Abstract
Bone is a dynamic tissue constantly renewed through a regulated balance between bone formation and resorption. Excessive bone degradation by osteoclasts leads to pathological decreased bone density characteristic of osteolytic diseases such as post-menopausal osteoporosis or bone metastasis. Osteoclasts are multinucleated cells derived from hematopoietic stem cells via a complex differentiation process. Their unique ability to resorb bone is dependent on the formation of the actin-rich sealing zone. Within this adhesion structure, the plasma membrane differentiates into the ruffled border where protons and proteases are secreted to demineralize and degrade bone, respectively. On the bone surface, mature osteoclasts alternate between stationary resorptive and migratory phases. These are associated with profound actin cytoskeleton reorganization, until osteoclasts die of apoptosis. In this review, we highlight the role of Rho GTPases in all the steps of osteoclasts differentiation, function, and death and conclude on their interest as targets for treatment of osteolytic pathologies.
Keywords: osteoclast, bone and bones, podosome, Rho GTPase, Rac, Cdc42, RhoU, actin, RANK ligand, guanine nucleotide exchange factor
Introduction
Bone is a dynamic tissue continuously renewed through the collaborative activity of osteoclasts, which resorb mineralized bone, and osteoblasts, which form the new bone matrix. During this process, some osteoblasts get embedded into the new bone where they further differentiate into osteocytes.1 Communication between these cell types is essential for bone remodeling, a crucial process for the integrity of the skeleton that allows auto-repair and adaptation of intrinsic properties of bone to external constraints.2 The compensatory activities of osteoblasts and osteoclasts must be tightly regulated because any disruption of this balance may lead to pathological bone defects.
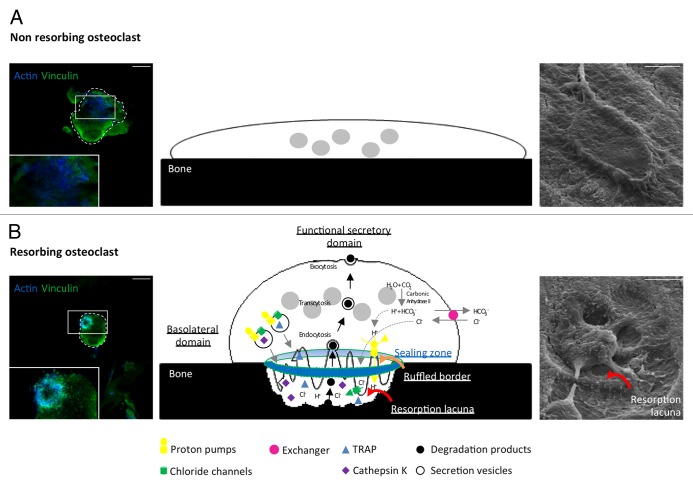
Osteoclasts are multinucleated motile cells derived from hematopoietic stem cells during a multistep differentiation process called osteoclastogenesis through cell-cell contact or the secretion of local factors. It is mainly mediated by two cytokines, the Macrophage Colony-Stimulating Factor-1 (M-CSF) and the Receptor Activator of Nuclear factor-kB Ligand (RANKL) which belongs to the Tumor Necrosis Factor (TNF) superfamily.3 Osteoclasts maturation is characterized by their subsequent polarization. They first assemble the sealing zone, an actin ring made of densely packed podosomes. Within this structure, the plasma membrane in contact with the bone reorganizes into a ruffled border where the cell secretes protons and chloride ions to acidify the extracellular medium and dissolve bone hydroxyapatite. The osteoclast also produces acidic proteases (Cathepsin K, MMP9) and phosphatases (Tartrate Resistant Acid Phosphatase TRAP) that will further degrade bone matrix proteins (Fig. 1). To exert their function, osteoclasts cycle between resorption and migration phases along the bone surface, which require deep actin cytoskeleton reorganization.
Figure 1. Resorption function of osteoclasts. Actin (blue) and vinculin (green) immunofluorescent staining (Axioplan2/LSM 510 META confocal microscope, Zeiss, left), schemes (middle), and scanning electron micrographs (S4000 scanning microscope, Hitachi, right) showing spread non-resorbing osteoclasts (A) and polarized resorbing osteoclasts (B) on bone. Polarized osteoclasts are characterized by 4 domains called sealing zone, ruffled border, basolateral domain, and functional secretory domain. Carbonic anhydrase II generates protons (H+) and HCO3-/Cl- exchangers increase chloride ions (Cl-) concentration in the osteoclasts cytoplasm while maintaining electroneutrality. Inside the sealing zone (blue and green circle), proton pumps and chloride channels export these ions to acidify the surface in contact with the ruffled border (orange curved arrow). As a consequence, the mineral component of bone matrix is dissolved allowing phosphatases and proteolytic enzymes such as TRAP and Cathepsin K to degrade the organic component of bone, therefore creating a resorption lacuna (red curved arrows). Bone degradation products are endocytosed, transported by transcytosis (black arrows) and evacuated at the functional secretory domain by exocytosis. Scale bars = 15 µm.
GTPases of the Rho subfamily, which are best known as critical regulators of the actin cytoskeleton,4 belong to the super family of Ras GTPases and include the far best characterized RhoA, Rac1, and Cdc42. These classical GTPases switch between an active guanine triphosphate (GTP)-bound state and an inactive guanine diphosphate (GDP)-bound state. Guanine-nucleotide exchange factors (GEFs), promote the release of GDP in exchange of GTP. They activate the Rho GTPases allowing their interaction with downstream effector proteins to propagate transduction signals.5,6 They return to their inactive conformation by hydrolyzing GTP into GDP thanks to their intrinsic phosphatase activity, a process that can be accelerated by GTPase activating proteins (GAPs).7 Rho GTPases are prenylated at their C-terminus and associate with cell membranes. Guanine-nucleotide dissociation inhibitors (GDIs) extract them from membranes and inhibit the dissociation of GDP, keeping the GTPases in an inactive state and preventing their activation by GEFs.8 Rho GTPases, are also regulated through their expression level, stability and post translational modifications, in particular atypical Rho GTPases such as RhoE/Rnd3, which lacks intrinsic GTPase activity, or RhoU/Wrch-1, which shows a high nucleotide exchange rate.9 In this review, we will focus on the regulatory role that Rho GTPases, and more particularly Rho, Cdc42 and Rac, exert on the actin cytoskeleton as well as on other cellular activities during osteoclasts differentiation and function.
Osteoclast Differentiation
M-CSF and RANKL play distinct roles in osteoclastogenesis. M-CSF is mainly essential for the commitment of hematopoietic stem cells in the osteoclast lineage, proliferation of precursors but also survival of the entire osteoclast lineage. RANKL is responsible for the induction of a specific transcriptional program allowing the fusion of the precursors and the maturation of osteoclasts into bone resorbing cells.
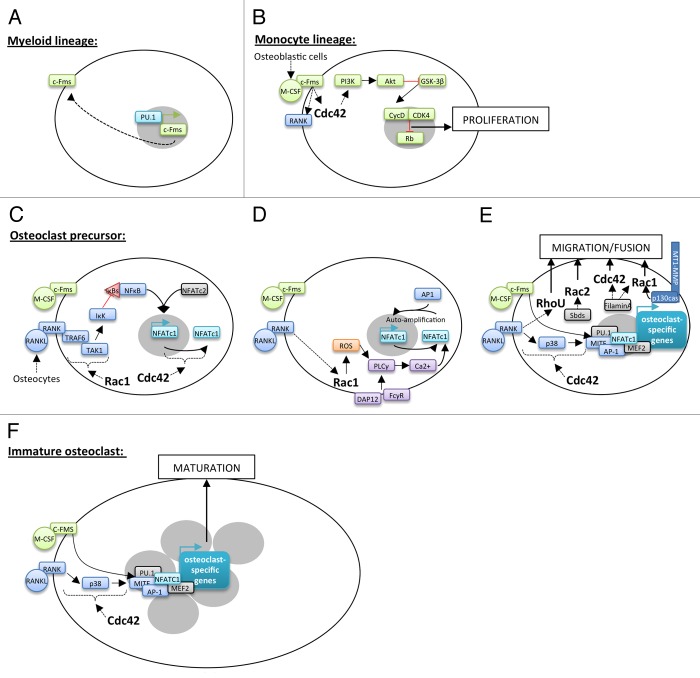
At the initial phase of monocytic differentiation pathway, the transcription factor PU.1 induces the expression of M-CSF receptor, c-Fms, in hematopoietic stem cells of the myeloid lineage (Fig. 2A).10 Secreted by osteoblastic cells, M-CSF then drives myeloid stem cells into the monocyte lineage as well as the proliferation and survival of these precursors called Bone Marrow Macrophages (BMMs).11 Indeed, M-CSF binding to c-Fms, a member of the receptor tyrosine kinase family, results in its dimerization and auto-phosphorylation of its cytoplasmic tail at specific tyrosine residues. The following recruitment of Src Homology 2 (SH2) or phosphotyrosine-binding (PTB) domains-containing proteins initiates the assembly of molecular complexes involved in various signaling pathways with key transducers such as phospholipase Cγ (PLCγ), Ras/p40/42 ERK, and phosphatidylinositol 3-kinase (PI3K)/Akt.12 Cdc42 is required for M-CSF-induced BMMs proliferation through activation of PI3K/Akt axis but not ERKs. Akt activation and the following GSK-3β inhibition increase cyclin D levels and its association with cyclin-dependent kinase 4 during G1 to S phase. This complex phosphorylates Rb, a negative regulator of cell proliferation, which is then inhibited and allows cell cycle entry (Fig. 2B).13-15
Figure 2. Implication of Rho GTPases in osteoclastogenesis. Hematopoietic stem cells differentiation into osteoclasts takes place through several steps controlled by keys factors. PU.1 induces the expression of c-Fms in hematopoietic stem cells of the myeloid lineage (A). M-CSF secreted by osteoblastic cells allows to the induction of myeloid stem cell into the monocyte lineage. The interaction between M-CSF and c-Fms leads to the activation of Cdc42 and the PI3K/Akt axis resulting in the proliferation of these cells (B). M-CSF/c-Fms binding is also responsible for the presence of RANK at their surface (B). RANKL secreted by osteocytes leads to the commitment of monocyte stem cells into osteoclast precursors. RANKL binding to RANK is followed by TRAF6 recruitment and activation of Rac1-dependent TAK1 pathway leading to NFκB nuclear translocation into the nucleus. NFκB and NFATc2 are then able to initiate the expression of the master regulator of osteoclastogenesis, NFATc1 (C). By unknown mechanisms, Cdc42 also regulates NFATc1 expression (C). RANKL and costimulatory signals, from DAP12 and FCγR, lead to sustained intracellular calcium influx that activates NFATc1. It is in part dependent on Rac1-mediated ROS production and PLCγ stimulation (D). Then, NFATc1 in cooperation with other transcription factors allows to the expression of osteoclast-specific target genes involved in precursor fusion (D). This process is dependent on precursor migration and contact, which implicate Rac1, Rac2, Cdc42, and RhoU through different signaling pathways (E). In multinucleated cells, NFATc1 associates with co-activators to induce the expression of osteoclast-specific target genes involved in osteoclast maturation and function (F).
M-CSF interaction to c-Fms also results in the expression of Receptor Activator of NFkappa B (RANK), a member of the TNF receptor family, at the BMMs surface.16 BMMs exit the cell cycle and RANKL interaction with RANK initiates their engagement into the osteoclast lineage.17,18 The main source of RANKL required for the formation of osteoclasts in bone remodeling comes from osteocytes.18,19 RANK activation induces its trimerization and the recruitment of the adaptor molecule TRAF6. This leads to the activation of downstream kinases such as inhibitors of κB (IκBs) kinase (IκK) and the mitogen activated kinases p38, JNK and ERK. These signaling pathways induce the expression of transcription factors like nuclear factor κB (NFκB), c-Fos, and nuclear factor of activated T cells 1 (NFATc1). Rac1 activates NFκB through the classical pathway, i.e., by releasing NFκB dimers from their inhibitory interaction with IκBs in the cytoplasm.20 This effect is mediated by TGFβ-activated kinase 1 (TAK1), a member of the MAP3K family, which is activated after its binding to TRAF6 via adaptors called TAK1-binding proteins 2 and 3.20-22 Activated TAK1 phosphorylates IκK which targets IκBs for proteasomal degradation resulting in NFκB activation.23 TAK1 plays an essential role in osteoclast differentiation since TAK1-deficient mice have a high bone density due to BMMs increased apoptosis and, as a consequence, to fewer osteoclasts.24 In collaboration with NFATc2, activated NFκB induces the expression of NFATc1, the master regulator of osteoclastogenesis (Fig. 2C).25 Upon calcium influx from RANKL and costimulatory signals involving immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors DAP12 and FCγR, NFATc1 is activated and binds with AP-1 on its own promoter to induce its auto-amplification.26-28 Rac1 is an essential regulator of NFATc1 activation. Indeed, the GTPase is activated downstream of RANKL and produces reactive oxygen species (ROS) which stimulate the PLCγ to induce sustained calcium oscillations that drive NFATc1 activation (Fig. 2D).29,30 Not surprisingly, BMMs lacking Rac1, which show an impaired osteoclastogenesis, are unable to generate RANKL-dependent ROS.31 NFATc1 induction is crucial for the expression of osteoclast-specific target genes involved in the fusion of mononuclear precursors, such as DC-STAMP and Atp6v0d2, and in osteoclast function such as Oscar, Acp5, Cathepsin K, and ITGB3. NFATc1–mediated transcriptional activation in osteoclasts is complex and involves other transcription factors such as AP-1, myocyte enhancer factor 2 (MEF2), PU.1 or microphtalmia transcription factor (MITF).32,33 First, M-CSF promotes the assembly of MITF-PU.1 complexes at the promoter of target genes (Oscar, Acp5, Cathepsin K) without activating their transcription. Then, RANKL increases MITF expression and its phosphorylation on ser307 by p38 MAPK.28 This allows the recruitment of the SWI/SNF chromatin-remodeling complex and the initiation of target gene transcription.34,35 NFATc1 is then recruited to maintain their transcription in differentiated cells (Fig. 2E).36 Cdc42 also promotes osteoclast differentiation by controlling MITF phosphorylation and NFATc1 expression but the precise mechanism remains to be elucidated.13
Another feature of osteoclast late differentiation is the fusion of mononuclear precursors to form multinucleated osteoclasts. Rho GTPases regulate the cytoskeleton reorganization associated with precursors’ migration into close proximity of neighboring cells as well as membrane protrusions thereby increasing fusion probability. Indeed, Rac2 activation downstream the signaling pathway of Sbds, a poorly characterized ribosome-related protein, and Rac1 activation upon interaction of the cytoplasmic tail of matrix metalloprotease MT1-MMP with p130Cas, are required for precursors migration.37,38 Similarly, filamin A regulates actin dynamics that control M-CSF-dependent precursors migration via Rac1 and Cdc42 combined activation.39 Besides, we showed that the atypical Rho GTPase RhoU, which expression is strongly induced during early osteoclastogenesis, is involved in osteoclast precursors fusion.40,41 RhoU likely promotes this process by inhibiting precursors adhesion to the extracellular matrix and stimulating cell-cell contact (Fig. 2E–F).40,42
Osteoclast Function
Mature multinucleated osteoclasts have the unique ability to degrade bone, a physiological process involved in developmental bone morphogenesis as well as in bone remodeling all life through to repair microdamages or to adapt to mechanical loads. On the bone surface, osteoclasts alternate between stationary resorptive and migratory phases, until they die of apoptosis. The resorption phase starts with the formation of a sealing zone, followed by apico-basal polarization and formation of the ruffled border. Below the area of the ruffled border delineated by the sealing zone, the activity of the osteoclast generates a resorption pit. Bone degradation products are then removed by endocytosis, trafficked through the osteoclasts by transcytosis, and released in the extracellular medium. Triggered by signals that remain to be identified, the sealing zone then disassembles and the cell body spreads away from the resorption pit. A new adhesion structure is then formed next to the former resorption pit, and the osteoclast repolarizes. This alternation of resorption and migration phases results in the formation of resorption pit trails at the bone surface.43
Adhesion structures
The sealing zone is essential for bone resorption. The inability of osteoclasts to assemble this structures results in high bone mass due to impaired bone resorption, as for instance in mice mutant for the tyrosine kinases Src or Pyk2.44,45 Indeed, the sealing zone seals the osteoclast to the substrate and isolates the acidic environment of the resorption lacuna from the extracellular medium to allow efficient bone resorption.46 The sealing zone is a thick ring of actin, 4-μm wide and 4-μm high, made of densely packed podosomes.47-49 Each podosome is made of an F-actin-rich core extending perpendicularly to the substrate which is surrounded by an F-actin cloud connected to the core by dome-like radial fibers.50 Both are associated with proteins mainly involved in actin polymerization for the core (cortactin, (N)WASp, Arp2/3, Cdc42…) and in cytoskeletal signaling for the cloud (Src, Pyk2, vinculin, RhoU…).51 Interestingly, they express distinct receptors: CD44 in the core and αvβ3 integrins in the cloud, suggesting that these structures may emanate from distinct signaling pathways.52 It is noteworthy that CD44 localization at the core surface is regulated by Rho-mediated Rho kinase (ROK-α) activation.53
Podosome organization depends on the osteoclast differentiation stage and the substrate it is seeded on. During osteoclast differentiation on non-mineralized matrix, podosomes evolve from clusters into unstable small podosome rings, which fuse and expand toward the periphery of the cell to form a belt, 2- to 3-μm wide in mature osteoclasts (Fig. 3).72 The sealing zone is a denser version of the podosome belt that only forms in osteoclasts sitting on a mineralized matrix, such as bone or dentine. Sealing zone and podosome belt only differ in their podosomes density and degree of interconnectivity.50 Osteoclast cytoskeletal organization is dependent on attachment to the matrix via αvβ3, the major integrin in osteoclasts which recognizes arginine-glycine-aspartic acid (RGD)-containing ligands such as vitronectin or osteopontin.52 Upon binding, various proteins such as c-Src and Pyk2 are recruited with scaffold proteins to transduce a signal required for actin ring formation and bone degradation.44,45,54,55,56 Interestingly, Rho and Rac activations in response to M-CSF are deficient in αvβ3 integrins knockout osteoclasts.57 Different approaches have been used to determine the importance of Rho, Cdc42 and Rac in osteoclasts adhesion structures organization but they have often produced contradictory results. The first evidence of Rho involvement in podosomes stability was published in 1995. It showed that osteoclasts treatment with C3, an exoenzyme isolated from Clostridium botulinum which ADP-ribosylates and inactivates RhoA, B, and C, disrupts the sealing zone and inhibits resorption on dentin slices.58 Similarly, C3 treatment of avian osteoclast-like cells showed podosomes dissolution as soon as 2h after the toxin administration.59,60 Considering the previous results, it would be expected that Rho activation stabilizes podosomes. However, microinjection or HIV-Tat-delivery of constitutively active V14RhoA also resulted in podosomes disappearance.59,60 This apparent discrepancy rather reveals the requirement of a tight regulation of RhoA activity during podosomes formation and patterning. Furthermore, C3 toxin-mediated Rho inhibition results in sealing zone disruption on bone58 but causes its reorganization in podosome belts on apatite matrix47 suggesting a substrate effect. At last, indirect RhoA-mediated effects on the actin cytoskeleton add an extra level of complexity. Podosome belt and sealing zone, which share the same molecular components, are stabilized by microtubules.61 RhoA, through its effector mDia2, induces microtubules deacetylation by HDAC6.62 The activity of RhoA is downregulated by Pyk2 to maintain a sufficient level of acetylation in order to stabilize microtubules and then the podosome belt and/or sealing zone.44,55 Similarly, analysis of actin distribution in avian osteoclast-like cells transduced with Cdc42 showed podosome belt disruption60 whereas mature Cdc42-lacking osteoclasts are able to form sealing zones on dentine.13 Although the results are somehow conflicting and their precise function not elucidated yet, both Rac1 and Rac2 seem to be important for the formation of the podosome belt and for bone resorption.31,63-66 This confirms earlier studies showing podosome belt disruption in osteoclasts where Rac1 and Rac2 have been neutralized by specific antibodies.67 Using DNA microarray, we analyzed the expression profile of 76 GEFs of the Dbl and Dock families in osteoclasts. We found that 46 are potentially expressed in osteoclasts among which the Rac GEFs, Vav3, Dock5, and FARP2.40 Interestingly, the osteoclasts derived from mice knockout for these GEFs could not assemble sealing zones on bone and showed an impaired resorptive activity associated with a decreased bone density.68-70 However, even if Dock5 and Vav3 are both required for cytoskeleton organization, they regulate Rac function through distinct signaling pathways. We and others showed that Dock5 is part of αvβ3 integrins downstream signaling by forming a Src/Pyk2/p130Cas/Dock5 complex which ultimately leads to Rac1 activation and localization to the sealing zone.56,70 On the other hand, Vav3 is activated by M-CSF and adhesion and it is recruited with Rac1 at the plasma membrane of osteoclasts.71 So Dock5 and Vav3 seem to regulate Rac1 activation at distinct locations in osteoclasts and at different phases of the bone-resorption cycle.
Figure 3. Dynamic of podosomes organization. Schemes and immunofluorescent staining of actin (podosome core in blue) and vinculin (podosome cloud in green) observed with Axioplan2/LSM 510 META confocal microscope (Zeiss), showing osteoclasts differentiation through the evolution of podosomes organization. On non-mineralized support, they first assemble into podosome clusters then into rings, which finally fuse into belts. On mineralized support, the sealing zone is the ultimate structure present in differentiated osteoclasts. It is characterized by a strong density and an important degree of podosome interconnectivity. Scale bar = 5 µm. Figure adapted from reference 72, with permission from the editor American Society for Cell Biology (ASCB).
Podosomes are highly dynamic structures.47,72 Polymerization of monomeric globular (G)-actin into filamentous (F)-actin is controlled by the Arp2/3 complex, which nucleate filaments and organize them into branched networks.73 It is activated by the nucleation promoting factors (NPFs), among which are the (N)-WASP and WASP-family verprolin homolog (WAVE) proteins.73 Following αvβ3 stimulation by osteopontin, phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) and the transducer of Cdc42-dependent actin assembly (Toca-1) cooperate to induce the Cdc42-mediated targeting of WASP to the plasma membrane where it is activated.74 The interaction of PIP2 with WASP was also suggested to be Rho-dependent as it is decreased by C3 toxin treatment.75 Activated WASP conformation enables access of Tyr291 (human)/Tyr294 (mouse) to modifying enzymes,76,77 increasing WASP affinity to Arp2/3 complex and ultimately leading to sealing zone formation and bone resorption.52,78 Surprisingly, WASP-null osteoclasts retain the capacity to polymerize actin but fail to assemble podosomes replaced by large actin-rich plaques.79 Even if Cdc42 has been shown to be an important regulator of (N)-WASP,80,81 notably because it is necessary for an efficient phosphorylation of Tyr291/Tyr294,77 microinjection of activated Cdc42 alone is not sufficient to form a podosome belt in osteoclasts.75 Interestingly, analysis of Cdc42 knockout in mature osteoclasts shows that Cdc42 is dispensable for ultimate formation of actin rings but regulates the rate at which these structures are generated.13 Similarly, osteoclasts differentiated from RhoE gene trap mice bone marrow precursors showed that RhoE is indispensable to maintain podosomes fast actin turnover and patterning. The GTPase acts indirectly by inhibiting the kinase Rock to maintain cofilin, an actin severing protein that regulates F-actin assembly and disassembly, in its non-phosphorylated active form.82,83 On the other hand, Rac1/Rac2 double knockout osteoclasts do not form sealing zones.63 This process might depend on Rac activation of the WAVE complex which requires simultaneous interactions with prenylated Rac-GTP, acidic phospholipids and a specific state of phosphorylation.84
Polarization and bone resorption
Following sealing zone formation, the osteoclasts round up and reorganize their membrane into 3 other distinct domains called ruffled border, basolateral domain and functional secretory domain (Fig. 1).85 This polarized reorganization of their morphology is critical for bone resorption.
The ruffled border is a highly convoluted domain formed by fusion of late endosomal/lysosomal vesicles at the plasma membrane within the sealing zone.85 It is mainly controlled by the small GTPases of the Rab family (for a detailed review, see ref. 86) and more particularly Rab7 which regulates the late stages of the endosomal pathway from the basolateral membrane to the ruffled border.87 In resorbing osteoclasts, Rac1 and Rab7 colocalization and interaction at the vesicle fusion zone of the ruffled border suggest a possible role of Rac1 in the formation of this structure.88 During this process, vacuolar H+-ATPases (v-ATPase) and chloride channels (ClC-7) are inserted into the ruffled border membrane where they pump protons and chloride ions from the cytoplasm to the resorption lacuna.89 The resulting acidification dissolves the mineral phase of the bone matrix and exposes the organic phase susceptible to degradation by acidic proteases and phosphatases such as cathepsin K and TRAP.89 Degraded collagen fragments, calcium and phosphate that accumulate at high concentration in the lacuna are endocytosed from the ruffled border, transported through the osteoclasts by transcytotic vesicles (in which they are further degraded by TRAP and cathepsin K) and released in the extracellular medium via the functional secretory domain.
Although bone-resorbing osteoclasts are polarized, the protein constituents that establish and sustain this polarity are unknown. In epithelial cells, activated Cdc42 binds the Cdc42/Rac interactive binding (Crib) domain of the adaptor Par6 which in turn interacts with Par3 and atypical PKC (aPKC), forming a quaternary complex involved in polarity maintenance.90,91 Interestingly, Ito et al. showed that all these proteins are able to form an active Cdc42/Par3/Par6/aPKC complex stimulated by RANKL.13
Spreading and associated-migration
After bone resorption, the osteoclasts spread initiating the first step of migration. Cytoskeleton rearrangement allows the dissolution of old sealing zones and the formation of new ones. The following polarization induces a contraction of the cells, which is responsible for the second step of migration. As the osteoclasts progress in the resorption cycle, the pits visualized on bone produce a typical trail reminiscent of inchworm-like migration.47
Osteoclast spreading is dependent on M-CSF signaling. This cytokine binding to c-Fms allows PI3K recruitment and Vav3 activation in part by facilitating exchange of the inhibitory molecule PIP2 for the stimulatory molecule phosphatidylinositol (3,4,5)-trisphosphate (PIP3) on Vav3 pleckstrin homology (PH) domain.71 Vav3 in turn leads to Rac activation by stimulating the on-loading of GTP and ultimately spreading. Accordingly, Vav3-deficient osteoclasts are incapable of spreading.92 The critical role of Rac in this process is further comforted by the observations that osteoclast-like cells microinjected with constitutively activated V12Rac1 show extensive spreading whereas Rac1 inhibition leads to retraction.59 The same study also shows the antagonistic effect of Rho whose inhibition by C3 triggers spreading.
Apoptosis regulation
Osteoclasts cycle between resorption and non-resorption phases, until they die of apoptosis. This process is directly activated partly by an intrinsic pathway implicating Bim, a pro-apoptotic Bcl-2 family member, which promotes the release of cytochrome c from mitochondria followed by caspases activation.93 Osteoclasts lacking Cdc42 express higher levels of Bim, activated caspases-3/9 and die more rapidly than wild-type osteoclasts upon M-CSF and RANKL withdrawal, showing that Cdc42 is crucial to inhibit apoptosis in these cells.13 Besides, apoptosis can also be indirectly regulated by the survival pathways involving PI3K/Akt.94-96 Indeed, adenovirus-mediated overexpression of dominant negative Rac1 abrogates the M-CSF-induced prosurvival effect through the decrease of Akt activation in osteoclasts. Conversely, constitutively active Rac1 delays apoptosis in these cells.97
Conclusion
Rho GTPases important role in osteoclast differentiation and function revealed by in vitro experiments (Fig. 4) is confirmed by in vivo studies. Indeed, deletion of various Rho GTPases or GEFs in osteoclasts leads to a significant osteopetrosis caused by either a reduced number of osteoclasts or a defect in their function (Table 1). Independent studies focused on the effect of Rac deficiency on bone physiology. Although the authors agree on the increase in bone density, there are discrepancies regarding the mechanisms involved that will need further investigation.31,63-65 Interestingly, the absence of a single Rac GEF, such as Vav3, Dock5, or FARP2, is sufficient to result in osteoclasts impaired resorption in vivo, through distinct mechanisms.68-70
Figure 4. Implication of Rho GTPases in the cyclic resorption function of osteoclasts. Scanning electron micrographs (S4000 scanning microscope, Hitachi, sides), schemes and immunofluorescent staining of actin (blue), and vinculin (green) staining (Axioplan2/LSM 510 META confocal microscope, Zeiss, middle) showing morphologies and cytoskeleton structures of osteoclasts alternating between stationary resorption and migration phases. Polarized osteoclasts have the unique ability to resorb bone (1). Then, the sealing zone (SZ) is disassembled and osteoclasts can spread under a Rac/Rho effect (2). Rac/Cdc42/RhoE are implicated in actin reorganization (3) into another sealing zone stabilized by Rac/Rho and followed by osteoclasts polarization involving Cdc42 (4). Thereby, osteoclasts can resorb bone again and form another resorption lacuna (red arrows) (5). This cyclic process takes place until osteoclasts die of apoptosis implicating Cdc42 and Rac (6). Scale bars = 15 µm. Figure adapted from reference 47 with permission of the editor ASCB.
Table 1. Genetically modified mice for Rho GTPases and GEFs with osteopetrotic phenotypes.
| Gene | Strategy | Modified cell types | Cause(s) of osteopetrosis | Ref |
| Cdc42 | Conditional knockout - pCtsK-Cre |
Osteoclasts | Fewer osteoclasts (increased osteoclasts apoptosis) | 13 |
| Rac1 | Conditional knockout - pLysM-Cre |
Myeloid cells | Defect in osteoclastogenesis (fewer osteoclasts) Defect in osteoclast function in aged females (not investigated further) |
31 65 |
| Rac2 | Global knockout | All | Defect in osteoclast function in males only (absence of sealing zone) Defect in osteoclast function in aged females (not investigated further) |
64 65 |
| Rac1/Rac2 | Rac1 conditional knockout - pLysM-Cre/Rac2 global knockout | Myeloid cells (Rac1)/all (Rac2) | Defect in osteoclast function (absence of sealing zone) Defect in osteoclastogenesis (absence of osteoclasts) |
63 31 |
| Rac1/Rac2 | Rac1 conditional knockout pCtsK-Cre/Rac2 global knockout | Osteoclasts (Rac1)/all (Rac2) | Non identified | 63 |
| Vav1 | Global knockout | All | Not investigated | 68 |
|
Vav3 (Rac GEF) |
Global knockout | All | Defect in osteoclast function (absence of sealing zone) | 68 |
|
Dock5 (Rac GEF) |
Gene trap | All | Defect in osteoclast function (absence of sealing zone) | 70 |
|
FARP2 (Rac GEF) |
Global knockout | All | Defect in osteoclast function (absence of sealing zone) | 69 |
pCtsK, Cathepsin K promoter that is expressed in osteoclasts; pLysM, Lysozyme M promoter that is expressed in all myeloid cells
The higher bone density observed in mice deficient for Rho GTPase signaling highlights them as targets in diseases with increased bone resorption such as post-menopausal osteoporosis or cancer-associated osteolysis.98,99 Besides, the nitrogen-containing bisphosphonates (N-BPs),100 which are the major class of antiresorptive drugs used to treat these diseases, target the small GTPases.101 By inhibiting enzymes of the mevalonate pathway,102,103 they impair the GTPases prenylation that is essential for their correct localization to the membrane.104 The consequent cytosolic accumulation of unprenylated activated Rho, Rac, and Cdc42105 induces the inappropriate activation of downstream signaling, ultimately leading to osteoclasts apoptosis and reduced bone resorption.106 However, on top of N-BPs unwanted side effects,107,108 osteoclast-induced cell death abrogates the osteoblasts-osteoclasts communication essential for bone remodeling and integrity.2 Novel therapeutic strategies are being developed to target specifically osteoclast function leaving osteoclast differentiation and survival unaffected. In particular, ONO-5334 and odanacatib, 2 inhibitors of Cathepsin K, the main bone-degrading protease expressed in osteoclasts,109,110 are currently in phase II and III clinical trials respectively. They indeed show encouraging results with decreased bone resorption and preserved bone formation.111-115 An alternative approach would be to target among Rho GTPase signaling pathway, proteins specifically involved in the control of the formation of the sealing zone. In this context, we identified N-(3,5-dichlorophenyl)benzenesulfonamide (C21) as an inhibitor of Rac activation by Dock5. Similar to Dock5 deficiency, C21 treatment impairs bone resorption without being toxic for osteoclasts.70 Thus, the modulation of Rho GTPase activity shows promising results for future treatment of osteolytic diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by research grants from the Institut National du Cancer (grant # INCa-4361 to A.B.) and the Agence Nationale de la Recherche (ANR grant # ANR-2011-BLAN-006 to A.B.).
References
- 1.Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–9. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 4.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–82. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 5.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 6.Ihara K, Muraguchi S, Kato M, Shimizu T, Shirakawa M, Kuroda S, Kaibuchi K, Hakoshima T. Crystal structure of human RhoA in a dominantly active form complexed with a GTP analogue. J Biol Chem. 1998;273:9656–66. doi: 10.1074/jbc.273.16.9656. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Chernoff J, Zheng Y. Interaction of Rac1 with GTPase-activating proteins and putative effectors. A comparison with Cdc42 and RhoA. J Biol Chem. 1998;273:8776–82. doi: 10.1074/jbc.273.15.8776. [DOI] [PubMed] [Google Scholar]
- 8.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 9.Aspenström P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–9. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DE, Hetherington CJ, Chen HM, Tenen DG. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994;14:373–81. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2:585–91. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]
- 12.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J, Ross FP, Zhao H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J Clin Invest. 2010;120:1981–93. doi: 10.1172/JCI39650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yavropoulou MP, Yovos JG. Osteoclastogenesis--current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–16. [PubMed] [Google Scholar]
- 15.Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–99. doi: 10.4161/cc.9.4.10611. [DOI] [PubMed] [Google Scholar]
- 16.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasawara T, Katagiri M, Yamamoto A, Hoshi K, Takato T, Nakamura K, Tanaka S, Okayama H, Kawaguchi H. Osteoclast differentiation by RANKL requires NFkappaB-mediated downregulation of cyclin-dependent kinase 6 (Cdk6) J Bone Miner Res. 2004;19:1128–36. doi: 10.1359/jbmr.2004.19.7.1128. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 19.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee NK, Choi HK, Kim D-K, Lee SY. Rac1 GTPase regulates osteoclast differentiation through TRANCE-induced NFkappa B activation. Mol Cell Biochem. 2006;281:55–61. doi: 10.1007/s11010-006-0333-y. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, Sakurai N. Receptor activator of NFkappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22:992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besse A, Lamothe B, Campos AD, Webster WK, Maddineni U, Lin S-C, Wu H, Darnay BG. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem. 2007;282:3918–28. doi: 10.1074/jbc.M608867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 24.Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33:582–95. doi: 10.1128/MCB.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–56. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 26.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–63. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 27.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–9. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danks L, Takayanagi H. Immunology and bone. J Biochem. 2013;154:29–39. doi: 10.1093/jb/mvt049. [DOI] [PubMed] [Google Scholar]
- 29.Lee NK, Choi YG, Baik JY, Han SY, Jeong D-W, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–9. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Yang Y-M, Son A, Tian YS, Lee S-I, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010;285:6913–21. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M. Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res. 2008;23:260–70. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- 32.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Feng H, Cheng T, Steer JH, Joyce DA, Pavlos NJ, Leong C, Kular J, Liu J, Feng X, Zheng MH, et al. Myocyte enhancer factor 2 and microphthalmia-associated transcription factor cooperate with NFATc1 to transactivate the V-ATPase d2 promoter during RANKL-induced osteoclastogenesis. J Biol Chem. 2009;284:14667–76. doi: 10.1074/jbc.M901670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S-Y, Li M, Lin Y-L. Mitf induction by RANKL is critical for osteoclastogenesis. Mol Biol Cell. 2010;21:1763–71. doi: 10.1091/mbc.E09-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NFkappa B ligand signaling. J Biol Chem. 2002;277:11077–83. doi: 10.1074/jbc.M111696200. [DOI] [PubMed] [Google Scholar]
- 36.Sharma SM, Bronisz A, Hu R, Patel K, Mansky KC, Sif S, Ostrowski MC. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007;282:15921–9. doi: 10.1074/jbc.M609723200. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalo P, Guadamillas MC, Hernández-Riquer MV, Pollán A, Grande-García A, Bartolomé RA, Vasanji A, Ambrogio C, Chiarle R, Teixidó J, et al. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev Cell. 2010;18:77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung R, Cuddy K, Wang Y, Rommens J, Glogauer M. Sbds is required for Rac2-mediated monocyte migration and signaling downstream of RANK during osteoclastogenesis. Blood. 2011;117:2044–53. doi: 10.1182/blood-2010-05-282574. [DOI] [PubMed] [Google Scholar]
- 39.Leung R, Wang Y, Cuddy K, Sun C, Magalhaes J, Grynpas M, Glogauer M. Filamin A regulates monocyte migration through Rho small GTPases during osteoclastogenesis. J Bone Miner Res. 2010;25:1077–91. doi: 10.1359/jbmr.091114. [DOI] [PubMed] [Google Scholar]
- 40.Brazier H, Stephens S, Ory S, Fort P, Morrison N, Blangy A. Expression profile of RhoGTPases and RhoGEFs during RANKL-stimulated osteoclastogenesis: identification of essential genes in osteoclasts. J Bone Miner Res. 2006;21:1387–98. doi: 10.1359/jbmr.060613. [DOI] [PubMed] [Google Scholar]
- 41.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 2001;15:1796–807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazier H, Pawlak G, Vives V, Blangy A. The Rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol. 2009;41:1391–401. doi: 10.1016/j.biocel.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Saltel F, Destaing O, Bard F, Eichert D, Jurdic P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15:5231–41. doi: 10.1091/mbc.E04-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J Cell Biol. 2007;178:1053–64. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 46.Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol. 2012;44:1422–35. doi: 10.1016/j.biocel.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Saltel F, Destaing O, Bard F, Eichert D, Jurdic P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15:5231–41. doi: 10.1091/mbc.E04-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–77. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Chabadel A, Bañon-Rodríguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, Jurdic P, Anton IM, Saltel F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell. 2007;18:4899–910. doi: 10.1091/mbc.E07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chellaiah MA, Kizer N, Biswas R, Alvarez U, Strauss-Schoenberger J, Rifas L, Rittling SR, Denhardt DT, Hruska KA. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell. 2003;14:173–89. doi: 10.1091/mbc.E02-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nagai Y, Osawa K, Fukushima H, Tamura Y, Aoki K, Ohya K, Yasuda H, Hikiji H, Takahashi M, Seta Y, et al. p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. J Bone Miner Res. 2013;28:2449–62. doi: 10.1002/jbmr.1936. [DOI] [PubMed] [Google Scholar]
- 57.Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108:2285–92. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- 59.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113:1177–88. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 60.Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–2002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 61.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–11. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 62.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–9. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- 63.Croke M, Ross FP, Korhonen M, Williams DA, Zou W, Teitelbaum SL. Rac deletion in osteoclasts causes severe osteopetrosis. J Cell Sci. 2011;124:3811–21. doi: 10.1242/jcs.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Itokowa T, Zhu ML, Troiano N, Bian J, Kawano T, Insogna K. Osteoclasts lacking Rac2 have defective chemotaxis and resorptive activity. Calcif Tissue Int. 2011;88:75–86. doi: 10.1007/s00223-010-9435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magalhaes JKRS, Grynpas MD, Willett TL, Glogauer M. Deleting Rac1 improves vertebral bone quality and resistance to fracture in a murine ovariectomy model. Osteoporos Int. 2011;22:1481–92. doi: 10.1007/s00198-010-1355-6. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg SR, Georgiou J, Glogauer M, Grynpas MD. A 3D scanning confocal imaging method measures pit volume and captures the role of Rac in osteoclast function. Bone. 2012;51:145–52. doi: 10.1016/j.bone.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 67.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–55. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 68.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–90. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 69.Takegahara N, Kang S, Nojima S, Takamatsu H, Okuno T, Kikutani H, Toyofuku T, Kumanogoh A. Integral roles of a guanine nucleotide exchange factor, FARP2, in osteoclast podosome rearrangements. FASEB J. 2010;24:4782–92. doi: 10.1096/fj.10-158212. [DOI] [PubMed] [Google Scholar]
- 70.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté J-F, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai H, Chen Y, Itokawa T, Yu K-P, Zhu M-L, Insogna K. Activated c-Fms recruits Vav and Rac during CSF-1-induced cytoskeletal remodeling and spreading in osteoclasts. Bone. 2006;39:1290–301. doi: 10.1016/j.bone.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Destaing O, Saltel F, Géminard J-C, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–16. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho H-YH, Rohatgi R, Lebensohn AM, Le Ma, Li J, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell. 2004;118:203–16. doi: 10.1016/j.cell.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 75.Chellaiah MA. Regulation of actin ring formation by rho GTPases in osteoclasts. J Biol Chem. 2005;280:32930–43. doi: 10.1074/jbc.M500154200. [DOI] [PubMed] [Google Scholar]
- 76.Buck M, Xu W, Rosen MK. Global disruption of the WASP autoinhibited structure on Cdc42 binding. Ligand displacement as a novel method for monitoring amide hydrogen exchange. Biochemistry. 2001;40:14115–22. doi: 10.1021/bi0157215. [DOI] [PubMed] [Google Scholar]
- 77.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell. 2003;11:1215–27. doi: 10.1016/S1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 78.Ma T, Samanna V, Chellaiah MA. Dramatic inhibition of osteoclast sealing ring formation and bone resorption in vitro by a WASP-peptide containing pTyr294 amino acid. J Mol Signal. 2008;3:4. doi: 10.1186/1750-2187-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J, Chambers T, Thrasher AJ. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood. 2004;103:3552–61. doi: 10.1182/blood-2003-04-1259. [DOI] [PubMed] [Google Scholar]
- 80.Abdul-Manan N, Aghazadeh B, Liu GA, Majumdar A, Ouerfelli O, Siminovitch KA, Rosen MK. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–83. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 81.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–31. doi: 10.1016/S0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 82.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Pérez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveals RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol Biol Cell. 2014;25:380–96. doi: 10.1091/mbc.E13-07-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blangy A, Touaitahuata H, Cres G, Pawlak G. Cofilin activation during podosome belt formation in osteoclasts. PLoS One. 2012;7:e45909. doi: 10.1371/journal.pone.0045909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–24. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mulari M, Vääräniemi J, Väänänen HK. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech. 2003;61:496–503. doi: 10.1002/jemt.10371. [DOI] [PubMed] [Google Scholar]
- 86.Itzstein C, Coxon FP, Rogers MJ. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases. 2011;2:117–30. doi: 10.4161/sgtp.2.3.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao H, Laitala-Leinonen T, Parikka V, Väänänen HK. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J Biol Chem. 2001;276:39295–302. doi: 10.1074/jbc.M010999200. [DOI] [PubMed] [Google Scholar]
- 88.Sun Y, Büki KG, Ettala O, Vääräniemi JP, Väänänen HK. Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J Biol Chem. 2005;280:32356–61. doi: 10.1074/jbc.M414213200. [DOI] [PubMed] [Google Scholar]
- 89.Lacombe J, Karsenty G, Ferron M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle. 2013;12:2744–52. doi: 10.4161/cc.25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–9. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 91.Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–90. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 93.Fesik SW. Insights into programmed cell death through structural biology. Cell. 2000;103:273–82. doi: 10.1016/S0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 94.Lee ZH, Lee SE, Kim C-W, Lee SH, Kim SW, Kwack K, Walsh K, Kim H-H. IL-1alpha stimulation of osteoclast survival through the PI 3-kinase/Akt and ERK pathways. J Biochem. 2002;131:161–6. doi: 10.1093/oxfordjournals.jbchem.a003071. [DOI] [PubMed] [Google Scholar]
- 95.Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem. 2001;276:49343–9. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- 96.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–79. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- 97.Fukuda A, Hikita A, Wakeyama H, Akiyama T, Oda H, Nakamura K, Tanaka S. Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res. 2005;20:2245–53. doi: 10.1359/JBMR.050816. [DOI] [PubMed] [Google Scholar]
- 98.Armas LAG, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am. 2012;41:475–86. doi: 10.1016/j.ecl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y-C, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010;12:215. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Russell RGG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 101.Giger EV, Castagner B, Leroux J-C. Biomedical applications of bisphosphonates. J Control Release. 2013;167:175–88. doi: 10.1016/j.jconrel.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 102.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–78. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 103.van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–4. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- 104.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 105.Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J Bone Miner Res. 2006;21:684–94. doi: 10.1359/jbmr.060118. [DOI] [PubMed] [Google Scholar]
- 106.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 107.Agarwal P, Rao NN. Bisphosphonate-associated osteonecrosis of the jaws. Indian J Dent Res. 2012;23:107–11. doi: 10.4103/0970-9290.99051. [DOI] [PubMed] [Google Scholar]
- 108.Hirschberg R. Renal complications from bisphosphonate treatment. Curr Opin Support Palliat Care. 2012;6:342–7. doi: 10.1097/SPC.0b013e328356062e. [DOI] [PubMed] [Google Scholar]
- 109.Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Lüth A, Koskivirta I, Kleuser B, Vacher J, Vuorio E, et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123:666–81. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Motyckova G, Fisher DE. Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease. Curr Mol Med. 2002;2:407–21. doi: 10.2174/1566524023362401. [DOI] [PubMed] [Google Scholar]
- 111.Chapurlat RD. Odanacatib for the treatment of postmenopausal osteoporosis. Expert Opin Pharmacother. 2014;15:97–102. doi: 10.1517/14656566.2014.853038. [DOI] [PubMed] [Google Scholar]
- 112.Stoch SA, Zajic S, Stone JA, Miller DL, van Bortel L, Lasseter KC, Pramanik B, Cilissen C, Liu Q, Liu L, et al. Odanacatib, a selective cathepsin K inhibitor to treat osteoporosis: safety, tolerability, pharmacokinetics and pharmacodynamics--results from single oral dose studies in healthy volunteers. Br J Clin Pharmacol. 2013;75:1240–54. doi: 10.1111/j.1365-2125.2012.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brixen K, Chapurlat R, Cheung AM, Keaveny TM, Fuerst T, Engelke K, Recker R, Dardzinski B, Verbruggen N, Ather S, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98:571–80. doi: 10.1210/jc.2012-2972. [DOI] [PubMed] [Google Scholar]
- 114.Engelke K, Nagase S, Fuerst T, Small M, Kuwayama T, Deacon S, Eastell R, Genant H-K. The effect of the cathepsin K inhibitor ONO-5334 on trabecular and cortical bone in postmenopausal osteoporosis: The OCEAN study. J Bone Miner Res. 2014;29:629–38. doi: 10.1002/jbmr.2080. [DOI] [PubMed] [Google Scholar]
- 115.Eastell R, Nagase S, Small M, Boonen S, Spector T, Ohyama M, Kuwayama T, Deacon S. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J Bone Miner Res. 2014;29:458–66. doi: 10.1002/jbmr.2047. [DOI] [PubMed] [Google Scholar]