Abstract
Rho GTPases are key regulators of actin and microtubule dynamics and organization. Increasing evidence shows that many viruses have evolved diverse interactions with Rho GTPase signaling and manipulate them for their own benefit. In this review, we discuss how Rho GTPase signaling interferes with many steps in the viral replication cycle, especially entry, replication, and spread. Seen the diversity between viruses, it is not surprising that there is considerable variability in viral interactions with Rho GTPase signaling. However, several largely common effects on Rho GTPases and actin architecture and microtubule dynamics have been reported. For some of these processes, the molecular signaling and biological consequences are well documented while for others we just begin to understand them. A better knowledge and identification of common threads in the different viral interactions with Rho GTPase signaling and their ultimate consequences for virus and host may pave the way toward the development of new antiviral drugs that may target different viruses.
Keywords: Rho GTPase, actin, egress, entry, gene expression, immune system, transformation, virus
Introduction
Cellular rigidity and structure, as well as crucial cellular processes including phagocytosis, cellular movement or intracellular communication all depend on actin.1-4 Two different forms can be distinguished in the cell: monomeric globular actin (G-actin) and polymeric filamentous actin (F-actin), the latter consisting of two parallel strands of actin monomers.
The actin cytoskeleton is mainly regulated by the Rho family of GTPases (Rho GTPases).5 These monomeric low molecular weight proteins constitute a distinct family with 22 mammalian members within the superfamily of Ras-related small GTPases, which are subdivided in the Rac subfamily (Rac1, Rac2, Rac3, and RhoG), Cdc42 subfamily (Cdc42, TC10, TCL, Chip, and Wrch-1), RhoA subfamily (RhoA, RhoB, and RhoC) and other Rho GTPases (RhoE/Rnd3, RhoH/TTF, Rif, RhoBTB1, RhoBTB2, Miro-1, Miro-2, RhoD, Rnd1, and Rnd2). Rho GTPases regulate abovementioned cellular processes by linking membrane receptors to the actin cytoskeleton, thereby explaining their fundamentality and omnipresence throughout eukaryotic cells.6 Because they regulate numerous different functions, Rho GTPases are strictly controlled in a spatiotemporal manner and can be viewed as molecular switches that cycle between an active GTP-bound and an inactive GDP-bound form. This switch is tightly regulated by three sets of proteins: guanine nucleotide-exchange factors (GEFs) that catalyze the activating exchange of GDP for GTP,7 GTP-ase activating proteins (GAPs) that stimulate the intrinsic GTPase activity to inactivate the switch8 and guanine nucleotide-dissociation inhibitors (GDIs), whose role appears to be to block spontaneous activation.9
In their active GTP-bound state, Rho GTPases act through a conformation-specific interaction with their target effector proteins. The best characterized members of the Rho GTPases are RhoA, Rac1, and Cdc42, which show high degrees of conservation over higher vertebrates. Activation of RhoA leads to formation of actin stress fibers and focal adhesion assembly, Rac1 typically regulates the formation of lamellipodia or membrane ruffles, while Cdc42 induces the formation of protrusive filopodia. Importantly, Rho GTPase signaling not only affects the actin cytoskeleton but also stability, dynamics, and polarization of the microtubule network.10
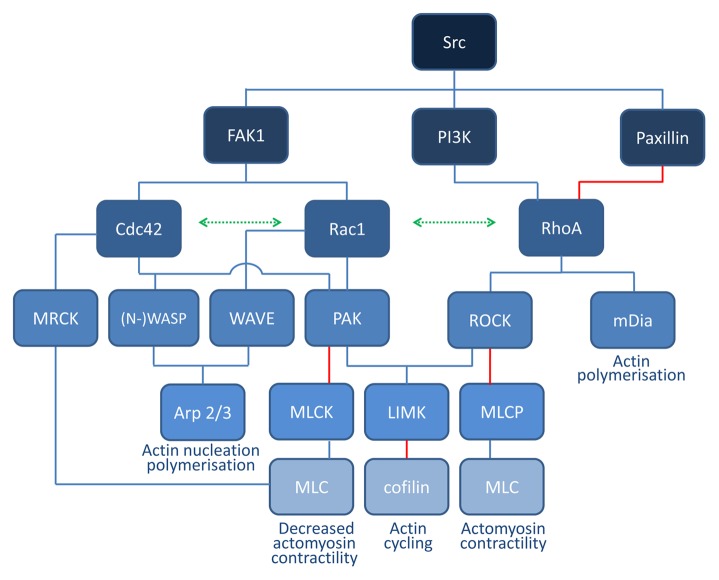
Over the past decades, many of the molecular details of the signal transduction pathways that connect Rho GTPase activity to rearrangements of the actin cytoskeleton have been unraveled. Nevertheless, identification of downstream targets of Rho GTPases has proven to be challenging. A combination of affinity chromatography, protein purification and yeast-two hybrid screening revealed over 100 potential Rho GTPase targets.11 RhoA, Rac1, and Cdc42 alone are capable of interacting with about 20–30 different proteins in a GTP-dependent manner, underscoring their broad signal transduction potential. Some of the main interaction partners of RhoA, Cdc42, and Rac1 are summarized in Figure 1.
Figure 1. Schematic overview of Rho GTPase signaling. Cdc42 and Rac1 signaling leads to activation of the common effector protein p21-activated kinase (PAK) and scaffold proteins belonging to the WASP/WAVE family, which are key regulators of actin nucleation and polymerization. Active PAK phosphorylates myosin light chain kinase (MLCK), thereby inactivating it and inhibiting myosin light chain (MLC) phosphorylation and contractility. PAK also phosphorylates and activates LIMK which may lead to phosphorylation of cofilin, thereby inhibiting its actin-severing function. Downstream targets of RhoA include the serine/threonine kinase ROCK which is mainly involved in the formation of stress fibers and focal adhesions. ROCK phosphorylates downstream MLC, leading to actin–myosin contractility. At the same time, ROCK inhibits MLC dephosphorylation by inhibiting MLC phosphatase (MLCP). LIMK is also a downstream effector of ROCK. The mammalian homolog of diaphanous (mDia) is another important RhoA effector mediating actin nucleation.
In brief, RhoA interacts directly with the formin mDia, which promotes the polymerization of actin into linear filaments.12 RhoA also activates ROCK (Rho-associated kinase) which increases myosin light chain (MLC) phosphorylation directly and through inactivation of MLC phosphatase, resulting in activation of myosin II.13 LIMK is another downstream effector of ROCK, which phosphorylates and inhibits cofilin.14 Cofilin is an F-actin-severing protein. Mainly depending on the local availability of G-actin monomers, cofilin activity may lead to net actin depolymerization or increased actin polymerization and branching. Other RhoA effectors include members of the ezrin/radixin/moesin (ERM) proteins15.
Rac1 is thought to release WAVE from an inhibited complex, thereby activating the actin-polymerizing complex Arp2/3.16,17 Active Arp2/3 mediates branching and elongation of existing actin filaments, generating a meshwork. Cdc42 also activates Arp2/3 through a direct interaction with the Arp2/3 activators Wiskott-Aldrich syndrome protein (WASP) or N-WASP.18 Both Rac1 and Cdc42 also activate group I serine/threonine p21 activating kinases (PAK). These different signalization branches are interconnected. In general, RhoA pathway signaling counteracts the Rac1 and Cdc42 signaling axes and vice versa.
Because Rho GTPase signaling is involved in a plethora of cellular processes, it is perhaps not surprising that several viral gene products have evolved to interfere with and modulate these signaling axes. Indeed, several viruses interfere with the actin cytoskeleton and Rho GTPase signaling during many steps of their replication cycle. During entry and egress, these interactions may allow or facilitate viral particle passage across the cortical actin barrier and to rearrange actin to conformations that promote virus infection and spread, while other viral processes, such as intracellular movement, gene expression and latency, but also interaction with the immune system or oncogenesis may also depend to some extent on Rho GTPase signaling and associated actin rearrangements. In this review, the current knowledge of the interactions of different viruses with Rho GTPase signaling will be discussed, with an emphasis on processes that are conserved or similar in different viruses.
Entry
A first step during viral infection of a host cell consists of attachment of the virus particle to the surface of a host cell. In some cases, the initial host factors to which the virus particle adheres serve as genuine entry receptors and in other cases entry receptors must still be recruited upon this initial attachment phase.
A wide variety of strategies of viral entry in host cells have been described. Irrespective of the entry route, at some point, the plasma membrane itself and the cortical actin barrier that is located just beneath the plasma membrane need to be overcome.19,20 Many viruses make use of cellular internalization processes for transport across the cell membrane and the cortical actin layer (endocytic viruses). Some viruses, however, enter by fusing their envelope with the plasma membrane (fusogenic viruses).21 During the latter type of entry, the cortical actin barrier is often remodeled by viral proteins to allow access of the particle to the cytoplasm.
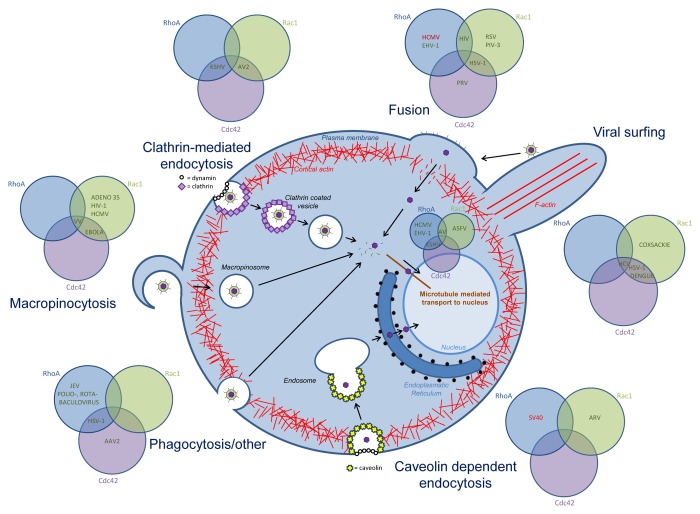
It may come as no surprise that Rho GTPase signaling is involved in the entry process of many viruses (Fig. 2) but the exact contribution of Rho GTPase signaling appears to depend largely on the entry route and the specific cell type. Indeed, Rho GTPase signaling is involved in the intrinsic signaling pathways that drive different endocytic uptake processes that may be (mis)used by viruses, particularly phagocytosis and macropinocytosis.22-34 On the other hand, fusogenic uptake may not directly depend on Rho signaling, but, upon fusion, local actin rearrangements might be required to allow passage of the capsids through the cortical actin meshwork underneath the plasma membrane.
Figure 2. Overview of the role of Rho GTPase signaling in viral entry. Rho GTPases are involved in the different entry mechansims used by diverse viruses: membrane fusion, clathrin-mediated endocytosis, caveolin-dependent endocytosis, phagocytosis and other entry processes, and in viral transport to the nucleus. For each entry process, the involvement of Cdc42, Rac1 and/or RhoA is indicated, in green if the Rho GTPase is upregulated or activated, in red when the Rho GTPase is downregulated or inhibited. Abbreviations; Kaposi’s sarcoma-associated herpesvirus (KSHV), adenovirus 2 (AV2), human cytomegalovirus (HCMV), equine herpesvirus (EHV-1), human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), parainfluenza virus 3 (PIV-3), herpes simplex virus (HSV-1), pseudorabies virus (PRV), hepatitis c virus (HCV), simian virus 40 (SV40), avian reovirus (ARV), Japanese encephalitis virus (JEV), adeno-associated virus 2 (AAV2), vaccinia virus (VV).
Viral surfing and receptor clustering
Several viruses, including retroviruses, papillomaviruses, herpesviruses, poxviruses, dengue virus and vesicular stomatitis virus (VSV), engage Rho GTPase signaling even before entering the cell. In order to reach the cell body, virions attach to filopodia-like structures, leading to unidirectional movements toward the cell, followed by viral entry. This process, called viral surfing, was first described by Lehmann and colleagues for murine leukemia virus (MLV), avian leukosis virus, human immunodeficiency virus (HIV) and VSV in HEK-293-T cells35 and relies on activation of myosin II and actin rearrangements (Fig. 4A).35-37 Herpes simplex virus 1 (HSV-1) and dengue virus not only travel along filopodia during entry, but may also actively induce filopodia formation at this stage through activation of Cdc42 and Rac1 signaling.38,39
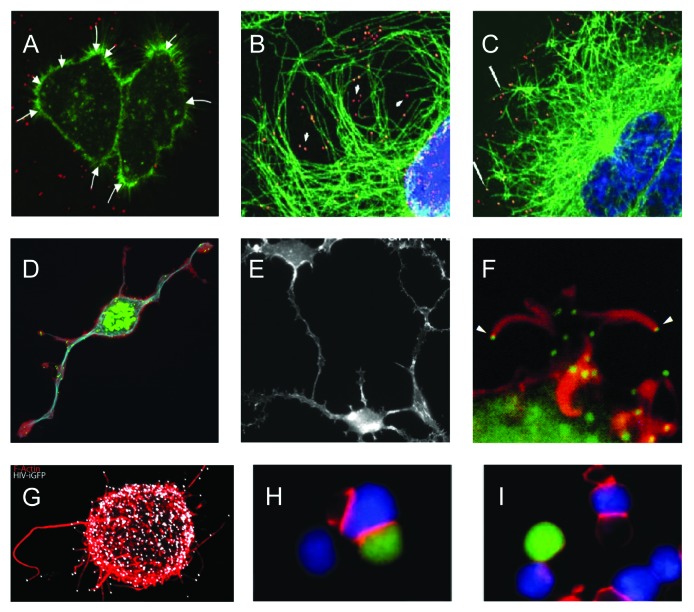
Figure 4. Illustrations of viral use and manipulation of the cytoskeleton through Rho GTPase signaling during virus entry, egress, and interaction with immune cells. (A) Actin(green)-dependent surfing of Avian Leukosis Virus particles (red) along filopodia to the cell body during virus entry. (B-C) Rac1-mediated microtubule(green)-based transport of adenovirus particles (red) to the nucleus (blue) during virus entry. Cells in (C) were treated with the Rac1 inhibitor NSC23766 and show reduced association of virus particles with microtubules and reduced accumulation of virus particles at the nuclear rim. (D-E) Similar phenotype of actin-based cell projections induced by the alphaherpesvirus pseudorabies virus US3 protein kinase (D) and vaccinia virus F11 protein (E). (D) shows actin (red), microtubules (blue) and virus particles (green), (E) shows actin cytoskeleton. (F) Actin(red)-dependent expulsion of vaccinia virus particles (green) from infected cells during virus egress. (G-I) Effects of HIV Nef-PAK2 signaling on HIV interaction with immune cells. (G) Nef-mediated production of virus particle (white)-tipped filopodia (red) in dendritic cells. (H-I) HIV (H) is unable to disturb actin-dependent immunological synapse (red) formation between infected Jurkat T cells (green) and Raji B cells (blue), whereas wild-type HIV disturbs immunological synapse formation (I). Image sources: (A) Lehmann et al., 2005, J Cell Biol, (B,C) Warren and Cassimeris, 2007, Cell Mot Cytoskeleton, (D) Image courtesy of Herman Favoreel, (E) Valderrama et al., 2006, Science, (F) Cudmore et al., 1995, Nature, (G) Aggarwal et al., 2012, PLoS Path, (H, I) Rudolph et al., 2009, J Virol.
Rho GTPases also play a crucial role in another viral pre-entry process. Virion entry requires interactions with specific receptors and coreceptors that are not always readily accessible. Therefore, several viruses have evolved interactions that induce specific Rho GTPase signaling leading to transport of the virus particle to sites of viral uptake. Binding of the hepatitis C virus (HCV) E2 protein to its CD81 receptor on hepatocytes increases the levels of activated RhoA, Rac1, and Cdc42. This induces actin rearrangements that lead to lateral movement of the virus-receptor complex to the tight junctions, the site where its coreceptor claudin is expressed.40 Internalization then occurs via a clathrin dependent endocytosis pathway.41
The cellular coxsackievirus and adenovirus receptor (CAR) is also located at tight junctions and therefore inaccessible. Group B coxsackieviruses first attach to the GPI-anchored protein decay-accelerating factor (DAF), which activates Abl kinase. This leads to Rac1-dependent actin rearrangements, permitting viral movement to the tight junctions, where virions are subsequently internalized via caveolin-associated vesicles.42
Membrane fusion
Certain enveloped viruses, such as HSV-1, Sendai virus and many retroviruses, like the HIV-1, possess pH-independent fusion proteins, which allow direct fusion with the plasma membrane in particular cell types. Although direct fusion with the plasma membrane has been shown to lead to a productive infection for these viruses, this does not exclude the possibility that these viruses may also enter via endocytosis in the same and certainly in other cell types.
HIV infection is initiated by binding of gp120 to the CD4 receptor and to chemokine coreceptors like CXCR4, which results in membrane fusion. Binding of gp120 to CD4 initiates signaling to moesin, Arp2/3 and filamin A, resulting in actin-dependent entry receptor clustering.43,44 This clustering initiates Rho GTPase signaling, which in turn results in actin rearrangements facilitating the movement of virions through the cortical actin meshwork. Both activation of RhoA and Rac1 are involved in these processes.45-47 A possible explanation is that Rac1 is activated through the CXCR4 pathway while RhoA activation results from CD4 engagement. During this process, the activity of the actin severing protein cofilin is regulated in a bi-phasic manner. First, cofilin is phosphorylated and thus inactivated, possibly contributing to receptor clustering, followed by cofilin activation, which might contribute to loosening the cortical actin barrier and thus facilitating nuclear translocation.48-50
Herpesviruses may also enter via fusion at the plasma membrane. The first report suggesting the involvement of Rho GTPases in alphaherpesvirus entry showed that HSV-1 entry in MDCKII cells was associated with activation of Cdc42 and Rac1, and that overexpression of genetically engineered Rho GTPases with altered activity influenced HSV-1 infectivity.51 This is in line with findings that pseudorabies virus (PRV), another alphaherpesvirus, induces Cdc42-dependent signaling upon infection in sensory neurons.52 For yet another alphaherpesvirus, equine herpesvirus 1 (EHV-1), entry into equine dermal (ED) or rabbit kidney (RK13) cells depends on activation of the RhoA effector ROCK, as inhibitors of this kinase or overexpression of a negative regulator of ROCK interfered with EHV-1 infection.53 Attachment of the betaherpesvirus human cytomegalovirus (HCMV) to fibroblasts is associated with integrin- and EGF receptor-mediated signaling through Src and PI3K respectively to downregulate RhoA activity. This leads to a decreased phosphorylation of cofilin and the subsequent disruption of stress fibers.54 However, other researchers have questioned a role for EGF receptor and associated signaling in HCMV entry in fibroblasts.55
The respiratory syncytial virus (RSV) and parainfluenza virus type-3 (PIV-3) both belong to the paramyxoviruses. Their F glycoproteins mediate two different forms of fusion: fusion of the virus with the plasma membrane, enabling viral entry and fusion of the infected cell membrane with adjacent cell membranes, leading to syncytia formation. In both processes, RhoA involvement has been reported but the underlying mechanisms are still unclear.56-59
In conclusion, Rho GTPase activation is a common mechanism involved in viral uptake, facilitating passage of the virus through the cortical actin layer and into the cytoplasm. Despite these apparent similarities, important differences exist, as there are different and sometimes contrasting contributions of the RhoA, Rac1, and Cdc42 signaling axes during these processes.
Endocytosis
Many mammalian viruses take advantage of different endocytosis mechanisms for their entry and infection.23,26,61 Endocytosis is advantageous for the virus, as it delivers the virus particle/genome deep into the cytoplasm, bypassing the cortical actin barrier.60
Endocytosis can be subdivided in clathrin-dependent and clathrin-independent endocytosis. Both types are hijacked by viruses and often involve Rho GTPase signaling.
Clathrin mediated endocytosis (CME)
During clathrin-mediated endocytosis (CME), actin-nucleation promoting factors steer the formation of an Arp2/3-dependent network of branched actin filaments where a clathrin-coated pit forms, providing forces for invagination.24,25 Studies have reported that CME involves a large number of conserved proteins, whose order of action and sequential recruitment is of uttermost importance. The GTPase dynamin is critically involved in CME, as it is necessary for maturation of the clathrin-coated pit as well as pinching off the clathrin-coated vesicle.61
Adenovirus 2 entry through CME depends on actin assembly and is triggered by activation of PI3K and, subsequently, Rac1 and Cdc42. Peculiarly, PAK1, a central downstream target of Cdc42 and Rac1, is not activated during viral entry.62
Kaposi’s sarcoma herpesvirus (KSHV) or human herpesvirus 8 have been reported to enter fibroblasts through CME.63 The interaction of KSHV gB with its receptor α3β1integrin on these cells induces integrin signaling which results, via FAK, Src, and PI3K, in temporal Cdc42 activation followed by sustained RhoA and ezrin activation.64,65 The importance of RhoA activation and Src phosphorylation for KSHV entry was later confirmed in overexpression experiments with dominant negative/active variants in HEK-293-T cells.66
Rho GTPase signaling may not only affect clathrin-coated vesicle formation but also subsequent movement of vesicles. Particularly, during CME and other internalization processes, Src signaling mediates assembly of the plasma-membrane associated Ras activation complex, activating Rho and Rab GTPases, which not only is critical for the formation of various types of endocytic vesicles but also for their subsequent movement. The latter may play a role in intracellular movement of viruses, including KSHV, and will be discussed further on.67-69
Clathrin-independent endocytosis
Clathrin-independent endocytosis (CIE) pathways cover a diversity of internalization routes, such as phagocytosis, macropinocytosis and caveolin dependent endocytosis (CDE) pathways. As actin dynamics play a role in many of these processes, Rho GTPase signaling is likely to play an important role in CIE of viruses.
Caveolin-dependent endocytosis
CDE occurs via caveolae, which are flask-shaped invaginations of the plasma membrane that belong to the cholesterol-rich microdomains (lipid rafts). In these regions, cholesterol molecules are intercalated between lipid chains, causing a local decrease of membrane fluidity. Caveolin-1 is the main structural protein of caveolae, essential for their formation and stability. Simian Virus 40 (SV40) entry through caveolae is well-documented, and studies on SV40 entry have contributed essential information to our understanding of CDE. SV40 transiently binds to caveolae leading to a transient breakdown of actin stress fibers and subsequent temporal dynamin and actin recruitment. This is followed by the formation of actin processes, contributing to intracytoplasmic transport of the viral particles.70,71 During SV40 entry, integrin signaling activates the PI3K/AKT pathway leading to RhoA inactivation through the RhoGAP GRAF1. This results in the phosphorylation of ezrin and subsequent detachment of cortical actin from the plasma membrane, enabling subsequent viral uptake.72 Although it was initially thought that caveolae vesicles were delivered to an organelle dubbed the caveosome, more recent research has questioned the existence of such organelle.73,74 Little is known about avian reovirus (ARV) cell entry, but infection has been reported to lead to p38 MAPK and Src mediated Rac1 activation in the early stage of the viral replication cycle, beneficial for caveolin1-mediated endocytic virus entry and productive infection.75
Macropinocytosis
Over the past years, macropinocytosis has emerged as an uptake route used by a wide variety of viruses.31 Virus particles first activate signaling pathways that trigger actin-mediated membrane ruffling and blebbing. In the next stage, the ‘collapse’ of these cytoskeletal structures induces the formation of large vacuoles (macropinosomes) at the plasma membrane, resulting in internalization of virus particles and subsequent penetration into the cytosol. Not surprisingly, Rho GTPases play a central role in macropinocytosis. Although small variations on the theme are reported, the pathways inducing macropinocytosis are quite conserved, and involve Rac1 and Cdc42 activation that lead to actin ruffle and filopodia formation. PAK activation is indispensable during all stages of macropinocytosis. PAK activation contributes to the initial stages of actin remodeling and is later also involved in the macropinosome closure by activating C-terminal-binding protein-1/brefeldin A-ADP ribosylated substrate (CtBP-1 also known as BARS).31,32,37,76-79
Macropinocytic uptake has been reported for many viruses including, among others, poxviruses, Ebola virus, influenza virus, adenoviruses, herpesviruses and retroviruses. For example, several labs have reported on the role of Rho GTPases and macropinocytosis during vaccinia virus (VV) entry27,37,77,78,80 For an extensive overview of the (subtle) differences in macropinocytic uptake between the different viruses we refer to a review by Mercer and Helenius.31 More recently, actin-dependent macropinocytosis-like uptake has been described for human papillomavirus type 16.81 Also recently, macropinocytic uptake has been suggested for two herpesviruses, the betaherpesvirus HCMV and the gammaherpesvirus KSHV. The viral gH/gL/UL128–131 complex of HCMV triggers integrin/Src/paxillin-signaling in monocytes, which promotes viral internalization through the regulation of dynamin and actin rearrangements, suggesting a macropinocytosis-like route of entry into target monocytes.82 KSHV binding to HMVEC-d cells induces ROS production and subsequent Rac1 activation, thereby amplifying the signaling pathways that are needed for macropinocytic uptake of this virus in this cell type.83
Phagocytosis and other endocytic routes
For some herpesviruses, atypical phagocytosis-like endocytic uptake routes have been described, which are all clathrin- and caveolin-independent.
HSV-1 follows a phagocytosis-like uptake route in corneal fibroblasts (CF) and nectin1-overexpressing Chinese hamster ovary (CHO) cells, during which brief Cdc42 activation is followed by sustained RhoA activation.84 Interestingly, as described higher, KSHV induces very similar Rho GTPase signaling in fibroblasts, but in this case, signaling culminates in clathrin-mediated endocytosis. Obviously, Rho GTPase signaling is critical for initiation of different internalization processes, but the particular endocytosis route activated appears to be determined by additional, cell type-dependent factors. In line with the HSV-1 results, EHV-1 entry into CHO-K1 cells occurs via an endocytic or phagocytic mechanism that depends on ROCK activation.53
Atypical entry processes that involve Rho GTPase signaling have also been described for other viruses. For the picornavirus echovirus 1 (EV1), cell entry is initiated by integrin clustering, which induces PAK1 activation. This entry route is caveolin and clathrin independent but was reported to transport the virus to the caveosome.85 However, since, as mentioned higher, the existence of the caveosome is highly questionable, the latter may be revisited. Adeno associated virus 2 (AAV2) entry occurs via clathrin-, caveolin-, and dynamin-independent endocytosis that depends on Cdc42 signaling.86
For several viruses, involvement of Rho GTPase signaling in virus entry was reported, although the specific uptake routes are still unclear. For example, Japanese encephalitis virus (JEV),87 poliovirus,88 rotavirus,89,90 and baculovirus91 all appear to depend on RhoA activation for their entry. Porcine circovirus 2 (PCV-2) infection also appears to rely on small Rho GTPase activity for infection, as inhibition with Clostridium difficile toxin B reduced PCV-2 infection in different cell types.92
Movement to the nucleus
Many viruses that replicate in the nucleus rely on microtubule-based transport for long distance intracellular movement from the periphery to the nucleus, while actin filaments may play a role in short-range movements.19,93-97 Rho GTPase signaling may also contribute to this viral transport to the nucleus, particularly through Rho GTPase-mediated stabilization (acetylation/detyrosination) of microtubules.
As already mentioned above, RhoA and Cdc42 signaling is induced upon interaction of the KSHV glycoprotein gB with integrins and subsequent activation of integrin dependent focal adhesion kinase (FAK).64 Apart from facilitating entry, this activation also modulates microtubule organization, intracellular virus transport, and delivery of the viral genome to the nucleus.65,98 Along the same lines, for HCMV and EHV-1, RhoA signaling and concomitant stress fiber breakdown during entry has been associated with efficient virus translocation to the nucleus.54,99
Rho GTPase-mediated modulation of microtubules during entry is not confined to herpesviruses alone. In adenovirus infected fibroblasts, RhoA- and Rac1-dependent increased acetylation and detyrosination of microtubules has been observed, which is thought to enhance the ability of microtubules to “search and capture” incoming viral particles (Fig. 4B&C).100,101
Likewise, in African Swine Fever Virus (ASFV) infection, Rac1-mediated microtubule acetylation is required for microtubule stabilization during viral transport.102
Hence, several viruses make use of RhoA and/or Rac1 GTPase signaling to modulate microtubules for establishment of infection or nuclear delivery of the viral genome.
Gene Expression and Latency
Once the virus has entered the target cell and reached its compartment of replication, gene expression and subsequent production and assembly of new virus particles takes place. For several viruses, Rho GTPase signaling has been reported to influence gene expression.
Activation of RhoA by the guanine exchange factor p115-RhoGEF inhibits HIV-1 gene expression in T lymphocytes.103 This has been associated with the ability of RhoA to inhibit transcriptional activity of the NFAT transcription factor, which may affect HIV gene expression through the NFAT-binding site in the HIV long-terminal repeat.104 Conversely, Rac1-mediated signaling may enhance HIV gene expression. In support of this, CD28-mediated signaling via Vav and Rac1 contributes to HIV gene expression in T lymphocytes.105 Rho GTPase signaling may also affect viral gene expression in a more indirect manner. For example, the influenza virus protein NS1 leads to decreased pRb phosphorylation through RhoA inhibition, which results in G0/G1 cell cycle arrest, thereby creating optimal conditions for influenza virus replication.106
Also PAKs, central effector proteins in the Rac1/Cdc42 signaling axes appear to be involved in viral gene expression. Recently, PAKs were shown to physically interact with the viral transactivator Tax of the human T-lymphotropic virus type 1 (HTLV-1) which enhanced Tax’ ability to activate transcription of the HTLV-1 long-terminal repeats.107 Quite contrary, PAK1 was reported to be involved in suppression of HCV replication. The mTOR downstream effector p70S6 kinase activates PAK1 and contributes to PI3K- and ERK-mediated inhibition of HCV RNA replication.108
Viruses typically need full viral gene expression and a resulting productive viral replication at some point to spread to other hosts. However, some viruses also benefit from a stealth mechanism with very limited viral gene expression. Such a dormant infection, referred to as latency, allows them to persistently infect particular cell types in their host. Specific stimuli during the lifetime of the host may then trigger full viral gene expression and production and spread of virus. To favor latency, viruses have developed a number of strategies aimed at interfering with different aspects of the host cell signaling machinery, which may include Rho GTPase signaling, although the exact implication of Rho GTPase signaling in latency is currently poorly understood.
Herpesviruses are notorious examples of viruses that can cause latent infections, which typically ensures them of a lifelong infection of their host. In the case of gammaherpesviruses, latency occurs by maintenance of the viral genome in the nuclei of infected lymphocytes. M2, a protein encoded by murine gammaherpesvirus 68 (MHV-68), associates with the Rho exchange factor Vav, resulting in Vav hyperphosphorylation and Rac1 activation. In vivo studies showed that the interaction of M2 with Vav proteins is important for the establishment of a latent infection of MHV-68 in B cells.109,110
Retroviruses like HIV also may establish latency. In a transcriptome study in cell lines latently infected with HIV, Cdc42 expression was found to be downregulated, which perhaps may be involved in cellular maintenance of latency.111
Overall, although there is considerable evidence that Rho GTPase signaling may be involved in regulating viral gene expression during productive viral replication for different viruses, the potential contribution of Rho GTPase signaling during viral latency is less well documented thus far.
Virus Assembly and Egress
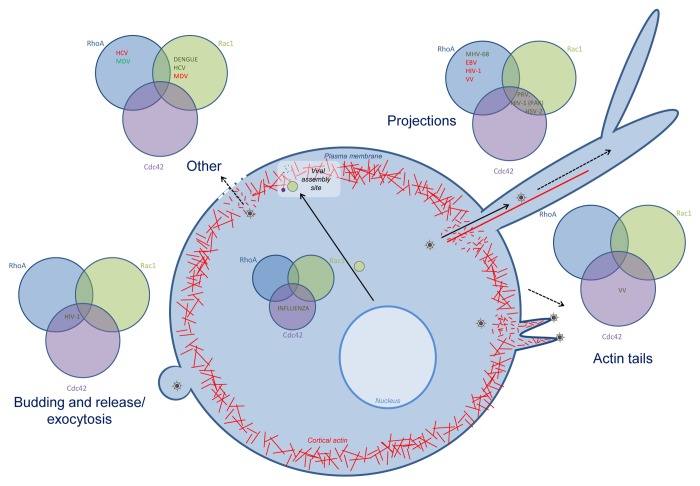
Many viruses also interfere with Rho GTPase signaling during later stages of infection, during virus particle assembly, egress, and/or spread (Fig. 3). In influenza virus infection, different mechanisms, all implying some kind of Rho GTPase signaling were reported to impact infectious virus production. Activation of Cdc42 increases transport of the viral envelope protein neuraminidase to the plasma membrane, the site of viral assembly, leading to increased virus production.112 Furthermore, interaction of Cdc42 with the YRKL domain of the influenza virus matrix protein M1 is beneficial for influenza virus production, since it influences the function of M1 during budding of the virus.113 RhoA, on the other hand, is involved in downregulation of HDAC6 activity, leading to increased microtubule acetylation, which in turn is associated with enhanced virus assembly and release.114
Figure 3. Overview of the role of Rho GTPase signaling during late stages of infection. Rho GTPases are involved in late stages of infection for different viruses: projection formation, budding and release and exocytosis. For each process, the involvement of Cdc42, Rac1 and/or RhoA is indicated, in green if the Rho GTPase is upregulated or activated, in red when the Rho GTPase is downregulated or inhibited. Abbreviations: hepatitis c virus (HCV), Marek’s disease virus (MDV), human immunodeficiency virus (HIV-1), murid herpesvirus 68 (MHV-68), Epstein-Barr virus (EBV), vaccinia virus (VV), pseudorabies virus (PRV), herpes simplex virus 2 (HSV-2).
In recent years, PAK1 was reported to contribute to ERK phosphorylation and thereby enhance influenza virus production, although the mechanism involved remained elusive.115,116 Another publication also reported ERK activation upon influenza infection, but showed that this was mediated by RhoA. Irrespective of the contributing triggers, further downstream, MLC phosphorylation appears to be at the crossroad of different signaling pathways induced by influenza infection, and is critically involved in virus replication. This might be explained by the fact that activated MLC mediates nuclear export of the ribonucleoprotein complex.19,116
During later stages of infection, and especially during egress, viruses encounter similar barriers as during entry. Indeed, in order to exit the cell, newly formed virus particles again have to overcome the cortical actin and the plasma membrane.19,20 In addition, during egress, viruses may manipulate the actin cytoskeleton to assist in viral spread to neighboring cells.
For herpesviruses, poxviruses and retroviruses these processes have been intensively studied and important steps in the signaling cascades driving these processes have been identified. For other viruses, Rho GTPase signaling has been associated with actin remodeling during later stages of infection, but the underlying mechanism or functional consequences remain unclear.
Over the past decade, it has become clear that lytic alphaherpesvirus infection results in alterations in Rho GTPase signaling that culminate in drastic rearrangements of the actin cytoskeleton. Common actin rearrangements include disassembly of actin stress fibers and/or the formation of cellular projections that are often branched and over 40 µm in length (Fig. 4D). Alphaherpesvirus-induced actin rearrangements have been associated with increased viral spread in cell cultures and could be attributed to the conserved viral US3 serine/threonine protein kinase for several alphaherpesviruses, including the porcine PRV, human HSV-2, bovine herpesviruses BHV1- and -5, and Marek’s disease virus (MDV) of poultry.117-121 For PRV, it has been shown that virus particles migrate inside the US3-induced projections to neighboring cells, allowing intercellular virus transmission, even in the presence of virus-neutralizing antibodies120.
A first suggestion that US3 may interfere with Rho GTPase signaling came from Murata and coworkers for HSV-2117, and has been unraveled in more detail for PRV US3. For PRV, it was shown that US3 leads to phosphorylation and activation of PAKs. Using PAK knockout cell lines, PAK1 was found to be involved in the formation of cell projections whereas PAK2 was crucial for actin stress fiber breakdown.122 In line with these findings, PAK signaling contributed to efficient cell-to-cell spread of PRV.122 Recently, Jacob and coworkers demonstrated that US3- and PAK-dependent cofilin activation plays a central role in the US3-induced actin rearrangements.123,124 HSV-1 was also found to activate cofilin although this appeared restricted to the early stages of infection.124 Interestingly, for HSV-1, US3 has recently been reported to stabilize microtubules, which further contributes to virus spread.125
Actin rearrangements by the US3 protein kinase typically depend on the kinase activity of this protein.126-129 However, an exception to this is the chicken alphaherpesvirus MDV. Intact and kinase-inactive US3 of MDV display equal abilities to induce actin rearrangements, although these are less dramatic compared with those of other alphaherpesviruses, as MDV US3 induces actin stress fiber disassembly but has not been reported to induce cell projections.130 Another indication that interference of MDV with Rho GTPase signaling is different compared with other alphaherpesviruses comes from the finding that, unlike for PRV, the Rac1/PAK signaling axis appears inhibitory to MDV spread, whereas the RhoA/ROCK signaling axis promotes spread.131
Actin containing projections that contribute to viral spread have also been reported for the gammaherpesviruses Epstein Barr virus (EBV) and MHV-68. Since these viruses do not encode a US3 kinase ortholog, the underlying mechanism is different, and depends on a viral integral membrane protein complex consisting of BDLF2/BMRF2 in EBV and its ortholog gp48/ORF58 in MHV-68. Experiments with dominant active and negative RhoA constructs suggest that Rho GTPase signaling is critically involved in the projection formation, although further research should clarify their specific role since different studies found opposing roles for RhoA.132,133
For poxviruses, particularly VV, the F11 protein has been reported to induce actin rearrangements that resemble those induced by alphaherpesviral US3. Indeed, comparable to US3 of alphaherpesviruses, F11 of VV is necessary and sufficient to induce actin stress fiber disassembly and formation of cellular projections (Fig. 4E). This has been associated with enhanced motility of infected cells and enhanced virus spread.134,135 Furthermore, F11 induces a reorganization of cortical actin and increased microtubule dynamics, resulting in an increased tendency of microtubules to reach the cell periphery, thereby promoting access of the virus to the plasma membrane.136,137 Although F11 was also shown to downregulate Cdc42 and Rac1 at 8hpi, only the ability of F11 to inhibit RhoA signaling to its downstream effectors appears to contribute to the effects on the actin and microtubule cytoskeleton.135-138 Recently, F11 was shown to interact with the Rho-GAP myosin 9A via a PDZ-like domain. This is the first described example of a functional PDZ-domain in a viral protein and its interaction with myosin 9A is essential to downregulate RhoA activity.136,139 Of particular interest, the ability of F11 to manipulate Rho GTPase signaling contributes to viral invasion in an in vivo intranasal infection model in mice.134
F11-mediated inhibition of RhoA signaling is not the sole mechanism whereby VV interferes with actin regulating signaling pathways. Indeed, newly formed cytoplasmic VV particles use an interaction between the viral integral membrane protein A36 and kinesin to travel along microtubules to the cell periphery, where they fuse with the plasma membrane to form extracellular cell-associated enveloped virus (CEV).140,141 These CEV sit on the cell surface and induce an outside-in signaling cascade initiated by the viral B5 protein to locally activate Src and Abl family kinases, resulting in tyrosine phosphorylation of the viral integral membrane protein A36.142-148 A36 phosphorylation triggers the recruitment of a signaling complex consisting of Grb2, Nck, WASP-interacting protein (WIP), N-WASP and Cdc42, a process that was recently shown to also depend on recruitment of clathrin.144,149-157 This signaling complex stimulates the actin-nucleating activity of the Arp2/3 complex, resulting in actin polymerization-driven propulsion of virus CEV particles to neighboring cells, thereby enhancing cell-to-cell spread of the virus (Fig. 4F).154,155,158-162 While extensive information is available about the viral and host factors that initiate actin polymerization, much less is known about the factors that contribute to subsequent virion release. The host phosphoinositide 5-phosphatase SHIP2 and the viral A34 protein were reported to act as gatekeepers to regulate virion release. SHP2 is recruited to the actin tails by Abl and Src family tyrosine kinases, N-WASP and the viral A34.163
There are indications that this type of virus signaling and spread may be conserved over different poxviruses, like monkeypox virus and variola virus.164 Similar actin-dependent propulsion of virus particles has also been seen upon infection with ASFV, but the underlying signaling pathways are still unknown, although they appear different from VV-induced signaling. Another important difference with VV is that the ASFV virus particles remain intracellular, whereas they are located extracellularly in VV.165
Perturbance of Rho GTPase signaling and concomitant actin rearrangements have also been extensively documented in HIV infections. The HIV Nef (negative factor) protein is a key regulator of these processes. Nef is an important pathogenicity factor in the progression to AIDS. This multifunctional protein is involved in a variety of processes involved in HIV pathogenesis, including replication, immune evasion and spread. Nef interacts with and activates PAK2 in a fragile multiprotein complex that also contains Rac1, and the GEF Vav1. Several potential cellular substrates for the Nef-PAK2 complex have been reported. These include Bad,166 Merlin,167 cofilin,168 and, as recently reported, also paxillin169 and Mek1.170 Nef has been implicated in changes in the actin cytoskeleton late in HIV-infection, consisting of the formation of long cellular extensions, which depend on the GEF activity of Vav.171 In podocytes, Nef induces disassembly of stress fibers and formation of lamellipodia via an interaction with diaphanous interacting protein (DIP), a regulator of RhoA and Rac1 signaling, which induces Vav and p190RhoGAP phosphorylation leading to Rac1 activation and RhoA inhibition.172 In line with this, Nef-expressing podocytes were recently shown to display enhanced Rac1 activity.173 In infected dendritic cells, HIV Nef contributes, via diaphanous 2 (Diaph2), to the formation of virus particle-tipped filopodia that make rapid and multiple contacts with CD4 T cells, which may contribute to HIV spread (Fig. 4G).174
Furthermore, the Nef-PAK2 interaction potently inhibits motility of fibroblasts through accumulation of phosphorylated, inactive cofilin, a process that is highly conserved among different nef alleles of HIV-1, HIV-2, and SIV.168,171,175,176 In addition, Nef has been reported to interact in a PAK2-dependent manner with components of the exocyst complex.177 The exocyst complex is an octameric complex that tethers vesicles at the plasma membrane, regulates polarized exocytosis, and recruits membranes and proteins required for the formation of nanotubes that interconnect cells. The authors therefore proposed that these interactions may be crucial for Nef’s ability to promote nanotube formation and enhance intercellular virus spread.177
Besides Nef, the Tat protein of HIV also causes stress fiber disassembly, in addition to peripheral retraction and ruffle formation in human umbilical vein endothelial cells (HUVEC) and human lung microvascular endothelial cells (HMVEC-L). This process occurs through PAK1 activation, but the biological consequences are not entirely clear.178 Tat can be released from HIV-infected cells and extracellular Tat targets different types of uninfected cells, including endothelial cells.179 The HIV-Tat/αvβ3 integrin interaction leads to activation of FAK, RhoA, NF-κB, and pp60src. This signaling cascade results in vivo in angiogenesis and possibly also in increased endothelial permeability, which may contribute to dissemination of HIV.180
In addition to these well-documented interactions of HIV proteins with Rho GTPase signaling, more general reports indicate that Rho GTPase signaling is involved in HIV egress. Active Cdc42 is involved in HIV budding and release, whereas citron K, a RhoA effector, enhances HIV exocytosis.181,182
Involvement of Rho GTPase signaling in late stages of infection for other viruses is much less documented. Rac1 is activated by the Dengue virus 2 E protein late in infection and may be involved in the interaction between actin and the viral E protein, although the biological consequences are not entirely clear.183 For HCV, Rho GTPase signaling is involved in loss of polarity of hepatocytes upon infection. The HCV core protein downregulates Dlg1 and SHIP2, thereby inhibiting RhoA activity but activating Rac1, which disrupts the apobasal polarity.184
Interactions with the Immune System
Rho GTPase signaling is also involved in the complex interplay between viruses and the immune system. Viral manipulation of Rho GTPase signaling may therefore also affect the ability of the host immune system to overcome a viral infection. As the HIV virus has been intensively studied and infects immune cells, most data in this part are based on HIV research. This section is subdivided in T-lymphocytes, dendritic cells, and monocytes/macrophages, according to the immune cells involved.
T lymphocytes
Since CD4+ T lymphocytes represent the main target cell population of the HIV virus, it is not surprising that the already described Nef-PAK2 interaction is involved in different aspects of the interplay between the virus and T-lymphocytes, affecting T-cell maturation and activation, T cell receptor (TCR) signaling and motility of T-lymphocytes, which all may contribute to the progressive loss of CD4+ T cells that leads to the acquired immunodeficiency syndrome (AIDS).185
At the interface between a T-cell and an antigen-presenting cell or target cell, TCR engagement by MHC-presented antigens triggers actin rearrangements that control receptor clustering and the formation of the immunological synapse (IS) at the contact area between both cells. The IS is highly enriched in Src tyrosine kinases and other signaling molecules that are critical for T cell activation and IS formation depends on cytoskeletal structures, particularly actin dynamics. Evolutionary conserved alterations of IS formation and function by Nef-PAK2 include interference with cell spreading and actin polymerization upon TCR engagement via inhibition of N-WASP and a reduced recruitment of the Src kinase Lck to the IS (Fig. 4H&I).186-188 Nef expression also results in endosomal Lck accumulation but this appears to be PAK independent.187 At the same time, the nucleocapsid protein of HIV-1 promotes localization of virus structural proteins to uropods of polarized T cells, which may contribute to virus transfer via T-cell–T-cell contacts through so-called virological synapses, a process that depends on cytoskeletal remodeling.189,190
Nef-PAK2 also affects T lymphocyte migration, as it leads to inactivation of cofilin, a downstream effector of PAK signaling, which results in reduced chemoattractant-induced T cell motility.168,176 Importantly, the Nef-PAK2 interaction also interferes with T-lymphocyte circulation in vivo. Inhibition of lymph node homing, subendothelial migration, and cell polarization, but not diapedesis, were all found to depend on Nef’s ability to inhibit host cell actin remodeling. Nef-mediated interference with in vivo recirculation of T lymphocytes may compromise T-cell help and therefore may function as a HIV pathogenicity factor.191
In general, increasing evidence supports the hypothesis that the combined effects of Nef-PAK2 on T cell activity and signaling allow sufficient T cell activation necessary for optimal virus replication in this cell type, but at the same time prevent excessive antiviral T cell activation, ultimately leading to an increase in virus replication and spread. This strategy allows Nef to optimize viral replication in CD4+ T lymphocytes and at the same time disturb efficient antiviral immunity.192
Measles virus (MV), a single-stranded, negative-sense, enveloped RNA virus also interferes with actin remodeling in T-cells. CD3/CD28-induced activation of the PI3K/AKT kinase pathway and proliferation is impaired in T cells upon contact with the measles virus glycoprotein (gp) complex. MV exposure results in an almost complete collapse of membrane protrusions in T cells, consistent with their inability to activate Rac1 and Cdc42 in response to TCR ligation. This is associated with reduced phosphorylation levels of cofilin and ERM proteins. Moreover, these events prevent CD3 from being efficiently clustered and redistributed to the central region of the immunological synapse. Thus, by inducing microvillar collapse and interfering with cytoskeletal remodeling, MV signaling disturbs the ability of T cells to adhere, spread, and cluster receptors essential for sustained T-cell activation.193
Dendritic cells
Apart from its effect on T-lymphocytes, HIV also manipulates the dendritic cells (DC) arm of the immune response by triggering DC differentiation and migration. These processes may enhance HIV transmission, as virus can be transferred from DC to the main target cell, CD4+ T lymphocytes. Binding of HIV to DC, especially the interaction between the viral gp120 envelope protein and cellular receptors like DC-SIGN and CCR5, induces Rho GTPase signaling that may contribute to this. Although different types of signaling have been reported upon interaction between HIV and DC-SIGN, they generally appear to be associated with increased viral dissemination. For example, HIV interaction with DC-SIGN has been reported to lead to Rho GTPase signaling via the Rho-GEF LARG, which contributes to viral transfer to T lymphocytes via virus-induced DC-T cell synapses.194 On the other hand, HIV interaction with DC-SIGN on immature dendritic cells has been reported to induce Src/Cdc42/PAK1/WASP signaling, resulting in the formation of membrane extensions that facilitate the transfer of virus particles from DC to CD4+ T-lymphocytes.195 In addition, interaction between HIV-1 gp120 and CCR5 induces a similar signaling pathway through c-Src which results in the activation of several GTPases, including Cdc42. Activated Cdc42 induces complexing of Wasp, LSP1, and the Arp2/3 complex with β-actin, thereby enhancing podosome formation and migration of immature dendritic cells.196,197 Also, through targeting of Vav and Rac1 signaling, recombinant Nef stimulates maturation of immature human DC and increased clustering with and activation of CD4+ T cells, which has been hypothesized to increase viral transmission.198,199 On the other hand, in a murine DC line stably expressing Nef, the Nef-PAK2 connection was found to inhibit DC maturation and antigen presentation, which may impair development of antiviral immunity in vivo.200 Clearly, although the molecular players are diverse and may depend on particular cell types, maturation stages or circumstances, HIV manipulates Rho GTPase signaling in DC in several ways to increase virus transmission to its primary target cell, the CD4+ T lymphocyte and to avoid the development of a potent antiviral immune response.
Plasmacytoid dendritic cells (pDC) form a special subpopulation of DC that is of particular importance in viral infections, as these cells are major producers of type I interferon (IFN). Recently, IFN production upon recognition of HCV infected hepatoma cells by pDCs via CD81/CD9 tetraspanins was found to depend on Rac1 activation.201 This is in line with findings that Rac1 signaling may play a central role in type I IFN production by pDC, as this signaling axis was found to lead to IKK-α phosphorylation and subsequent nuclear translocation of IFN regulatory factor 7 (IRF7), which is a critical event in type I IFN production.202 IRF3 is a known determinant of a strong virus- and dsRNA-induced IFNβ response. For RNA viruses like the human H1N1 and avian H7N7 influenza A virus and Sendai virus, infection resulted in Rac1- and PAK1-dependent activation of IRF3, thereby inducing an antiviral interferon response.
Monocytes/macrophages
Also in monocytes and macrophages, expression of HIV Nef modulates Rho GTPase signaling and particularly leads to activation of the Rac1 signaling axis.
In monocytes, Nef-mediated Rac1 activation results in clathrin- and dynamin-independent endocytosis of immune costimulatory molecules CD80 and CD86, likely involving Rac1-based actin polymerization, which has been associated with viral immune evasion.203,204 Also in monocytes, the HIV-1 matrix protein p17 binds the IL-8 receptor CXCR1. In this way, p17 mimicks IL-8, triggering rapid adhesion and chemotaxis of monocytes through a pathway that involves RhoA/ROCK.205
In macrophages, HIV Nef expression induces Vav-mediated, small GTPase–dependent cytoskeleton remodeling resulting in the formation of cellular projections or nanotubes. Such nanotubes could also be observed in macrophages in tissue from HIV-infected patients, and contacted B-cells. There is evidence indicating that nanotubes may shuttle Nef from macrophages to B cells, which impairs IgG2A and IgA class switching.206
Human rhinovirus also activates Rac1 signaling in macrophages, leading to p38 activation and release of the pro-inflammatory cytokine CCL2, which may contribute to the inflammatory microenvironment in the human airway upon infection with rhinovirus.207
Other interactions with the immune system
A recent report described a fascinating additional mechanism how the Nef-PAK2 signaling axis may influence the immune system and viral replication. In transfection studies, Nef-PAK2 was found to lead to secretion of ADAM proteases in so-called extracellular vesicles (EV), reminiscent of what had been described earlier for melanoma cells. EV formation occurs through the integrin-associated adaptor protein paxillin, and the ADAM proteases in the EV can cleave pro-TNFα to active TNFα. As a consequence, peripheral blood mononuclear cells (PBMCs) that ingested these EV released TNFα, which may promote viral replication by creating a favorable microenvironment.169
Furthermore, HIV-mediated interference with Rho GTPase signaling in endothelial cells may affect brain homeostasis by disrupting the blood-brain barrier (BBB). The BBB prevents the entrance of circulating molecules and immune cells into the central nervous system by forming specialized brain endothelial cells that are connected by tight junctions (TJ). HIV infection of BBB endothelial cells induces activation of RhoA and its downstream effector ROCK. Activated ROCK phosphorylates the tight junction proteins occludin and claudin5 directly, resulting in diminished barrier tightness and enhanced monocyte migration across the BBB.208,209 The Tat protein of HIV is of particular importance in this respect, as it may affect BBB integrity through several mechanisms, including decreasing occludin expression through activation of the RhoA/ROCK signaling pathway, altering the expression of ZO-1 (another tight junction protein) through induction of RhoA signaling and phosphorylation and activation of transcription factor cAMP responsive element binding protein (CREB) and translocating ZO-1 to the nucleus through Rac1 activation.210,211 On the other hand, promotor activity and expression of the P-glycoprotein (P-gp), an ATP-dependent exporter essential for the proper function of the BBB, are upregulated upon Tat-mediated RhoA-expression.210
Transformation
Several virus families are implicated in carcinogenesis.212-215 Viruses that are associated with human cancers include hepatitis B virus (HBV) (liver cancer), papillomaviruses (cervical and other anogenital cancers), EBV (Burkitt lymphoma and nasopharyngeal carcinoma), KSHV (Kaposi sarcoma), and human T-cell lymphotropic virus (adult T-cell leukemia). Loss of cell polarity, increased motility and invasion are among the hallmarks of cancer progression and are associated with cytoskeletal rearrangements. It is therefore not surprising that for all oncoviral families, Rho GTPase signaling is involved to some extent in tumorigenesis.
HTLV-1 infection is associated with clonal expansion and transformation of mature T lymphocytes. The viral protein Tax plays a critical role in the pathogenesis of adult T cell leukemia. The modulation and deregulation of cellular signaling by Tax involves a range of pathways, including direct interaction with Rho GTPases.216
HBV is the major cause of hepatocellular carcinoma (HCC). Several studies reported that the HBX protein enhances cell motility and metastasis by activating the Rho GTPases Rac1 and RhoA/ROCK.217-219 Furthermore, HBX activates PAK1 and Src, thereby triggering Raf phosphorylation and translocation to the mitochondria. Mitochondrial localization of Raf protects cells from stress-induced apoptosis, thus promoting cell survival.220,221 Recently, it was shown that RhoE is frequently downregulated in HBV-associated HCC, and reduced RhoE correlated with more aggressive HCC since RhoE suppresses the RhoA/ROCK axis, leading to reduced invasiveness.222
In tumor cells transformed by human papillomaviruses HPV-16 and HPV-18, the RhoG-specific GEF SGEF was reported to contribute to their invasive capacity. The viral oncoprotein E6 complexes with SGEF and the tumor suppressor human Discs Larg (hDlg), resulting in a strong enhancement of RhoG activity and increased invasive capacity.223
LMP-1, the major EBV transforming protein affects Rho GTPase signaling, leading to the formation of filopodia and actin stress fibers. Interestingly, both processes are regulated by different domains of the LMP-1 protein. Filopodia formation is triggered by Cdc42 through the transmembrane spanning region, while stress fiber formation is induced by RhoA activation through the CTAR1 domain.224,225 This CTAR1 was shown to mediate PI3K/AKT activation which also contributes to the oncogenic properties of the virus, especially by promoting cell survival.224 For another oncogenic gammaherpesvirus, KSHV, the v-FLIP protein activates Rac1 by inducing COX-2 and its inflammatory metabolite PGE-2 to promote inflammation, angiogenesis and invasion.226,227 Another KSHV protein, viral G-protein coupled receptor (vGPCR), contributes to cell transformation by inducing the Rac1-PAK signaling pathway.222,228 Rac1 induction by vGPCR of KSHV infection also contributes to oncogenesis via the activation of ROS production, which promotes proliferation and angiogenesis.222 Also in mouse mammary tumor virus infection, Rac1 and ROS were suggested to be involved in tumorigenesis.229
The small tumor (st) antigen of the polyomavirus SV40 is required for the transforming activity of SV40 in epithelial cells. Expression of the st antigen leads to upregulation of Rac1 and Cdc42 and downregulation of RhoA and concomitant deregulation of the actin cytoskeleton230 and st-mediated activation of the PI3K-AKT-Rac1 signaling pathway involved in transformation.231
Similarly, the oncogenic envelope proteins of the betaretroviruses enzootic nasal tumor virus (ENTV) and Jaagsiekte sheep retrovirus (JSRV) and the oncolytic adenoviral E4ORF1 protein all depend on the PI3K-AKT-mTOR pathway to induce transformation.232,233 For adenovirus, E4ORF1 induces AKT activation through Tiam1 and Rac1.233
Some viruses on the other hand are oncolytic by selectively replicating in and killing tumor cells. Interestingly, for the oncolytic Newcastle disease virus (NDV), Rac1 expression was reported to be sufficient to render non-tumorigenic cells susceptible to NDV replication which identified Rac1 as a potential oncogene.234
Concluding remarks and future perspectives
Rho GTPase signaling and corresponding actin dynamics may be implicated in almost every step of the viral replication cycle. Indeed, accumulating evidence shows that Rho GTPase signaling is involved to a greater or lesser extent in viral entry, gene expression, egress and spread, transformation, and interactions with the immune system. Furthermore, viral infection affects microtubule dynamics and organization through Rho GTPases, which may affect transport of viral particles in and between cells. RhoA, Cdc42, and Rac1 are the most important viral targeted Rho GTPases, but surely other currently un(der)investigated Rho GTPases are also important for viral infection. Crosstalk between these different proteins and associated signaling axes further complicates this field of research.
Viral genomes are compact with limited coding capacity and must rely on host gene products for efficient replication. This interaction of viral and cellular proteins has evolved such that cellular signaling pathways are modulated or bypassed in often fascinating ways. These interactions not only teach us how these signaling molecules participate in the viral replication cycle but also enhance our understanding of the processes in which they are naturally involved. A good example hereof is the use of the macropinocytic entry route by a growing group of viruses. Indeed, as it is becoming increasingly clear that many viruses use macropinocytosis as one of their entry routes, increasing information becomes available about the viral and cellular molecular players involved, which also provides new insights into macropinocytosis in general and the signaling pathways associated with it. Rho GTPase signaling, and more specifically PAK signaling, plays a central role in this context and it is fascinating to see that evolutionary very distinct viruses show substantial similarity in interfering with this signaling pathway to facilitate their entry into host cells.
Manipulation of Rho GTPase signaling is a common aspect of viral entry, although the specific Rho GTPase signaling axes involved are highly variable, depending on the entry route used by the virus. Importantly, evidence is accumulating that entry routes not only differ between different viruses but also between different target cells. The specific impact of the different Rho GTPases and their sometimes conflicting roles in entry processes merit further attention in the future. More insights into the different entry routes and why different mechanisms are used in specific situations and cell types might lead to the development of antiviral drugs that block viral entry in pathogenically important target cells. Interestingly, a RhoA-derived peptide was shown to possess antiviral activity against RSV, PIV-3, and HIV by interfering with syncytium formation and entry,59,235 confirming the potential of targeting small Rho GTPases in antiviral strategies.
For several viruses, efficient intercellular spread depends on Rho GTPase signaling. Indeed, actin-based projection formation enabling the virus to reach and infect neighboring cells is well-documented for diverse viruses. Interestingly, the mechanisms leading to these structurally largely similar cell projections are quite diverse within and between virus families, highlighting the complexity of Rho GTPase signaling and indicating that different manipulations of these signaling networks may lead to similar morphological consequences. Eventually, Rho GTPase signaling often leads to the activation of common downstream proteins. It will therefore be important to obtain clear insights into the downstream targets of Rho signaling, as they may be valuable to identify putative common signaling nodes, potentially allowing the development of antiviral drugs that may interfere with several signaling cascades induced by a virus, or even be active against different virus families. In support of this notion, the identification of downstream effectors of Rho GTPase signaling revealed that MHC phosphorylation is at the crossroad of different signaling pathways induced by influenza A infection, making it a very interesting target in a strategy to reduce viral replication.115,116 Such a strategy to attempt to identify common signaling nodes may be of particular importance in the context of transforming viruses. Since Rho GTPase signaling is involved in the tumorigenic properties of all major oncogenic viruses, it will be particularly interesting to investigate potential signaling similarities. The PI3K/AKT signaling pathway appears to be promising in this respect, and may open important avenues in the search for novel anticancer therapeutics.
Another important question that merits further attention in future research is timing and subcellular localization of specific Rho GTPase activation. Indeed, consequences of Rho GTPase signaling may vary greatly depending on localization, timing and duration of the signal. These considerations add an extra layer of complexity to this field of research. For many viruses, the involvement of Rho GTPase signaling during infection is still largely based on inhibitor studies, making it difficult to extract the exact contribution of these central signaling axes in virus biology.
An aspect of viral interactions with Rho GTPase signaling that appears to be underinvestigated thus far is its contribution to antiviral immunity. This has been quite extensively studied for HIV, with particular emphasis on the Nef-PAK2 signaling axis, but undoubtedly also plays an important role in the biology and pathogenesis of other viruses. A clear view on how Rho GTPase signaling contributes to antiviral immunity and, conversely, how viruses manipulate Rho GTPase signaling to mislead, tamper with or hide from the immune system will be of utmost importance to obtain a realistic view on the antiviral potential of drugs targeting Rho GTPase signaling.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research of H.W.F. is supported by grants from the F.W.O.-Vlaanderen and the Special Research Fund of Ghent University. C.V.D.B. is supported by a post-doc grant of the F.W.O.-Vlaanderen. T. J. is supported by a PhD grant from the Agency for Innovation by Science and Technology in Flanders (IWT-Vlaanderen).
References
- 1.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disanza A, Steffen A, Hertzog M, Frittoli E, Rottner K, Scita G. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci. 2005;62:955–70. doi: 10.1007/s00018-004-4472-6. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 4.Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–6. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- 5.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Aspenström P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–37. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 8.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–54. doi: 10.1016/S0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 10.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 11.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh S, Tominaga T. mDia-interacting protein acts downstream of Rho-mDia and modifies Src activation and stress fiber formation. J Biol Chem. 2001;276:39290–4. doi: 10.1074/jbc.M107026200. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 14.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 15.Matsui T, Yonemura S, Tsukita S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol. 1999;9:1259–62. doi: 10.1016/S0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- 16.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LG, Li R. Actin polymerization: riding the wave. Curr Biol. 2004;14:R109–11. doi: 10.1016/j.cub.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–34. doi: 10.1016/S0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 19.Taylor MP, Koyuncu OO, Enquist LW. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol. 2011;9:427–39. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delorme-Axford E, Coyne CB. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses. 2011;3:2462–77. doi: 10.3390/v3122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barocchi MA, Masignani V, Rappuoli R. Opinion: Cell entry machines: a common theme in nature? Nat Rev Microbiol. 2005;3:349–58. doi: 10.1038/nrmicro1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–45. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 23.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–87. doi: 10.1016/S0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 25.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–20. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Humphries AC, Way M. The non-canonical roles of clathrin and actin in pathogen internalization, egress and spread. Nat Rev Microbiol. 2013;11:551–60. doi: 10.1038/nrmicro3072. [DOI] [PubMed] [Google Scholar]
- 27.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–33. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 28.Ghigo E. A dilemma for viruses and giant viruses: which endocytic pathway to use to enter cells? Intervirology. 2010;53:274–83. doi: 10.1159/000312912. [DOI] [PubMed] [Google Scholar]
- 29.Schelhaas M. Come in and take your coat off - how host cells provide endocytosis for virus entry. Cell Microbiol. 2010;12:1378–88. doi: 10.1111/j.1462-5822.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–20. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Mercer J, Helenius A. Gulping rather than sipping: macropinocytosis as a way of virus entry. Curr Opin Microbiol. 2012;15:490–9. doi: 10.1016/j.mib.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–20. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 33.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–43. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 34.Sayedyahossein S, Dagnino L. Integrins and small GTPases as modulators of phagocytosis. Int Rev Cell Mol Biol. 2013;302:321–54. doi: 10.1016/B978-0-12-407699-0.00006-6. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–25. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelhaas M, Ewers H, Rajamäki ML, Day PM, Schiller JT, Helenius A. Human papillomavirus type 16 entry: retrograde cell surface transport along actin-rich protrusions. PLoS Pathog. 2008;4:e1000148. doi: 10.1371/journal.ppat.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–5. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 38.Zamudio-Meza H, Castillo-Alvarez A, González-Bonilla C, Meza I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J Gen Virol. 2009;90:2902–11. doi: 10.1099/vir.0.014159-0. [DOI] [PubMed] [Google Scholar]
- 39.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–81. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brazzoli M, Bianchi A, Filippini S, Weiner A, Zhu Q, Pizza M, Crotta S. CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes. J Virol. 2008;82:8316–29. doi: 10.1128/JVI.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–72. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–31. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Jiménez-Baranda S, Gómez-Moutón C, Rojas A, Martínez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–46. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 44.Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontow S, Harmon B, Campbell N, Ratner L. Antiviral activity of a Rac GEF inhibitor characterized with a sensitive HIV/SIV fusion assay. Virology. 2007;368:1–6. doi: 10.1016/j.virol.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.del Real G, Jiménez-Baranda S, Mira E, Lacalle RA, Lucas P, Gómez-Moutón C, Alegret M, Peña JM, Rodríguez-Zapata M, Alvarez-Mon M, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–7. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmon B, Campbell N, Ratner L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010;6:e1000956. doi: 10.1371/journal.ppat.1000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, Liu J, Wang W, Vorster PJ, Agulto L, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–92. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly RP, Harman AN, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A. 2010;107:16934–9. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vorster PJ, Guo J, Yoder A, Wang W, Zheng Y, Xu X, Yu D, Spear M, Wu Y. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011;286:12554–64. doi: 10.1074/jbc.M110.182238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoppe S, Schelhaas M, Jaeger V, Liebig T, Petermann P, Knebel-Mörsdorf D. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J Gen Virol. 2006;87:3483–94. doi: 10.1099/vir.0.82231-0. [DOI] [PubMed] [Google Scholar]
- 52.De Regge N, Nauwynck HJ, Geenen K, Krummenacher C, Cohen GH, Eisenberg RJ, Mettenleiter TC, Favoreel HW. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J Cell Biol. 2006;174:267–75. doi: 10.1083/jcb.200510156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frampton AR, Jr., Stolz DB, Uchida H, Goins WF, Cohen JB, Glorioso JC. Equine herpesvirus 1 enters cells by two different pathways, and infection requires the activation of the cellular kinase ROCK1. J Virol. 2007;81:10879–89. doi: 10.1128/JVI.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–21. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isaacson MK, Feire AL, Compton T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. 2007;81:6241–7. doi: 10.1128/JVI.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastey MK, Crowe JE, Jr., Graham BS. RhoA interacts with the fusion glycoprotein of respiratory syncytial virus and facilitates virus-induced syncytium formation. J Virol. 1999;73:7262–70. doi: 10.1128/jvi.73.9.7262-7270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gower TL, Peeples ME, Collins PL, Graham BS. RhoA is activated during respiratory syncytial virus infection. Virology. 2001;283:188–96. doi: 10.1006/viro.2001.0891. [DOI] [PubMed] [Google Scholar]
- 58.Gower TL, Pastey MK, Peeples ME, Collins PL, McCurdy LH, Hart TK, Guth A, Johnson TR, Graham BS. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol. 2005;79:5326–36. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastey MK, Gower TL, Spearman PW, Crowe JE, Jr., Graham BS. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nat Med. 2000;6:35–40. doi: 10.1038/71503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh M, Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J Cell Sci. 1997;110:95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- 61.Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. Dissecting dynamin’s role in clathrin-mediated endocytosis. Biochem Soc Trans. 2009;37:1022–6. doi: 10.1042/BST0371022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–12. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol. 2003;77:7978–90. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–23. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79:1191–206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi’s sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol. 2006;80:11432–46. doi: 10.1128/JVI.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavalli V, Corti M, Gruenberg J. Endocytosis and signaling cascades: a close encounter. FEBS Lett. 2001;498:190–6. doi: 10.1016/S0014-5793(01)02484-X. [DOI] [PubMed] [Google Scholar]
- 68.Lanzetti L, Di Fiore PP, Scita G. Pathways linking endocytosis and actin cytoskeleton in mammalian cells. Exp Cell Res. 2001;271:45–56. doi: 10.1006/excr.2001.5369. [DOI] [PubMed] [Google Scholar]
- 69.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–92. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 70.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 71.Pelkmans L, Püntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 72.Stergiou L, Bauer M, Mair W, Bausch-Fluck D, Drayman N, Wollscheid B, Oppenheim A, Pelkmans L. Integrin-mediated signaling induced by simian virus 40 leads to transient uncoupling of cortical actin and the plasma membrane. PLoS One. 2013;8:e55799. doi: 10.1371/journal.pone.0055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–29. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parton RG, Howes MT. Revisiting caveolin trafficking: the end of the caveosome. J Cell Biol. 2010;191:439–41. doi: 10.1083/jcb.201009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang WR, Wang YC, Chi PI, Wang L, Wang CY, Lin CH, Liu HJ. Cell entry of avian reovirus follows a caveolin-1-mediated and dynamin-2-dependent endocytic pathway that requires activation of p38 mitogen-activated protein kinase (MAPK) and Src signaling pathways as well as microtubules and small GTPase Rab5 protein. J Biol Chem. 2011;286:30780–94. doi: 10.1074/jbc.M111.257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mercer J, Knébel S, Schmidt FI, Crouse J, Burkard C, Helenius A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc Natl Acad Sci U S A. 2010;107:9346–51. doi: 10.1073/pnas.1004618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt FI, Bleck CK, Helenius A, Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30:3647–61. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandgren KJ, Wilkinson J, Miranda-Saksena M, McInerney GM, Byth-Wilson K, Robinson PJ, Cunningham AL. A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells. PLoS Pathog. 2010;6:e1000866. doi: 10.1371/journal.ppat.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell. 2000;11:2497–511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell. 2000;11:2497–511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schelhaas M, Shah B, Holzer M, Blattmann P, Kühling L, Day PM, Schiller JT, Helenius A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012;8:e1002657. doi: 10.1371/journal.ppat.1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nogalski MT, Chan GC, Stevenson EV, Collins-McMillen DK, Yurochko AD. The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes. PLoS Pathog. 2013;9:e1003463. doi: 10.1371/journal.ppat.1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bottero V, Chakraborty S, Chandran B. Reactive oxygen species are induced by Kaposi’s sarcoma-associated herpesvirus early during primary infection of endothelial cells to promote virus entry. J Virol. 2013;87:1733–49. doi: 10.1128/JVI.02958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–21. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karjalainen M, Kakkonen E, Upla P, Paloranta H, Kankaanpää P, Liberali P, Renkema GH, Hyypiä T, Heino J, Marjomäki V. A Raft-derived, Pak1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol Biol Cell. 2008;19:2857–69. doi: 10.1091/mbc.E07-10-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–76. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalia M, Khasa R, Sharma M, Nain M, Vrati S. Japanese encephalitis virus infects neuronal cells through a clathrin-independent endocytic mechanism. J Virol. 2013;87:148–62. doi: 10.1128/JVI.01399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coyne CB, Kim KS, Bergelson JM. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. EMBO J. 2007;26:4016–28. doi: 10.1038/sj.emboj.7601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silva-Ayala D, López T, Gutiérrez M, Perrimon N, López S, Arias CF. Genome-wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry. Proc Natl Acad Sci U S A. 2013;110:10270–5. doi: 10.1073/pnas.1304932110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zambrano JL, Sorondo O, Alcala A, Vizzi E, Diaz Y, Ruiz MC, Michelangeli F, Liprandi F, Ludert JE. Rotavirus infection of cells in culture induces activation of RhoA and changes in the actin and tubulin cytoskeleton. PLoS One. 2012;7:e47612. doi: 10.1371/journal.pone.0047612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laakkonen JP, Mäkelä AR, Kakkonen E, Turkki P, Kukkonen S, Peränen J, Ylä-Herttuala S, Airenne KJ, Oker-Blom C, Vihinen-Ranta M, et al. Clathrin-independent entry of baculovirus triggers uptake of E. coli in non-phagocytic human cells. PLoS One. 2009;4:e5093. doi: 10.1371/journal.pone.0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Misinzo G, Delputte PL, Lefebvre DJ, Nauwynck HJ. Porcine circovirus 2 infection of epithelial cells is clathrin-, caveolae- and dynamin-independent, actin and Rho-GTPase-mediated, and enhanced by cholesterol depletion. Virus Res. 2009;139:1–9. doi: 10.1016/j.virusres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Lyman MG, Enquist LW. Herpesvirus interactions with the host cytoskeleton. J Virol. 2009;83:2058–66. doi: 10.1128/JVI.01718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Döhner K, Sodeik B. The role of the cytoskeleton during viral infection. Curr Top Microbiol Immunol. 2005;285:67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- 95.Radtke K, Döhner K, Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker’s guide to the cell. Cell Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 96.Greber UF, Way M. A superhighway to virus infection. Cell. 2006;124:741–54. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Dodding MP, Way M. Coupling viruses to dynein and kinesin-1. EMBO J. 2011;30:3527–39. doi: 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol. 2007;81:7941–59. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frampton AR, Jr., Uchida H, von Einem J, Goins WF, Grandi P, Cohen JB, Osterrieder N, Glorioso JC. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet Microbiol. 2010;141:12–21. doi: 10.1016/j.vetmic.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warren JC, Cassimeris L. The contributions of microtubule stability and dynamic instability to adenovirus nuclear localization efficiency. Cell Motil Cytoskeleton. 2007;64:675–89. doi: 10.1002/cm.20215. [DOI] [PubMed] [Google Scholar]
- 101.Warren JC, Rutkowski A, Cassimeris L. Infection with replication-deficient adenovirus induces changes in the dynamic instability of host cell microtubules. Mol Biol Cell. 2006;17:3557–68. doi: 10.1091/mbc.E05-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quetglas JI, Hernáez B, Galindo I, Muñoz-Moreno R, Cuesta-Geijo MA, Alonso C. Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection. J Virol. 2012;86:1758–67. doi: 10.1128/JVI.05666-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Zhang H, Solski PA, Hart MJ, Der CJ, Su L. Modulation of HIV-1 replication by a novel RhoA effector activity. J Immunol. 2000;164:5369–74. doi: 10.4049/jimmunol.164.10.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Helms WS, Jeffrey JL, Holmes DA, Townsend MB, Clipstone NA, Su L. Modulation of NFAT-dependent gene expression by the RhoA signaling pathway in T cells. J Leukoc Biol. 2007;82:361–9. doi: 10.1189/jlb.0206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cook JA, Albacker L, August A, Henderson AJ. CD28-dependent HIV-1 transcription is associated with Vav, Rac, and NF-kappa B activation. J Biol Chem. 2003;278:35812–8. doi: 10.1074/jbc.M302878200. [DOI] [PubMed] [Google Scholar]
- 106.Jiang W, Wang Q, Chen S, Gao S, Song L, Liu P, Huang W. Influenza A virus NS1 induces G0/G1 cell cycle arrest by inhibiting the expression and activity of RhoA protein. J Virol. 2013;87:3039–52. doi: 10.1128/JVI.03176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan CP, Siu YT, Kok KH, Ching YP, Tang HM, Jin DY. Group I p21-activated kinases facilitate Tax-mediated transcriptional activation of the human T-cell leukemia virus type 1 long terminal repeats. Retrovirology. 2013;10 doi: 10.1186/1742-4690-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishida H, Li K, Yi M, Lemon SM. p21-activated kinase 1 is activated through the mammalian target of rapamycin/p70 S6 kinase pathway and regulates the replication of hepatitis C virus in human hepatoma cells. J Biol Chem. 2007;282:11836–48. doi: 10.1074/jbc.M610106200. [DOI] [PubMed] [Google Scholar]
- 109.Rodrigues L, Pires de Miranda M, Caloca MJ, Bustelo XR, Simas JP. Activation of Vav by the gammaherpesvirus M2 protein contributes to the establishment of viral latency in B lymphocytes. J Virol. 2006;80:6123–35. doi: 10.1128/JVI.02700-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madureira PA, Matos P, Soeiro I, Dixon LK, Simas JP, Lam EW. Murine gamma-herpesvirus 68 latency protein M2 binds to Vav signaling proteins and inhibits B-cell receptor-induced cell cycle arrest and apoptosis in WEHI-231 B cells. J Biol Chem. 2005;280:37310–8. doi: 10.1074/jbc.M507478200. [DOI] [PubMed] [Google Scholar]
- 111.Krishnan V, Zeichner SL. Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. J Virol. 2004;78:9458–73. doi: 10.1128/JVI.78.17.9458-9473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S, Li H, Chen Y, Wei H, Gao GF, Liu H, Huang S, Chen JL. Transport of influenza virus neuraminidase (NA) to host cell surface is regulated by ARHGAP21 and Cdc42 proteins. J Biol Chem. 2012;287:9804–16. doi: 10.1074/jbc.M111.312959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hui EK, Barman S, Tang DH, France B, Nayak DP. YRKL sequence of influenza virus M1 functions as the L domain motif and interacts with VPS28 and Cdc42. J Virol. 2006;80:2291–308. doi: 10.1128/JVI.80.5.2291-2308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Husain M, Harrod KS. Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett. 2011;585:128–32. doi: 10.1016/j.febslet.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 115.Pascua PN, Lee JH, Song MS, Park SJ, Baek YH, Ann BH, Shin EY, Kim EG, Choi YK. Role of the p21-activated kinases (PAKs) in influenza A virus replication. Biochem Biophys Res Commun. 2011;414:569–74. doi: 10.1016/j.bbrc.2011.09.119. [DOI] [PubMed] [Google Scholar]
- 116.Haidari M, Zhang W, Ganjehei L, Ali M, Chen Z. Inhibition of MLC phosphorylation restricts replication of influenza virus--a mechanism of action for anti-influenza agents. PLoS One. 2011;6:e21444. doi: 10.1371/journal.pone.0021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murata T, Goshima F, Daikoku T, Takakuwa H, Nishiyama Y. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells. 2000;5:1017–27. doi: 10.1046/j.1365-2443.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 118.Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J Virol. 2003;77:9074–80. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calton CM, Randall JA, Adkins MW, Banfield BW. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes. 2004;29:131–45. doi: 10.1023/B:VIRU.0000032796.27878.7f. [DOI] [PubMed] [Google Scholar]
- 120.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci U S A. 2005;102:8990–5. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schumacher D, Tischer BK, Trapp S, Osterrieder N. The protein encoded by the US3 orthologue of Marek’s disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J Virol. 2005;79:3987–97. doi: 10.1128/JVI.79.7.3987-3997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci U S A. 2009;106:8707–12. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacob T, Van den Broeke C, van Troys M, Waterschoot D, Ampe C, Favoreel HW. Alphaherpesviral US3 kinase induces cofilin dephosphorylation to reorganize the actin cytoskeleton. J Virol. 2013;87:4121–6. doi: 10.1128/JVI.03107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiang Y, Zheng K, Ju H, Wang S, Pei Y, Ding W, Chen Z, Wang Q, Qiu X, Zhong M, et al. Cofilin 1-mediated biphasic F-actin dynamics of neuronal cells affect herpes simplex virus 1 infection and replication. J Virol. 2012;86:8440–51. doi: 10.1128/JVI.00609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naghavi MH, Gundersen GG, Walsh D. Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread. Proc Natl Acad Sci U S A. 2013;110:18268–73. doi: 10.1073/pnas.1310760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van den Broeke C, Deruelle M, Nauwynck HJ, Coller KE, Smith GA, Van Doorsselaere J, Favoreel HW. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology. 2009;385:155–60. doi: 10.1016/j.virol.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 127.Brzozowska A, Rychłowski M, Lipińska AD, Bieńkowska-Szewczyk K. Point mutations in BHV-1 Us3 gene abolish its ability to induce cytoskeletal changes in various cell types. Vet Microbiol. 2010;143:8–13. doi: 10.1016/j.vetmic.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 128.Finnen RL, Banfield BW. Subcellular localization of the alphaherpesvirus serine/threonine kinase Us3 as a determinant of Us3 function. Virulence. 2010;1:291–4. doi: 10.4161/viru.1.4.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Finnen RL, Roy BB, Zhang H, Banfield BW. Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology. 2010;397:23–33. doi: 10.1016/j.virol.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schumacher D, McKinney C, Kaufer BB, Osterrieder N. Enzymatically inactive U(S)3 protein kinase of Marek’s disease virus (MDV) is capable of depolymerizing F-actin but results in accumulation of virions in perinuclear invaginations and reduced virus growth. Virology. 2008;375:37–47. doi: 10.1016/j.virol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richerioux N, Blondeau C, Wiedemann A, Rémy S, Vautherot JF, Denesvre C. Rho-ROCK and Rac-PAK signaling pathways have opposing effects on the cell-to-cell spread of Marek’s Disease Virus. PLoS One. 2012;7:e44072. doi: 10.1371/journal.pone.0044072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loesing JB, Di Fiore S, Ritter K, Fischer R, Kleines M. Epstein-Barr virus BDLF2-BMRF2 complex affects cellular morphology. J Gen Virol. 2009;90:1440–9. doi: 10.1099/vir.0.009571-0. [DOI] [PubMed] [Google Scholar]
- 133.Gill MB, Edgar R, May JS, Stevenson PG. A gamma-herpesvirus glycoprotein complex manipulates actin to promote viral spread. PLoS One 2008; 3:e1808. [DOI] [PMC free article] [PubMed]
- 134.Morales I, Carbajal MA, Bohn S, Holzer D, Kato SE, Greco FA, Moussatché N, Krijnse Locker J. The vaccinia virus F11L gene product facilitates cell detachment and promotes migration. Traffic. 2008;9:1283–98. doi: 10.1111/j.1600-0854.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 135.Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science. 2006;311:377–81. doi: 10.1126/science.1122411. [DOI] [PubMed] [Google Scholar]
- 136.Arakawa Y, Cordeiro JV, Schleich S, Newsome TP, Way M. The release of vaccinia virus from infected cells requires RhoA-mDia modulation of cortical actin. Cell Host Microbe. 2007;1:227–40. doi: 10.1016/j.chom.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 137.Arakawa Y, Cordeiro JV, Way M. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe. 2007;1:213–26. doi: 10.1016/j.chom.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 138.Cordeiro JV, Guerra S, Arakawa Y, Dodding MP, Esteban M, Way M. F11-mediated inhibition of RhoA signalling enhances the spread of vaccinia virus in vitro and in vivo in an intranasal mouse model of infection. PLoS One. 2009;4:e8506. doi: 10.1371/journal.pone.0008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Handa Y, Durkin CH, Dodding MP, Way M. Vaccinia virus F11 promotes viral spread by acting as a PDZ-containing scaffolding protein to bind myosin-9A and inhibit RhoA signaling. Cell Host Microbe. 2013;14:51–62. doi: 10.1016/j.chom.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 140.Ward BM, Moss B. Vaccinia virus A36R membrane protein provides a direct link between intracellular enveloped virions and the microtubule motor kinesin. J Virol. 2004;78:2486–93. doi: 10.1128/JVI.78.5.2486-2493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rietdorf J, Ploubidou A, Reckmann I, Holmström A, Frischknecht F, Zettl M, Zimmermann T, Way M. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat Cell Biol. 2001;3:992–1000. doi: 10.1038/ncb1101-992. [DOI] [PubMed] [Google Scholar]
- 142.Katz E, Ward BM, Weisberg AS, Moss B. Mutations in the vaccinia virus A33R and B5R envelope proteins that enhance release of extracellular virions and eliminate formation of actin-containing microvilli without preventing tyrosine phosphorylation of the A36R protein. J Virol. 2003;77:12266–75. doi: 10.1128/JVI.77.22.12266-12275.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–31. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 144.Frischknecht F, Moreau V, Röttger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–9. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 145.Newsome TP, Scaplehorn N, Way M. SRC mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science. 2004;306:124–9. doi: 10.1126/science.1101509. [DOI] [PubMed] [Google Scholar]
- 146.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–8. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 147.Reeves PM, Bommarius B, Lebeis S, McNulty S, Christensen J, Swimm A, Chahroudi A, Chavan R, Feinberg MB, Veach D, et al. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat Med. 2005;11:731–9. doi: 10.1038/nm1265. [DOI] [PubMed] [Google Scholar]
- 148.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–55. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–8. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 150.Scaplehorn N, Holmström A, Moreau V, Frischknecht F, Reckmann I, Way M. Grb2 and Nck act cooperatively to promote actin-based motility of vaccinia virus. Curr Biol. 2002;12:740–5. doi: 10.1016/S0960-9822(02)00812-6. [DOI] [PubMed] [Google Scholar]
- 151.Snapper SB, Takeshima F, Antón I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3:897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- 152.Zettl M, Way M. The WH1 and EVH1 domains of WASP and Ena/VASP family members bind distinct sequence motifs. Curr Biol. 2002;12:1617–22. doi: 10.1016/S0960-9822(02)01112-0. [DOI] [PubMed] [Google Scholar]
- 153.Humphries AC, Dodding MP, Barry DJ, Collinson LM, Durkin CH, Way M. Clathrin potentiates vaccinia-induced actin polymerization to facilitate viral spread. Cell Host Microbe. 2012;12:346–59. doi: 10.1016/j.chom.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 154.Weisswange I, Newsome TP, Schleich S, Way M. The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature. 2009;458:87–91. doi: 10.1038/nature07773. [DOI] [PubMed] [Google Scholar]
- 155.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–8. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 156.Donnelly SK, Weisswange I, Zettl M, Way M. WIP provides an essential link between Nck and N-WASP during Arp2/3-dependent actin polymerization. Curr Biol. 2013;23:999–1006. doi: 10.1016/j.cub.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Humphries AC, Donnelly SK, Way M. Cdc42 and the RhoGEF Intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci. 2013 doi: 10.1242/jcs.141366. [DOI] [PubMed] [Google Scholar]
- 158.Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 2001;11:30–8. doi: 10.1016/S0962-8924(00)01871-7. [DOI] [PubMed] [Google Scholar]
- 159.Cudmore S, Reckmann I, Griffiths G, Way M. Vaccinia virus: a model system for actin-membrane interactions. J Cell Sci. 1996;109:1739–47. doi: 10.1242/jcs.109.7.1739. [DOI] [PubMed] [Google Scholar]
- 160.Hollinshead M, Rodger G, Van Eijl H, Law M, Hollinshead R, Vaux DJ, Smith GL. Vaccinia virus utilizes microtubules for movement to the cell surface. J Cell Biol. 2001;154:389–402. doi: 10.1083/jcb.200104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ward BM, Moss B. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J Virol. 2001;75:11651–63. doi: 10.1128/JVI.75.23.11651-11663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rodger G, Smith GL. Replacing the SCR domains of vaccinia virus protein B5R with EGFP causes a reduction in plaque size and actin tail formation but enveloped virions are still transported to the cell surface. J Gen Virol. 2002;83:323–32. doi: 10.1099/0022-1317-83-2-323. [DOI] [PubMed] [Google Scholar]
- 163.McNulty S, Powell K, Erneux C, Kalman D. The host phosphoinositide 5-phosphatase SHIP2 regulates dissemination of vaccinia virus. J Virol. 2011;85:7402–10. doi: 10.1128/JVI.02391-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reeves PM, Smith SK, Olson VA, Thorne SH, Bornmann W, Damon IK, Kalman D. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J Virol. 2011;85:21–31. doi: 10.1128/JVI.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jouvenet N, Windsor M, Rietdorf J, Hawes P, Monaghan P, Way M, Wileman T. African swine fever virus induces filopodia-like projections at the plasma membrane. Cell Microbiol. 2006;8:1803–11. doi: 10.1111/j.1462-5822.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 166.Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d’Aloja P, Schürmann A, Baur AS. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7:1217–24. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- 167.Wei BL, Arora VK, Raney A, Kuo LS, Xiao GH, O’Neill E, Testa JR, Foster JL, Garcia JV. Activation of p21-activated kinase 2 by human immunodeficiency virus type 1 Nef induces merlin phosphorylation. J Virol. 2005;79:14976–80. doi: 10.1128/JVI.79.23.14976-14980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stolp B, Reichman-Fried M, Abraham L, Pan X, Giese SI, Hannemann S, Goulimari P, Raz E, Grosse R, Fackler OT. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe. 2009;6:174–86. doi: 10.1016/j.chom.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 169.Lee JH, Wittki S, Bräu T, Dreyer FS, Krätzel K, Dindorf J, Johnston IC, Gross S, Kremmer E, Zeidler R, et al. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol Cell. 2013;49:668–79. doi: 10.1016/j.molcel.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 170.Kouwenhoven A, Minassian VD, Marsh JW. HIV-1 Nef mediates Pak phosphorylation of Mek1 serine298 and elicits an active phospho-state of Pak2. Curr HIV Res. 2013;11:198–209. doi: 10.2174/1570162X113119990039. [DOI] [PubMed] [Google Scholar]
- 171.Fackler OT, Luo W, Geyer M, Alberts AS, Peterlin BM. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–39. doi: 10.1016/S1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 172.Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–82. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tan R, Patni H, Tandon P, Luan L, Sharma B, Salhan D, Saleem MA, Mathieson PW, Malhotra A, Husain M, et al. Nef interaction with actin compromises human podocyte actin cytoskeletal integrity. Exp Mol Pathol. 2013;94:51–7. doi: 10.1016/j.yexmp.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aggarwal A, Iemma TL, Shih I, Newsome TP, McAllery S, Cunningham AL, Turville SG. Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells. PLoS Pathog. 2012;8:e1002762. doi: 10.1371/journal.ppat.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, Machu C, Renaud O, Prévost MC, Hivroz C, Schwartz O, et al. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J Virol. 2010;84:2282–93. doi: 10.1128/JVI.02230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stolp B, Abraham L, Rudolph JM, Fackler OT. Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling. J Virol. 2010;84:3935–48. doi: 10.1128/JVI.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology. 2012;9:33. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu RF, Gu Y, Xu YC, Mitola S, Bussolino F, Terada LS. Human immunodeficiency virus type 1 Tat regulates endothelial cell actin cytoskeletal dynamics through PAK1 activation and oxidant production. J Virol. 2004;78:779–89. doi: 10.1128/JVI.78.2.779-789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rusnati M, Presta M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis. 2002;5:141–51. doi: 10.1023/A:1023892223074. [DOI] [PubMed] [Google Scholar]
- 180.Urbinati C, Mitola S, Tanghetti E, Kumar C, Waltenberger J, Ribatti D, Presta M, Rusnati M. Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:2315–20. doi: 10.1161/01.ATV.0000186182.14908.7b. [DOI] [PubMed] [Google Scholar]
- 181.Audoly G, Popoff MR, Gluschankof P. Involvement of a small GTP binding protein in HIV-1 release. Retrovirology. 2005;2:48. doi: 10.1186/1742-4690-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Loomis RJ, Holmes DA, Elms A, Solski PA, Der CJ, Su L. Citron kinase, a RhoA effector, enhances HIV-1 virion production by modulating exocytosis. Traffic. 2006;7:1643–53. doi: 10.1111/j.1600-0854.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang JL, Zhang JL, Chen W, Xu XF, Gao N, Fan DY, An J. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Awad A, Sar S, Barré R, Cariven C, Marin M, Salles JP, Erneux C, Samuel D, Gassama-Diagne A. SHIP2 regulates epithelial cell polarity through its lipid product, which binds to Dlg1, a pathway subverted by hepatitis C virus core protein. Mol Biol Cell. 2013;24:2171–85. doi: 10.1091/mbc.E12-08-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stove V, Naessens E, Stove C, Swigut T, Plum J, Verhasselt B. Signaling but not trafficking function of HIV-1 protein Nef is essential for Nef-induced defects in human intrathymic T-cell development. Blood. 2003;102:2925–32. doi: 10.1182/blood-2003-03-0833. [DOI] [PubMed] [Google Scholar]
- 186.Rudolph JM, Eickel N, Haller C, Schindler M, Fackler OT. Inhibition of T-cell receptor-induced actin remodeling and relocalization of Lck are evolutionarily conserved activities of lentiviral Nef proteins. J Virol. 2009;83:11528–39. doi: 10.1128/JVI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haller C, Rauch S, Fackler OT. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS One. 2007;2:e1212. doi: 10.1371/journal.pone.0001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haller C, Rauch S, Michel N, Hannemann S, Lehmann MJ, Keppler OT, Fackler OT. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J Biol Chem. 2006;281:19618–30. doi: 10.1074/jbc.M513802200. [DOI] [PubMed] [Google Scholar]
- 189.Jolly C. T cell polarization at the virological synapse. Viruses. 2010;2:1261–78. doi: 10.3390/v2061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Llewellyn GN, Hogue IB, Grover JR, Ono A. Nucleocapsid promotes localization of HIV-1 gag to uropods that participate in virological synapses between T cells. PLoS Pathog. 2010;6:e1001167. doi: 10.1371/journal.ppat.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stolp B, Imle A, Coelho FM, Hons M, Gorina R, Lyck R, Stein JV, Fackler OT. HIV-1 Nef interferes with T-lymphocyte circulation through confined environments in vivo. Proc Natl Acad Sci U S A. 2012;109:18541–6. doi: 10.1073/pnas.1204322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abraham L, Fackler OT. HIV-1 Nef: a multifaceted modulator of T cell receptor signaling. Cell Commun Signal. 2012;10:39. doi: 10.1186/1478-811X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Müller N, Avota E, Schneider-Schaulies J, Harms H, Krohne G, Schneider-Schaulies S. Measles virus contact with T cells impedes cytoskeletal remodeling associated with spreading, polarization, and CD3 clustering. Traffic. 2006;7:849–58. doi: 10.1111/j.1600-0854.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 194.Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–77. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 195.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–52. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Anand AR, Prasad A, Bradley RR, Deol YS, Nagaraja T, Ren X, Terwilliger EF, Ganju RK. HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1. Blood. 2009;114:3588–600. doi: 10.1182/blood-2009-02-206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Prasad A, Kuzontkoski PM, Shrivastava A, Zhu W, Li DY, Groopman JE. Slit2N/Robo1 inhibit HIV-gp120-induced migration and podosome formation in immature dendritic cells by sequestering LSP1 and WASp. PLoS One. 2012;7:e48854. doi: 10.1371/journal.pone.0048854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Quaranta MG, Mattioli B, Spadaro F, Straface E, Giordani L, Ramoni C, Malorni W, Viora M. HIV-1 Nef triggers Vav-mediated signaling pathway leading to functional and morphological differentiation of dendritic cells. FASEB J. 2003;17:2025–36. doi: 10.1096/fj.03-0272com. [DOI] [PubMed] [Google Scholar]
- 199.Quaranta MG, Tritarelli E, Giordani L, Viora M. HIV-1 Nef induces dendritic cell differentiation: a possible mechanism of uninfected CD4(+) T cell activation. Exp Cell Res. 2002;275:243–54. doi: 10.1006/excr.2002.5497. [DOI] [PubMed] [Google Scholar]
- 200.Mann J, Patrick CN, Cragg MS, Honeychurch J, Mann DA, Harris M. Functional analysis of HIV type 1 Nef reveals a role for PAK2 as a regulator of cell phenotype and function in the murine dendritic cell line, DC2.4. J Immunol. 2005;175:6560–9. doi: 10.4049/jimmunol.175.10.6560. [DOI] [PubMed] [Google Scholar]
- 201.Zhang S, Kodys K, Babcock GJ, Szabo G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology. 2013;58:940–9. doi: 10.1002/hep.25827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med. 2010;207:721–30. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chaudhry A, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S. A two-pronged mechanism for HIV-1 Nef-mediated endocytosis of immune costimulatory molecules CD80 and CD86. Cell Host Microbe. 2007;1:37–49. doi: 10.1016/j.chom.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 204.Chaudhry A, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S. HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic. 2008;9:1925–35. doi: 10.1111/j.1600-0854.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- 205.Giagulli C, Magiera AK, Bugatti A, Caccuri F, Marsico S, Rusnati M, Vermi W, Fiorentini S, Caruso A. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood. 2012;119:2274–83. doi: 10.1182/blood-2011-06-364083. [DOI] [PubMed] [Google Scholar]
- 206.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–17. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schreiber MT, Schuler B, Li L, Hall DJ. Activation of the small G-protein Rac by human rhinovirus attenuates the TLR3/IFN-α axis while promoting CCL2 release in human monocyte-lineage cells. Innate Immun. 2013;19:278–89. doi: 10.1177/1753425912460709. [DOI] [PubMed] [Google Scholar]
- 208.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–33. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–80. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhong Y, Hennig B, Toborek M. Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells. J Cereb Blood Flow Metab. 2010;30:522–33. doi: 10.1038/jcbfm.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 2012;1436:13–9. doi: 10.1016/j.brainres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 212.Avanzi S, Alvisi G, Ripalti A. How virus persistence can initiate the tumorigenesis process. World J Virol. 2013;2:102–9. doi: 10.5501/wjv.v2.i2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Münger K, Hayakawa H, Nguyen CL, Melquiot NV, Duensing A, Duensing S. Viral carcinogenesis and genomic instability. EXS. 2006;•••:179–99. doi: 10.1007/3-7643-7378-4_8. [DOI] [PubMed] [Google Scholar]
- 214.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 215.Duelli DM, Padilla-Nash HM, Berman D, Murphy KM, Ried T, Lazebnik Y. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr Biol. 2007;17:431–7. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 216.Hall WW, Fujii M. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene. 2005;24:5965–75. doi: 10.1038/sj.onc.1208975. [DOI] [PubMed] [Google Scholar]
- 217.Feng H, Zhang J, Li X, Chen WN. HBX-mediated migration of HBV-replicating HepG2 cells: insights on development of hepatocellular carcinoma. J Biomed Biotechnol 2009; 2009:930268. [DOI] [PMC free article] [PubMed]
- 218.Lara-Pezzi E, Serrador JM, Montoya MC, Zamora D, Yáñez-Mó M, Carretero M, Furthmayr H, Sánchez-Madrid F, López-Cabrera M. The hepatitis B virus X protein (HBx) induces a migratory phenotype in a CD44-dependent manner: possible role of HBx in invasion and metastasis. Hepatology. 2001;33:1270–81. doi: 10.1053/jhep.2001.1270. [DOI] [PubMed] [Google Scholar]
- 219.Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600–12. doi: 10.1016/j.jhep.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 220.Chen S, Belikova NA, Subbaiah PV. Structural elucidation of molecular species of pacific oyster ether amino phospholipids by normal-phase liquid chromatography/negative-ion electrospray ionization and quadrupole/multiple-stage linear ion-trap mass spectrometry. Anal Chim Acta. 2012;735:76–89. doi: 10.1016/j.aca.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen J, Siddiqui A. Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J Virol. 2007;81:6757–60. doi: 10.1128/JVI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ma W, Wong CC, Tung EK, Wong CM, Ng IO. RhoE is frequently down-regulated in hepatocellular carcinoma (HCC) and suppresses HCC invasion through antagonizing the Rho/Rho-kinase/myosin phosphatase target pathway. Hepatology. 2013;57:152–61. doi: 10.1002/hep.25987. [DOI] [PubMed] [Google Scholar]
- 223.Narayan N, Subbaiah VK, Banks L. The high-risk HPV E6 oncoprotein preferentially targets phosphorylated nuclear forms of hDlg. Virology. 2009;387:1–4. doi: 10.1016/j.virol.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 224.Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694–704. doi: 10.1074/jbc.M209840200. [DOI] [PubMed] [Google Scholar]
- 225.Puls A, Eliopoulos AG, Nobes CD, Bridges T, Young LS, Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF(alpha) and IL-1, and by the Epstein-Barr virus transforming protein LMP1. J Cell Sci. 1999;112:2983–92. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- 226.Sharma-Walia N, Patel K, Chandran K, Marginean A, Bottero V, Kerur N, Paul AG. COX-2/PGE2: molecular ambassadors of Kaposi’s sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis. 2012;1 doi: 10.1038/oncsis.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003;63:8837–47. [PubMed] [Google Scholar]
- 229.McHenry PR, Sears JC, Herrick MP, Chang P, Heckman-Stoddard BM, Rybarczyk M, Chodosh LA, Gunther EJ, Hilsenbeck SG, Rosen JM, et al. P190B RhoGAP has pro-tumorigenic functions during MMTV-Neu mammary tumorigenesis and metastasis. Breast Cancer Res. 2010;12:R73. doi: 10.1186/bcr2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol. 2003;77:2807–18. doi: 10.1128/JVI.77.5.2807-2818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, Hahn WC. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–95. doi: 10.1016/S1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 232.Maeda N, Fan H. Signal transduction pathways utilized by enzootic nasal tumor virus (ENTV-1) envelope protein in transformation of rat epithelial cells resemble those used by jaagsiekte sheep retrovirus. Virus Genes. 2008;36:147–55. doi: 10.1007/s11262-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 233.Thomas MA, Broughton RS, Goodrum FD, Ornelles DA. E4orf1 limits the oncolytic potential of the E1B-55K deletion mutant adenovirus. J Virol. 2009;83:2406–16. doi: 10.1128/JVI.01972-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Puhlmann J, Puehler F, Mumberg D, Boukamp P, Beier R. Rac1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene. 2010;29:2205–16. doi: 10.1038/onc.2009.507. [DOI] [PubMed] [Google Scholar]
- 235.Maselko M, Ward C, Pastey M. A RhoA-derived peptide inhibits human immunodeficiency virus-1 entry in vitro. Curr HIV Res. 2011;9:1–5. doi: 10.2174/157016211794582605. [DOI] [PubMed] [Google Scholar]