Abstract
Information on the structure of binding sites of wheat germ agglutinin was obtained on the basis of fluorescence and phosphorescence changes of tryptophan residues induced by the binding of several thiomercuribenzoate derivatives of glycosides. The thiomercuribenzoate derivatives bind selectively to wheat germ agglutinin in the same way as the corresponding sugars. Using the thiomercuribenzoate of di-N-acetyl-β-chitobiose, it was found that: (i) the fluorescence of tryptophan residues was drastically quenched at both 298 and 77 K; (ii) the phosphorescence intensity was strongly enhanced at 77 K; (iii) the phosphorescence lifetime was markedly decreased. A similar effect was observed with the thiomercuribenzoate of N-acetyl-β-D-glucosamine. These changes were completely reversed upon addition of 1-O-methyl-di-N-acetyl-β-chitobioside. The thiomercuribenzoate of β-D-glucose had no effect at all, and the thiomercuribenzoate of tri-N-acetyl-β-chitotriose had a limited effect. These results are interpreted as a specific heavy atom effect due to a close contact between one tryptophan residue of the protein and the heavy atom of the bound ligand. They are consistent with the view that: (i) binding sites of wheat germ agglutinin may be divided in three subsites, A, B, and C; (ii) a tryptophan residue is in the binding site at subsite C; and (iii) this residue and the ligand are in close contact. This new method, using the enhancement of spin-orbit coupling due to the selective perturbation induced in a tryptophan residue by a ligand containing a heavy atom, has proved to be suitable for locating the tryptophan residue in the binding site of wheat germ agglutinin and can probably be extended to other sugar-binding proteins.
Keywords: heavy atom effect, lectin, protein-sugar interaction
Full text
PDF
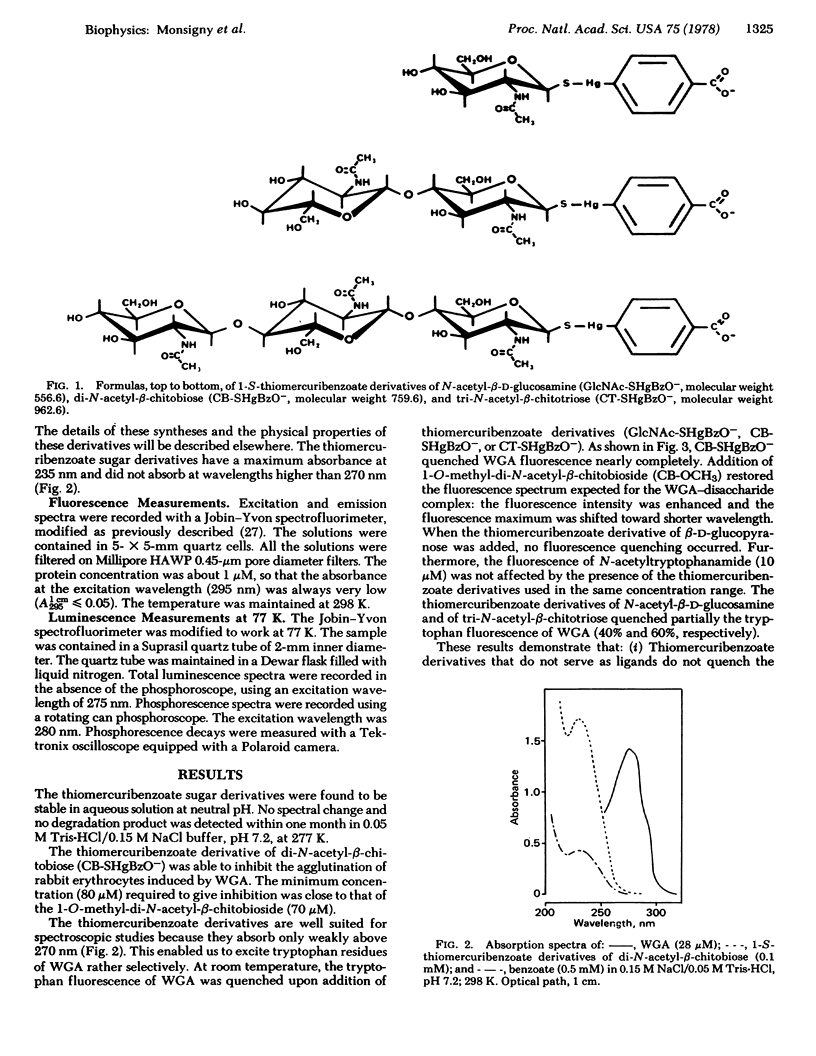
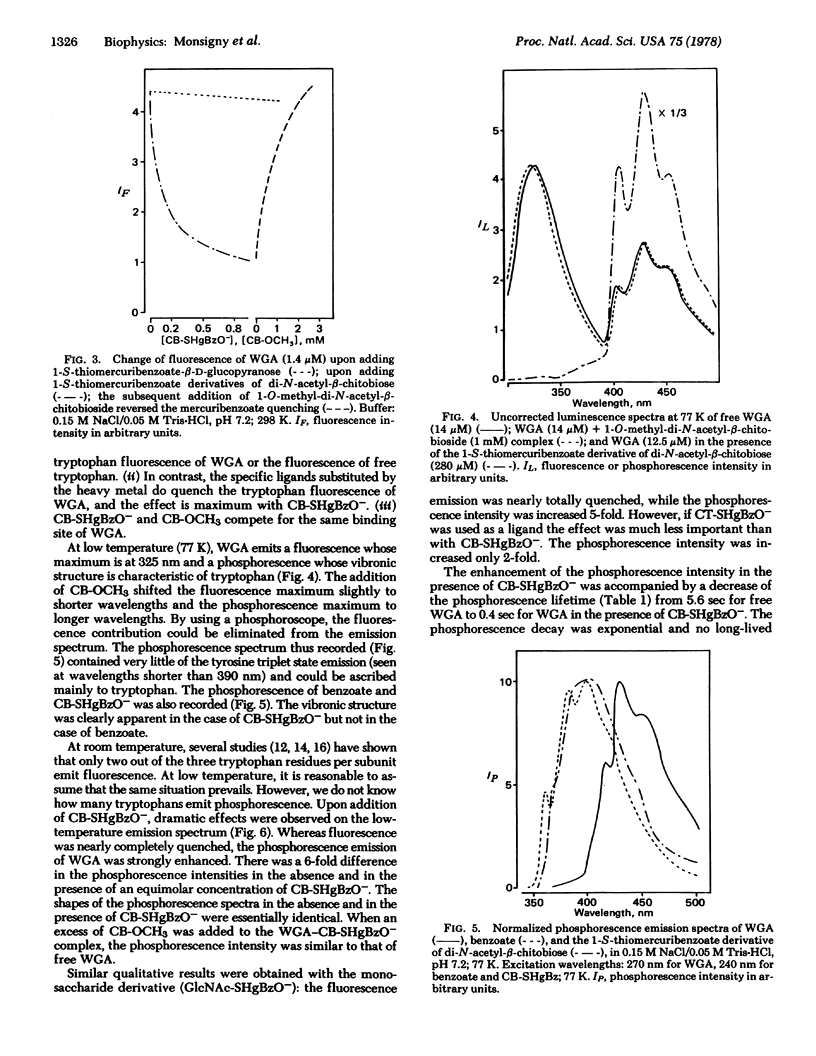
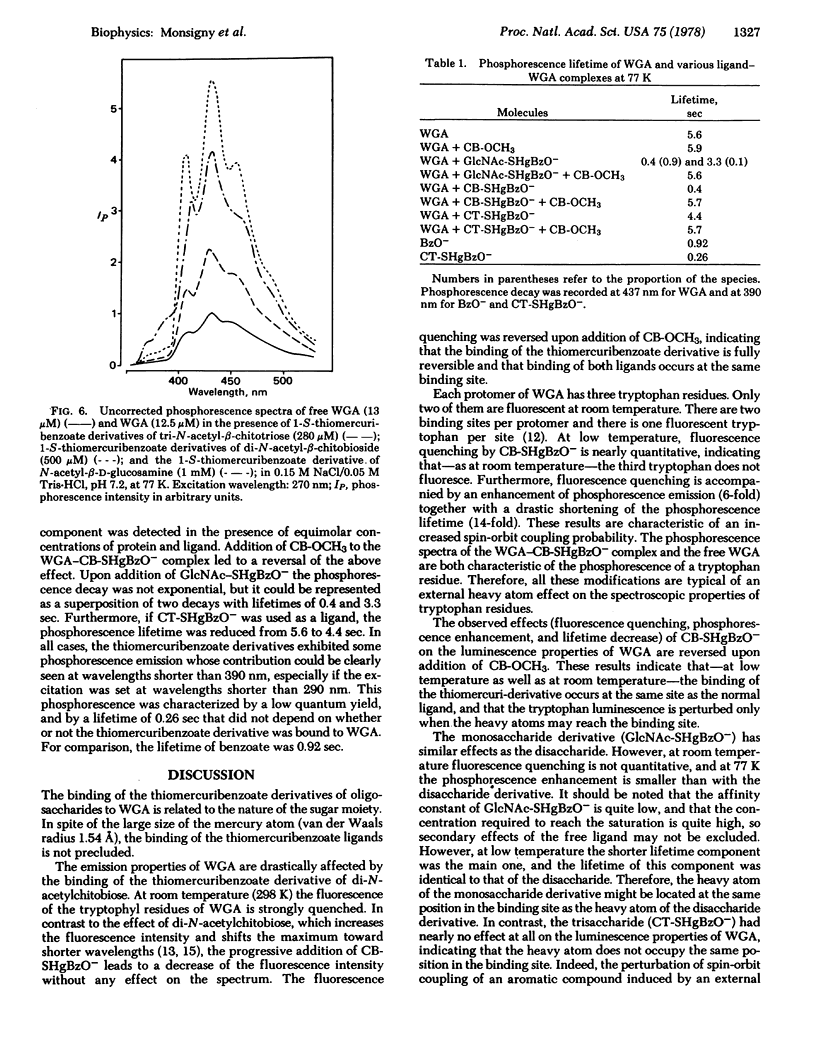

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUB J. C., TIESLAU C., LANKESTER A. REACTIONS OF NORMAL AND TUMOR CELL SURFACES TO ENZYMES. I. WHEAT-GERM LIPASE AND ASSOCIATED MUCOPOLYSACCHARIDES. Proc Natl Acad Sci U S A. 1963 Oct;50:613–619. doi: 10.1073/pnas.50.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R., Burger M. M. Purification of wheat germ agglutinin using affinity chromatography on chitin. Biochem Biophys Res Commun. 1974 May 7;58(1):13–19. doi: 10.1016/0006-291x(74)90884-5. [DOI] [PubMed] [Google Scholar]
- Bouchard P., Moroux Y., Tixier R., Privat J. P., Monsigny M. An improved method for purification of wheat germ agglutinin (lectin) by affinity chromatography. Biochimie. 1976;58(10):1247–1253. doi: 10.1016/s0300-9084(76)80124-1. [DOI] [PubMed] [Google Scholar]
- Brun F., Toulmé J. J., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. Fluorescence studies of the binding of oligopeptides containing tryptophan and tyrosine residues to polynucleotides. Biochemistry. 1975 Feb 11;14(3):558–563. doi: 10.1021/bi00674a015. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley W. C., Purkey R. M. Spin-orbital probes of biomolecular structure. A model DNA-acridine system. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2198–2202. doi: 10.1073/pnas.69.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Sharon N., Mirelman D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur J Biochem. 1975 Jun 16;55(1):257–262. doi: 10.1111/j.1432-1033.1975.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Lotan R., Sharon N. The fluorescence of wheat germ agglutinin and of its complexes with saccharides. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1340–1346. doi: 10.1016/s0006-291x(73)80041-5. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Privat J. P., Delmotte F., Mialonier G., Bouchard P., Monsigny M. Fluorescence studies of saccharide binding to wheat-germ agglutinin (lectin). Eur J Biochem. 1974 Aug 15;47(1):5–14. doi: 10.1111/j.1432-1033.1974.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Delmotte F., Monsigny M. Protein-sugar interactions. Association of beta-(1 leads to 4) linked N-acetyl-D-glucosamine oligomer derivatives with wheat germ agglutinin (lectin). FEBS Lett. 1974 Sep 15;46(1):224–228. doi: 10.1016/0014-5793(74)80373-x. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Delmotte F., Monsigny M. Protein-sugar interactions. Association of wheat germ agglutinin (lectin) and O-(4-methyl-umbelliferyl)-glycosides. FEBS Lett. 1974 Sep 15;46(1):229–232. doi: 10.1016/0014-5793(74)80374-1. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Lotan R., Bouchard P., Sharon N., Monsigny M. Chemical modification of the tryptophan residues of wheat-germ agglutinin. Effect on fluorescence and saccharide-binding properties. Eur J Biochem. 1976 Sep 15;68(2):563–572. doi: 10.1111/j.1432-1033.1976.tb10844.x. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Monsigny M. Luminescence studies of saccharide binding to wheat germ agglutinin (lectin). Eur J Biochem. 1975 Dec 15;60(2):555–567. doi: 10.1111/j.1432-1033.1975.tb21034.x. [DOI] [PubMed] [Google Scholar]
- Privat J. P., Wahl P., Monsigny M., Auchet J. C. Nanosecond-pulse fluorimetry of wheat-germ agglutinin (lectin). Eur J Biochem. 1976 Sep 15;68(2):573–580. doi: 10.1111/j.1432-1033.1976.tb10845.x. [DOI] [PubMed] [Google Scholar]
- Rahn R. O., Battista M. D., Landry L. C. Influence of mercuric ions on the phosphorescence and photochemistry of DNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1390–1397. doi: 10.1073/pnas.67.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. W., Walborg E. F., Jr, Jirgensons B. Circular dichroism and saccharide-induced conformational transitions of wheat germ agglutinin. Arch Biochem Biophys. 1977 Jan 30;178(2):625–630. doi: 10.1016/0003-9861(77)90234-x. [DOI] [PubMed] [Google Scholar]
- Van Landschoot A., Loontiens R. G., Clegg R. M., Sharon N., De Bruyne C. K. Binding of 4-methylumbelliferyl n-acetyl-chitooligosaccharides to wheat-germ agglutinin. A reinvestigation of equilibrium studies. Eur J Biochem. 1977 Sep 15;79(1):275–283. doi: 10.1111/j.1432-1033.1977.tb11807.x. [DOI] [PubMed] [Google Scholar]
- Wright C. S. The crystal structure of wheat germ agglutinin at 2-2 A resolution. J Mol Biol. 1977 Apr 25;111(4):439–457. doi: 10.1016/s0022-2836(77)80063-6. [DOI] [PubMed] [Google Scholar]


