have a generally widespread tissue distribution,20,97,98 the relative expression of these components undoubtedly varies between specific cell types. Hence, certain GEFs and GAPs are likely to play a more important role in some cells than in others, which may account for some of the apparent discrepancies concerning the involvement of specific proteins in adhesion and migration.
Here we summarize what is currently known about the involvement of Rho family GEFs and GAPs in different regions of polarized cells, and speculate on how these regulatory molecules may fit in with the emerging model of cell migration that shows RhoA, Rac1, and Cdc42 all localizing to the leading edge.
RhoA activation at the leading edge
RhoA activation at the anterior edge of migrating cells may be stimulated by RhoA-specific GEFs such as p190RhoGEF, GEF-H1, Syx, and LARG (Fig. 2). Knockdown or genetic ablation of p190RhoGEF inhibits the migration of MEFs by suppressing RhoA activity.76,77 This GEF has also been shown to regulate lamellipodia formation in growth factor-stimulated cells via RhoC.99 RhoC is a Rho GTPase that localizes slightly behind the leading edge of migrating cells and controls actin dynamics via cofilin, thereby contributing to the efficient formation of lamellipodia.99,100 GEF-H1 has been identified to regulate the localization of RhoA to the leading edge of migrating cells and its depletion also affects migration.101 Interestingly, GEF-H1 has been linked to the phosphorylation of FAK and paxillin101 and may therefore influence the accumulation of other GEFs and GAPs to the front of the cell. As noted above, Syx selectively couples RhoA to its effector mDia at the leading edge and is required for efficient polarity and migration.94 The GEFs LARG and p115RhoGEF are involved in promoting RhoA activity in response to fibronectin adhesion and, in migrating cells, LARG has been implicated in regulating RhoA-dependent lamellipodia formation.79,99 The role of p115RhoGEF in migration is currently unclear.
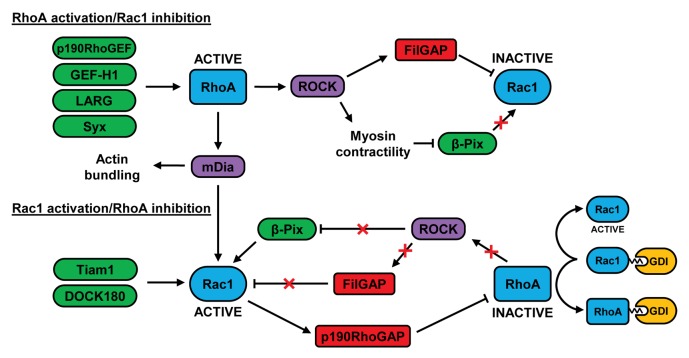
Figure 2. Crosstalk between RhoA and Rac1 in migrating cells. At the leading edge of migrating cells, RhoA can be activated by GEFs such as p190RhoGEF, GEF-H1, LARG, and Syx. At the cell rear, RhoA can restrict Rac1 activity via FilGAP and by negatively regulating the localization of β-Pix. RhoA-stimulated mDia activity may contribute to the subsequent increase in Rac1 activity at the leading edge, possibly by activating Src-dependent GEFs such as Tiam1 and DOCK180. Rac1 can inhibit RhoA via p190RhoGAP and the decrease in RhoA activity may further activate Rac1 by preventing FilGAP activation and by relieving the inhibition of β-Pix. The association of inactive RhoA with RhoGDI could also increase Rac1 activity as a result of the competitive binding of these 2 GTPases to GDI. See text for further details.
RhoA-mediated suppression of Rac
At the cell rear, RhoA has been shown to restrict the formation of lateral protrusions.102 The Rac GEFs β-Pix and DOCK180 are negatively regulated by ROCK-mediated myosin II contractility and, given the role of RhoA in driving myosin II activity, it therefore seems likely that the low activity of Rac at the cell posterior derives in part from the activity of RhoA.86,100,103 Similarly, the ROCK-mediated phosphorylation and activation of the filamin A-associated protein FilGAP may contribute to the suppression of Rac1 activity downstream of RhoA in the cell rear (Fig. 2).82,84
At the leading edge, RhoA activity precedes that of Rac1, suggesting that RhoA may initially suppress Rac activation.92 FilGAP has been suggested to downregulate Rac1 activity in this region in response to RhoA activity during the early stages of migration, although this has yet to be experimentally proven.104 FilGAP is closely related to another Rac-specific GAP, ArhGAP22, and both proteins have been shown to play a role in regulating the plasticity of tumor cell migration.
Tumor cells can migrate using either an ‘amoeboid’ or a ‘mesenchymal’ mode of movement. Amoeboid migration is characterized by a rounded morphology and elevated RhoA activity, whereas mesenchymal migration is associated with an elongated cell morphology and high Rac1 activity. Notably, the balance of Rho and Rac activity is a key determinant of migratory mode, and mutual antagonism between these 2 GTPases dictates the prevailing cell morphology. In mesenchymal melanoma cells, Rac1 is activated by a complex containing the GEF DOCK3 and the p130Cas-related protein NEDD9. However, in amoeboid cells, Rac activity is inhibited by ArhGAP22 in a RhoA- and ROCK-dependent manner.105 FilGAP has also been shown to promote mesenchymal-to-amoeboid transition downstream of ROCK.106
In addition to FilGAP and ArhGAP22, RacGAP1 has recently been identified as another Rac-specific GAP that can control migration.85,107 Under conditions of α5β1 recycling, RacGAP1 is phosphorylated by Akt, causing its recruitment to IQGAP1 at the edge of invasive pseudopods where it can inhibit Rac. Interestingly, this process also leads to an increase in RhoA activation, resulting in enhanced invasion into 3D fibronectin matrices.107 The mechanism through which RacGAP1 causes this elevation in RhoA activity is currently unclear.
Rac1 activation and RhoA suppression
As shown by Machacek et al., the initially high activity of RhoA at the leading edge subsides as the activities of Rac1 and Cdc42 increase.92 Furthermore, a photoactivatable construct of a constitutively active variant of Rac1 is capable of suppressing RhoA when expressed and activated to promote lamellipodia formation in fibroblasts.108
It has been proposed that RhoA activity contributes to membrane ruffling by selectively coupling to its effector, mDia1, a formin protein which is capable of nucleating actin filaments and stabilizing microtubule formation.93,109,110 Interestingly, microtubule growth has been linked to the activation of Rac.111 Furthermore, mDia1 can influence Rac activity by controlling the localization of Src to focal adhesions and promoting the subsequent phosphorylation of p130Cas.109,112 Hence, it is possible that RhoA initiates Rac activity at the leading edge of migrating cells via mDia1 (Fig. 2). The GEFs involved in activating Rac in this putative RhoA-dependent mechanism could be speculated to include those that are activated downstream of Src and p130Cas, including Tiam1 and DOCK180. Tiam1 associates with the PAR complex and has been shown to control cell polarity downstream of Cdc42.113 Upon binding to talin, this Tiam1-PAR complex has also been implicated in the activation of Rac1 during cell migration.35
As Rac1 activity gradually rises, it is possible that the activity of RhoA at the leading edge may decline as a result of the Rac-mediated activation of p190RhoGAP (Fig. 2).27,71,72 This Rho-specific GAP is localized to the leading edge in a complex with FAK and p120RasGAP.70 p190RhoGAP also inhibits RhoC, and has been shown to counteract p190RhoGEF in the control of RhoC activity behind the leading edge.99 Another way in which Rac1 may suppress RhoA activity is via the GEF Asef2, which can stimulate Rac activity in migrating fibrosarcoma cells, causing RhoA inhibition through an unknown mechanism.114 At the leading edge of epithelial cells, PKA-mediated phosphorylation of the RhoA residue serine 188 increases the affinity of RhoA for RhoGDI, thus reducing the interaction of RhoA with the plasma membrane.115 Hence, RhoGDIs may contribute to the suppression of RhoA at the leading edge. Since RhoA and Rac1 undergo competitive binding to RhoGDI,116 it is possible that the increased RhoA-RhoGDI interaction may facilitate the release and activation of Rac (Fig. 2).117,118
As RhoA activity decreases in migrating cells, it is conceivable that the associated decrease in contractility may also promote Rac activity, by relieving the spatially restricted localization of β-Pix (Fig. 2). This GEF can activate Rac in the lamellipodium but its localization to nascent adhesions is inhibited in a myosin-dependent manner.86 The tension-mediated localization of FilGAP could also be speculated to modulate Rac activity downstream of RhoA.104
The Role of Force in Adhesion
Cells are constantly exposed to tension that can either arise from external sources or intracellularly, from the cell’s own actomyosin contractile system. Recent work has shown that cells respond in many ways to mechanical force and the physical characteristics of the environment, and tension is increasingly recognized as a key regulator of cell adhesion and migration. Tension can influence Rho GTPase activity in several ways, and a number of GEFs and GAPs have been shown to be regulated by force. One way in which mechanical tension has long been accepted to control adhesion is in the regulation of focal adhesion assembly and growth. Indeed, the progression of focal complexes into focal adhesions is usually attributed to mechanical tension.75,119,120 Recent evidence is beginning to question this dogma, however.121,122
The idea that mechanical tension promotes focal adhesion assembly originally arose from the finding that active RhoA induces myosin activity and that blocking either RhoA or contractility abolished focal adhesion assembly.75 However, some of the inhibitors of contractility that were used in this early work were relatively non-specific and some were subsequently shown to inhibit ROCK rather than myosin directly. The most elegant evidence for the role of mechanical force in inducing adhesion assembly came from experiments in which the growth of focal adhesions was observed in real-time upon the direct application of force to cells with inhibited RhoA activity.120 The observation that adhesion size correlates with the force transmitted at that site is also consistent with the notion of focal adhesion growth being dictated by tension.123,124
The recent challenge to this view has emerged from several lines of evidence. First, Beningo et al. observed that the tension transmitted at focal adhesions to the substratum did not correlate to the size of the adhesions within the cell, and that the greatest tension occurred at small adhesions (focal complexes) found at the cell front rather than at the large adhesions further back.125 Similar results have been obtained by Gardel’s group.126 In a separate study, Tan et al. identified one subset of adhesions that exhibited a correlation between tension and size, and another set of smaller adhesions that were involved in transmitting high forces and therefore did not fit this correlation.127 Hence, there may be 2 adhesion populations: one that undergoes growth and maturation in response to force, and one that does not. Nevertheless, the overall argument of these results is that tension is not a critical determinant of adhesion size.
Further evidence against tension leading to adhesion maturation comes from the Gardel lab’s manipulation of mDia and α-actinin. Decreasing the expression of either of these proteins resulted in increased tension on the adhesions but a decrease in adhesion size and diminished maturation, as determined by decreased FAK and phospho-paxillin accumulation.121 Similarly, using inhibitors of contractility such as the ROCK inhibitor Y27632, focal adhesion size has been shown to be unaffected over a considerable range of myosin-generated tension.122 The complete loss of myosin activity, however, causes focal adhesion disassembly. Hence, a low threshold level of myosin activation appears to be required for focal adhesion maintenance and maturation. It is notable that adhesion maturation in myosin II-depleted cells can be rescued by re-expression of a myosin II that is defective in its motor function but is still able to bind and crosslink actin.24 The idea that myosin-mediated crosslinking of actin filaments contributes to stress fibers and focal adhesions has previously been proposed.128 The F-actin crosslinking function of myosin is also downstream of RhoA activity, and may be more important in the assembly of adhesions than its force-generating activity.
The requirement for myosin II in the formation, but not the maturation of large focal adhesions is supported by a recent study conducted by Schiller et al.17 Using pan-integrin-depleted fibroblasts reconstituted with the αv- or the β1-integrin subunit (thereby generating cells which expressed αvβ3 and αvβ5 but not β1 integrins, or α5β1 but not αv integrins), they found that cells which expressed αv integrins alone exhibited large focal adhesions, but low myosin II activity and traction force. Myosin II was however required for focal adhesion formation, as these structures failed to form in the presence of the myosin II inhibitor blebbistatin.17
The same study employed a proteomic approach to analyze the composition of the focal adhesions induced by either αv or α5β1 integrins downstream of fibronectin binding, and strikingly delineated the individual roles of each. Cells reconstituted with the αv subunit had high levels of RhoA activity but could not couple RhoA to ROCK, hence the low myosin II activity of these cells. Instead, mDia1 was enriched in αv focal adhesions and is proposed to induce stress fiber-dependent focal adhesion maturation downstream of RhoA in these cells. In contrast, α5β1-expressing cells were capable of inducing ROCK-dependent myosin II activity, but did not appear to develop large ventral stress fibers.17 Instead, these cells seem to contain peripheral dorsal ‘arcs’, a form of stress fiber that moves centripetally from the cell periphery toward the cell nuclear region. While arcs are moving rearward, they are not usually linked directly to focal adhesions. However, in some situations dorsal arcs can link up with focal adhesions and give rise to large prominent ventral stress fibers.129 This phenomenon is not apparent in the study by Schiller et al. but, notably, α5β1-expressing cells exhibited small adhesions that formed independently of myosin II. It will be interesting to explore the mechanisms through which αv and β1 integrins selectively couple to mDia and ROCK, respectively, especially since previous work, which did not take integrin type or expression into account, has yielded opposing results with respect to the role of mDia and ROCK in stress fiber formation.130
A final finding of the study by Schiller et al. is that the Rho-specific exchange factor GEF-H1 associates with the αvβ3 integrin, but that the activation of this GEF is dependent on α5β1, possibly via ERK activation. Hence, in normal cells adhering to fibronectin, the αvβ3 and α5β1 integrins appear to synergize to promote the activity of GEF-H1, as well as to elicit efficient stress fiber formation and myosin II activation.17
GEF-H1 is activated upon the external application of force to integrins and has been shown to regulate RhoA-dependent cell stiffening downstream of FAK, Ras, and ERK. Fyn-stimulated activation of the Rho GEF LARG has also been implicated in promoting cell rigidity.81 These 2 GEFs are members of a relatively short list of GTPase regulators that have been shown to be regulated by force, which also includes β-Pix, Vav2, FilGAP, ArhGAP22, and p190RhoGAP (Table 1).83,86,105,131-133 Given the increasing interest in studying the role of force in mediating cell responses, and the fact that a number of Rho family GEFs and GAPs remain uncharacterized, it seems inevitable that others will be added to this list in due course.
Conclusions
In this review we have outlined the GEFs and GAPs that are involved in regulating RhoA and Rac1 in the control of integrin-mediated cell adhesion and migration. We have also attempted to reassess 2 topics that have proven controversial in light of surprising new evidence: first, the dynamics of GTPase activation at the leading edge of migrating cells and second, the role of tension in promoting focal adhesion maturation. While our understanding of the mechanisms that control adhesion and migration is continually advancing, much remains to be revealed about the specific roles of Rho GTPases, GEFs, and GAPs in these processes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
C.D.L. is grateful to the US. Army Medical Research and Materiel Command for a Breast Cancer Research Program Postdoctoral Fellowship Award (W81XWH-14-1-0033). K.B. thanks the Kenan Foundation and the National Institutes of Health (Grant GM029860) for support.
Glossary
Abbreviations:
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FRET
fluorescence resonance energy transfer
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- ILK
integrin linked kinase
- MEF
mouse embryonic fibroblast
- RGD
arginine-glycine-aspartic acid
- ROCK
Rho kinase
References
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41:494–7. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–51. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–88. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 12.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–72. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 15.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Théry M, et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15:625–36. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 18.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 19.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 21.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2013 doi: 10.1038/onc.2013.362. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente-Manzanares M, Horwitz AR. Adhesion dynamics at a glance. J Cell Sci. 2011;124:3923–7. doi: 10.1242/jcs.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–22. doi: 10.1016/S0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 29.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 30.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–26. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–71. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–56. doi: 10.1016/S0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 34.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, Ye F, Sato K, Murase K, Sugiyama I, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol. 2012;199:331–45. doi: 10.1083/jcb.201202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Ruest PJ, Roy S, Shi E, Mernaugh RL, Hanks SK. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 2000;11:41–8. [PubMed] [Google Scholar]
- 39.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamorte L, Rodrigues S, Sangwan V, Turner CE, Park M. Crk associates with a multimolecular Paxillin/GIT2/beta-PIX complex and promotes Rac-dependent relocalization of Paxillin to focal contacts. Mol Biol Cell. 2003;14:2818–31. doi: 10.1091/mbc.E02-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–69. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–9. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–6. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiyokawa E, Hashimoto Y, Kurata T, Sugimura H, Matsuda M. Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J Biol Chem. 1998;273:24479–84. doi: 10.1074/jbc.273.38.24479. [DOI] [PubMed] [Google Scholar]
- 45.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–4. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 46.Katoh H, Hiramoto K, Negishi M. Activation of Rac1 by RhoG regulates cell migration. J Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 47.Meller J, Vidali L, Schwartz MA. Endogenous RhoG is dispensable for integrin-mediated cell spreading but contributes to Rac-independent migration. J Cell Sci. 2008;121:1981–9. doi: 10.1242/jcs.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Rijssel J, Hoogenboezem M, Wester L, Hordijk PL, Van Buul JD. The N-terminal DH-PH domain of Trio induces cell spreading and migration by regulating lamellipodia dynamics in a Rac1-dependent fashion. PLoS One. 2012;7:e29912. doi: 10.1371/journal.pone.0029912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medley QG, Buchbinder EG, Tachibana K, Ngo H, Serra-Pagès C, Streuli M. Signaling between focal adhesion kinase and trio. J Biol Chem. 2003;278:13265–70. doi: 10.1074/jbc.M300277200. [DOI] [PubMed] [Google Scholar]
- 50.van Rijssel J, van Buul JD. The many faces of the guanine-nucleotide exchange factor trio. Cell Adh Migr. 2012;6:482–7. doi: 10.4161/cam.21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–82. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawson CD, Donald S, Anderson KE, Patton DT, Welch HC. P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. J Immunol. 2011;186:1467–76. doi: 10.4049/jimmunol.1002738. [DOI] [PubMed] [Google Scholar]
- 53.Marignani PA, Carpenter CL. Vav2 is required for cell spreading. J Cell Biol. 2001;154:177–86. doi: 10.1083/jcb.200103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones MC, Machida K, Mayer BJ, Turner CE. Paxillin kinase linker (PKL) regulates Vav2 signaling during cell spreading and migration. Mol Biol Cell. 2013;24:1882–94. doi: 10.1091/mbc.E12-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–22. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández-Varas P, Coló GP, Bartolomé RA, Paterson A, Medraño-Fernández I, Arellano-Sánchez N, Cabañas C, Sánchez-Mateos P, Lafuente EM, Boussiotis VA, et al. Rap1-GTP-interacting adaptor molecule (RIAM) protein controls invasion and growth of melanoma cells. J Biol Chem. 2011;286:18492–504. doi: 10.1074/jbc.M110.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Toole TE, Bialkowska K, Li X, Fox JE. Tiam1 is recruited to β1-integrin complexes by 14-3-3ζ where it mediates integrin-induced Rac1 activation and motility. J Cell Physiol. 2011;226:2965–78. doi: 10.1002/jcp.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Guzman M, Dolfi F, Russello M, Vuori K. Cell adhesion regulates the interaction between the docking protein p130(Cas) and the 14-3-3 proteins. J Biol Chem. 1999;274:5762–8. doi: 10.1074/jbc.274.9.5762. [DOI] [PubMed] [Google Scholar]
- 59.Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem. 2003;278:34339–46. doi: 10.1074/jbc.M302960200. [DOI] [PubMed] [Google Scholar]
- 60.Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–32. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 62.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 63.Grashoff C, Thievessen I, Lorenz K, Ussar S, Fässler R. Integrin-linked kinase: integrin’s mysterious partner. Curr Opin Cell Biol. 2004;16:565–71. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol. 2012;24:607–13. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenberger G, Jantke I, Gal A, Kutsche K. Interaction of alphaPIX (ARHGEF6) with beta-parvin (PARVB) suggests an involvement of alphaPIX in integrin-mediated signaling. Hum Mol Genet. 2003;12:155–67. doi: 10.1093/hmg/ddg019. [DOI] [PubMed] [Google Scholar]
- 66.Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837–49. doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur J Cell Biol. 2006;85:265–74. doi: 10.1016/j.ejcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Boulter E, Grall D, Cagnol S, Van Obberghen-Schilling E. Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 2006;20:1489–91. doi: 10.1096/fj.05-4579fje. [DOI] [PubMed] [Google Scholar]
- 69.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–26. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–62. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bustos RI, Forget MA, Settleman JE, Hansen SH. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr Biol. 2008;18:1606–11. doi: 10.1016/j.cub.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–41. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 73.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 74.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 75.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller NL, Lawson C, Chen XL, Lim ST, Schlaepfer DD. Rgnef (p190RhoGEF) knockout inhibits RhoA activity, focal adhesion establishment, and cell motility downstream of integrins. PLoS One. 2012;7:e37830. doi: 10.1371/journal.pone.0037830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller NL, Lawson C, Kleinschmidt EG, Tancioni I, Uryu S, Schlaepfer DD. A non-canonical role for Rgnef in promoting integrin-stimulated focal adhesion kinase activation. J Cell Sci. 2013;126:5074–85. doi: 10.1242/jcs.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dubash AD, Wennerberg K, García-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–98. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 80.Rosenfeldt H, Castellone MD, Randazzo PA, Gutkind JS. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J Mol Signal. 2006;1:8. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–14. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 83.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–3. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shifrin Y, Arora PD, Ohta Y, Calderwood DA, McCulloch CA. The role of FilGAP-filamin A interactions in mechanoprotection. Mol Biol Cell. 2009;20:1269–79. doi: 10.1091/mbc.E08-08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacquemet G, Morgan MR, Byron A, Humphries JD, Choi CK, Chen CS, Caswell PT, Humphries MJ. Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J Cell Sci. 2013;126:4121–35. doi: 10.1242/jcs.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 89.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/S0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 90.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–60. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 92.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell. 2005;16:4294–303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, Feathers R, Anastasiadis PZ. The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol Cell Biol. 2013;33:4909–18. doi: 10.1128/MCB.00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee HH, Chang ZF. Regulation of RhoA-dependent ROCKII activation by Shp2. J Cell Biol. 2008;181:999–1012. doi: 10.1083/jcb.200710187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee HH, Tien SC, Jou TS, Chang YC, Jhong JG, Chang ZF. Src-dependent phosphorylation of ROCK participates in regulation of focal adhesion dynamics. J Cell Sci. 2010;123:3368–77. doi: 10.1242/jcs.071555. [DOI] [PubMed] [Google Scholar]
- 97.Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–12. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 98.Csépányi-Kömi R, Sáfár D, Grósz V, Tarján ZL, Ligeti E. In silico tissue-distribution of human Rho family GTPase activating proteins. Small GTPases. 2013;4:90–101. doi: 10.4161/sgtp.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bravo-Cordero JJ, Sharma VP, Roh-Johnson M, Chen X, Eddy R, Condeelis J, Hodgson L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J Cell Sci. 2013;126:3356–69. doi: 10.1242/jcs.123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–65. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070–82. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578–84. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 103.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol. 2011;193:381–96. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura F. FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration. Biochem J. 2013;453:17–25. doi: 10.1042/BJ20130290. [DOI] [PubMed] [Google Scholar]
- 105.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 106.Saito K, Ozawa Y, Hibino K, Ohta Y. FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. Mol Biol Cell. 2012;23:4739–50. doi: 10.1091/mbc.E12-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacquemet G, Green DM, Bridgewater RE, von Kriegsheim A, Humphries MJ, Norman JC, Caswell PT. RCP-driven α5β1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J Cell Biol. 2013;202:917–35. doi: 10.1083/jcb.201302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26:6844–58. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–9. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- 111.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 112.Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, et al. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol. 2002;157:819–30. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–7. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 114.Bristow JM, Sellers MH, Majumdar D, Anderson B, Hu L, Webb DJ. The Rho-family GEF Asef2 activates Rac to modulate adhesion and actin dynamics and thereby regulate cell migration. J Cell Sci. 2009;122:4535–46. doi: 10.1242/jcs.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tkachenko E, Sabouri-Ghomi M, Pertz O, Kim C, Gutierrez E, Machacek M, Groisman A, Danuser G, Ginsberg MH. Protein kinase A governs a RhoA-RhoGDI protrusion-retraction pacemaker in migrating cells. Nat Cell Biol. 2011;13:660–7. doi: 10.1038/ncb2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–83. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rolli-Derkinderen M, Toumaniantz G, Pacaud P, Loirand G. RhoA phosphorylation induces Rac1 release from guanine dissociation inhibitor alpha and stimulation of vascular smooth muscle cell migration. Mol Cell Biol. 2010;30:4786–96. doi: 10.1128/MCB.00381-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–9. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 119.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–92. doi: 10.1016/S0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 120.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–86. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol. 2012;196:363–74. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stricker J, Beckham Y, Davidson MW, Gardel ML. Myosin II-mediated focal adhesion maturation is tension insensitive. PLoS One. 2013;8:e70652. doi: 10.1371/journal.pone.0070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 124.Schwarz US, Balaban NQ, Riveline D, Bershadsky A, Geiger B, Safran SA. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys J. 2002;83:1380–94. doi: 10.1016/S0006-3495(02)73909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML. Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J. 2011;100:2883–93. doi: 10.1016/j.bpj.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 129.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 131.Yang B, Radel C, Hughes D, Kelemen S, Rizzo V. p190 RhoGTPase-activating protein links the β1 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arterioscler Thromb Vasc Biol. 2011;31:376–83. doi: 10.1161/ATVBAHA.110.217794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peng F, Zhang B, Ingram AJ, Gao B, Zhang Y, Krepinsky JC. Mechanical stretch-induced RhoA activation is mediated by the RhoGEF Vav2 in mesangial cells. Cell Signal. 2010;22:34–40. doi: 10.1016/j.cellsig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 133.Lessey EC, Guilluy C, Burridge K. From mechanical force to RhoA activation. Biochemistry. 2012;51:7420–32. doi: 10.1021/bi300758e. [DOI] [PMC free article] [PubMed] [Google Scholar]