Abstract
♦ Background: Acute Kidney Injury (AKI) is an important cause of morbidity and mortality in developing countries. Although continuous renal replacement therapy is gaining more popularity worldwide, peritoneal dialysis (PD) in children remains an appropriate therapy for AKI in children for all age groups including neonates.
♦ Methodology: We retrospectively reviewed all children who have been admitted with AKI at the pediatric nephrology unit, Soba University Hospital, Khartoum, during the period from January 2005 to December 2011.
♦ Results: Over 7 years we recorded 659 children of whom 362 (54.9%) were male. The spectrum of age was variable with the majority being neonates, 178 (27.1%). The average patient admission rate was 94 patients per year, with an estimated incidence of 9.8 patients/million population/year. Common causes of AKI were sepsis 202 (30.8%), acute glomerulonephritis 75 (11.5%) and obstructive uropathy due to stones 56 (8.5%). The most common dialysis modality used was PD, 343 (52.4%), and peritonitis was reported in 53 (15.4%) patients. Recovery from AKI was achieved in 450 (68.9%) children, 37 (5.7%) went into chronic kidney disease (CKD), 33 (5.1%) referred to the pediatric surgery and 194 (29.7%) died.
♦ Conclusion: In the setting of developing countries where AKI is a common cause of morbidity and mortality, reasonably equipped renal units with adequately trained medical staff may save many lives. International funding programs for communicable diseases and charity organizations should include AKI management in their programs. Acute PD remains the treatment modality of choice for AKI in developing countries.
Keywords: Acute kidney injury, peritoneal dialysis, children, Sudan, Africa
Sudan is the third largest country on the African continent (following Algeria and DR Congo) and the sixteenth largest in the world. The total land area is about 1,881,000 km2 and the population is 33,419,625 million after separation of the Republic of Southern Sudan (1). Fifty percent (50%) of the population lives in rural areas. The annual population growth rate is 2.4% and children under 15 years old constitute approximately 45% of the total population (1). It is well recognized that acute kidney injury (AKI) in developed countries affects the elderly more, while in developing countries AKI is a disease of the young and children in whom pre-renal causes are predominant (2,3).
In Sudan, AKI is an important cause of morbidity and mortality due to the high prevalence of many tropical diseases such as malaria, sepsis, diarrheal diseases and respiratory tract infections (4). Worldwide, it is estimated that of 10.6 million deaths reported in children under the age of 5 years between 2000 and 2003 in developing countries, 0.62 million died from malaria and 1.4 million from diarrheal diseases (5).
Peritoneal dialysis (PD) in developing countries is the preferred treatment for AKI in children for all age groups including neonates (6). In developed countries, although continuous renal replacement therapy (CRRT) is gaining more popularity and has certain advantages, particularly in patients who are hemodynamically unstable or who have multiorgan failure, PD in children remains an appropriate therapy for AKI from many causes, even in severely ill children requiring vasopressor support (7,8,9). It is cheap, can be utilized in many clinical settings and requires less sophisticated equipment than hemodialysis (HD), and PD access can be obtained relatively quickly and safely at bed side. Moreover, the Brazilian group from Sao Paolo using randomized trial design were able to show that results with PD in adults are at least as good as those with HD (10).
Although Sudan was the leading country in Africa to start peritoneal dialysis (it started in 1968 in the adult population), children had limited access to PD and only few older children were treated by physicians until 2001. Recently (2004), a specialized pediatric nephrology unit was established at Soba University Hospital in the capital, Khartoum (11). This unit delivers PD, HD and kidney transplant; it is supported by the Sudan government and charity organizations. All medications and renal replacement therapy are delivered free of charge for children.
Improved nephrology services and availability of cost-effective dialysis facilities in developing countries may reduce mortalities and save many children. We reviewed the data of all children with AKI who have been referred and treated at the pediatric nephrology unit - Soba University Hospital, Sudan.
Patients and Methods
This is a retrospective review of all children who have been admitted with AKI to the pediatric nephrology unit - Soba University Hospital, SUH. It is a 500-bed tertiary hospital in southern Khartoum. It is the main teaching hospital for the faculty of medicine - University of Khartoum and one of three big hospitals in Sudan. It was established in 1975 and hosts the largest medical subspecialties in the country. It serves as a referral hospital for about 5.3 million people in Khartoum state and receives patients from other states as well.
The hospital has an important international link with hospitals and institutions abroad and, through this link, two pediatricians were trained initially at the pediatric nephrology unit in Nottingham, UK, in early 2000, within the context of the Masters of Nephrology at the University of Sheffield (11,12). After returning, they started general nephrology clinics and limited facilities for acute PD and HD services within the existing adult unit. They made every possible contact to establish pediatric nephrology services, which came to reality in December 2004 after a generous donation from a donor from Qatar. Acute PD started in a 5-bed ward which acts as a filtering unit for all children with AKI from all over the country with 24 hour on-call service.
In May 2005, the Sudan PD program was established with an allocated budget for training, support and PD consumables under the umbrella of the ministry of health. The program attempted to encourage the use of PD, raise awareness of the availability of acute PD services and continuous ambulatory peritoneal dialysis (CAPD) through courses, practical sessions and community awareness (13). Structured PD training (Basic and Advanced PD courses) were started by the PD program with additional short training courses abroad through dialysis companies, the International Society for Peritoneal Dialysis (ISPD) and the International Pediatric Nephrology Association (IPNA).
Acute kidney injury was defined according to the RIFLE (Risk, Injury, Failure, Loss, End-stage renal disease) criteria/criteria of the international acute kidney injury work group (14). In summary, the criteria include creatinine ≥ 150% above normal levels for age and oliguria (≤0.5 mL/kg per hour for 6 hours) and/or the occurrence of acidosis, and/or urea, phosphate and potassium outside the normal range for age. Children were assessed initially by a trained resident doctor and the decision for treatment options were made in every case based on clinical and biochemical criteria after discussion with the pediatric nephrologist. These criteria included pulmonary oedema, ureamic pericarditis, intractable hypertension not responsive to drugs, ureamic symptoms and/or disturbed level of consciousness, severe acidosis, hyperkalemia (K > 7 mmol/L) and rapidly rising urea and creatinine.
Every attempt was made to treat the children conservatively with fluid management, bicarbonate, diuretics and antibiotics for sepsis before considering dialysis. If dialysis was indicated, all children were considered candidates for PD unless there was a reason to switch to HD such as older children, abdominal pathology or multiple scars, recent surgery, life-threatening hyperkalemia and parents’ refusal of PD.
Catheter Placement and Technique
Catheter placement was performed by a trained resident doctor, fellow or pediatric nephrologist according to local standard practice (reasonable aseptic technique, empty bladder, local anesthesia, IV line, etc.). General sedation in smaller children was used during the procedure. A hard catheter (Peritocat; peritoneal dialysis catheter, B. Braun Melsungen AG, Melsunge, Germany) was placed at the bedside using a blind technique. Dialysate consisted of commercially available lactate-based solution (Dianeal; Baxter Healthcare Corporation, Italy). All range of solutions, consumables and PD catheters were supported by the Sudan PD program and provided free for all children.
After cleaning the catheter site (usually lateral to the umbilicus) with povidine iodine, a small skin incision (slightly smaller than the diameter of the catheter) was made using a sharp pointed blade. We introduced the catheter with the stylette perpendicular to the abdominal wall while controlling the length with the dominant hand, until the peritoneum was pierced. The stylette was then withdrawn and the catheter gently pushed in, directing it towards either iliac fossa until all the perforations were well within the peritoneal cavity.
We then checked the catheter flow, fixed the catheter gently with one stitch suture and tightly fixed a plaster. Initially 2 - 3 quick cycles were done to ensure patency and good flow.
Dialysis was usually individualized. The exchange volume was done as follows:
Start at 20 - 30 mL/kg and observe for discomfort, cardio-respiratory changes or leakage at catheter site
The volume can be increased to a maximum of 35 ml/kg or 1,000 - 1,200 mL/m2 body surface area.
Fill volume is estimated in older children by weighing the fluid by hooking the whole bag on a spring scale and monitoring scale change during fill bag phase.
We make all other calculations and are sure that fluid chart and renal chart are in the patient notes.
Baseline laboratory studies are done and then on a daily basis until the patient achieves the desired fluid removal and solute clearance.
PD is performed manually by the nursing staff. It is initiated with 1.5% - if more rapid ultrafiltration is required, higher glucose concentrations (2.5% and 4.25% solutions) can be used.
It starts with quick hourly exchange cycles (10 minutes infusion and 15 - 20 minutes outflow) and then modified CAPD of 2 - 4-hour cycles is used for 3 - 5 days.
Peritonitis is common when dialysis is prolonged or when acute catheters are used for more than 5 days. Therefore, for patients who need a longer time on PD and are free of infection, a soft PD catheter is introduced or switched to HD.
Peritonitis was diagnosed according to the standard Sudan PD program guidelines which were adapted from the ISPD guidelines that include fever, abdominal pain and/or cloudy effluent and the biochemical features of WBCS of > 100 cells, gram stain and PD fluid culture (15). Empirical treatment of peritonitis was a combination of 1st generation of cephalosporin (Cephazolin) and 3rd generation (Ceftazidime) or Aminoglycoside (according to drug availability) and then we switched according to the result of culture and sensitivity. The treatment was free either through the PD program, the unit support or charity organizations.
Chronic kidney disease was defined as glomerular filtration rate (GFR) less than 50 mL/min per 1.73 m2 body surface area, estimated by the Schwartz formula (16).
Results
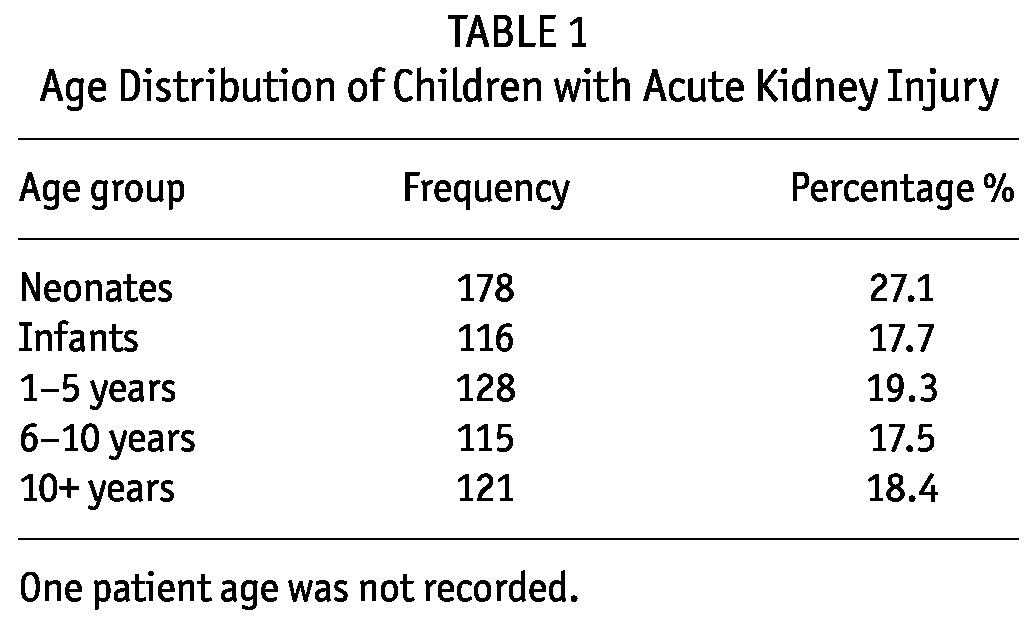
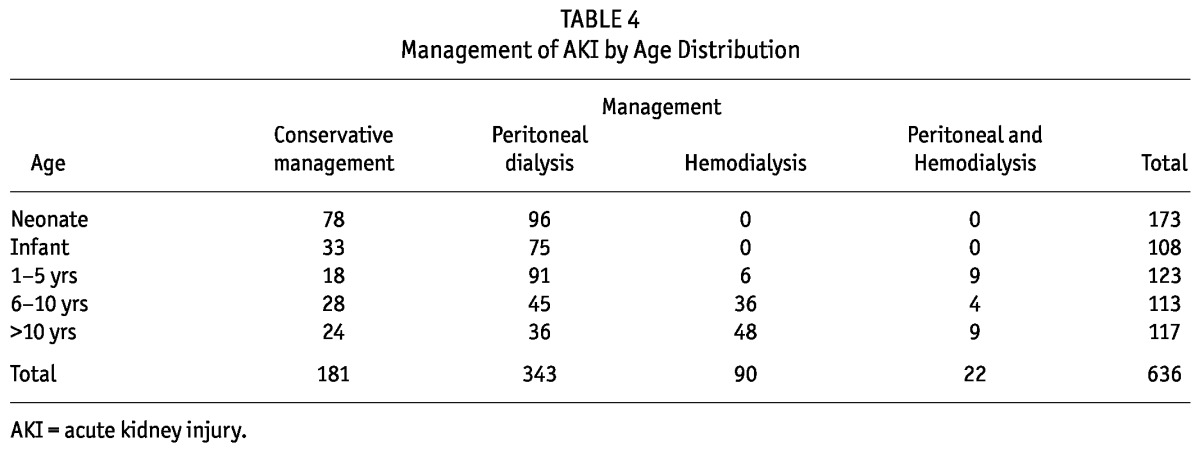
Over the 7-year period (January 2005 - December 2011), the unit received 659 children from different states of the country. There were 362 (54.9%) male and 297 (45.1%) female. The spectrum of age was variable, from neonates and small infants to adolescents, with the majority being neonates, 178 (27.1%), and the least common being children 6 - 10 years, 121 (17.5 %) (Table 1).
TABLE 1.
Age Distribution of Children with Acute Kidney Injury
Children were received from almost all states of the country with the majority coming from the capital Khartoum state 364 (60.7%) (Table 2). The average patient admission was 94 patients per year with only 25 admissions at the start of the service in 2005 compared to 128 patients in 2011 (Figure 1).
TABLE 2.
Number of Patients Admitted from Different States
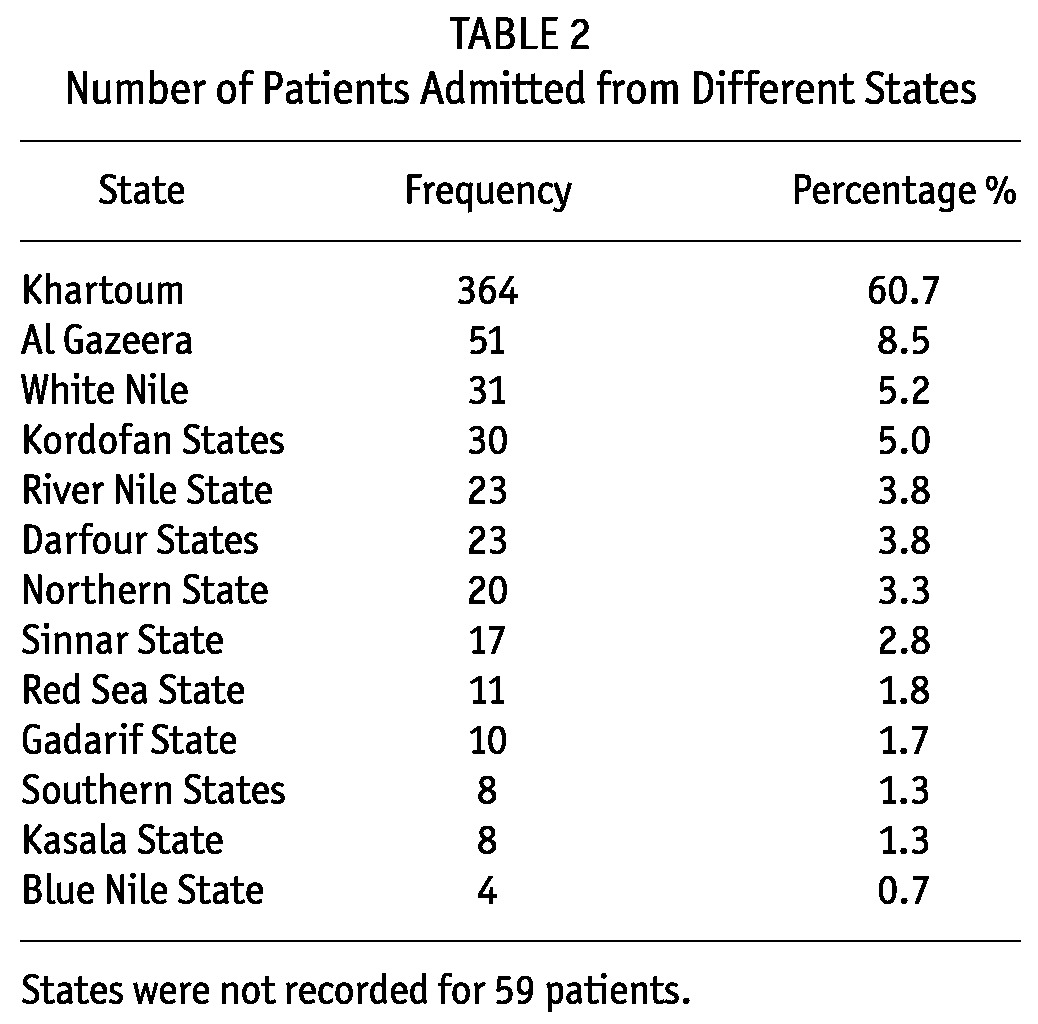
Figure 1 —
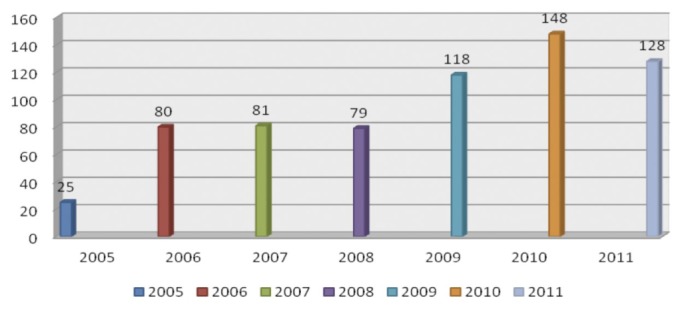
Admissions of children with AKI per year. AKI = Acute Kidney Injury.
The incidence of AKI for the whole country (before country separation) was estimated to be 2.6 patients/million population per year. However, because only a small number of children with AKI had access to services from other states compared to the capital, Khartoum, due to transportation difficulties, the use of traditional/herbal medicines and poverty, this incidence may not well represent the whole country. The population of cosmopolitan Khartoum is 5.3 million, and 364 children with AKI were from Khartoum state, giving an estimated incidence of 9.8 patients/million population/year.
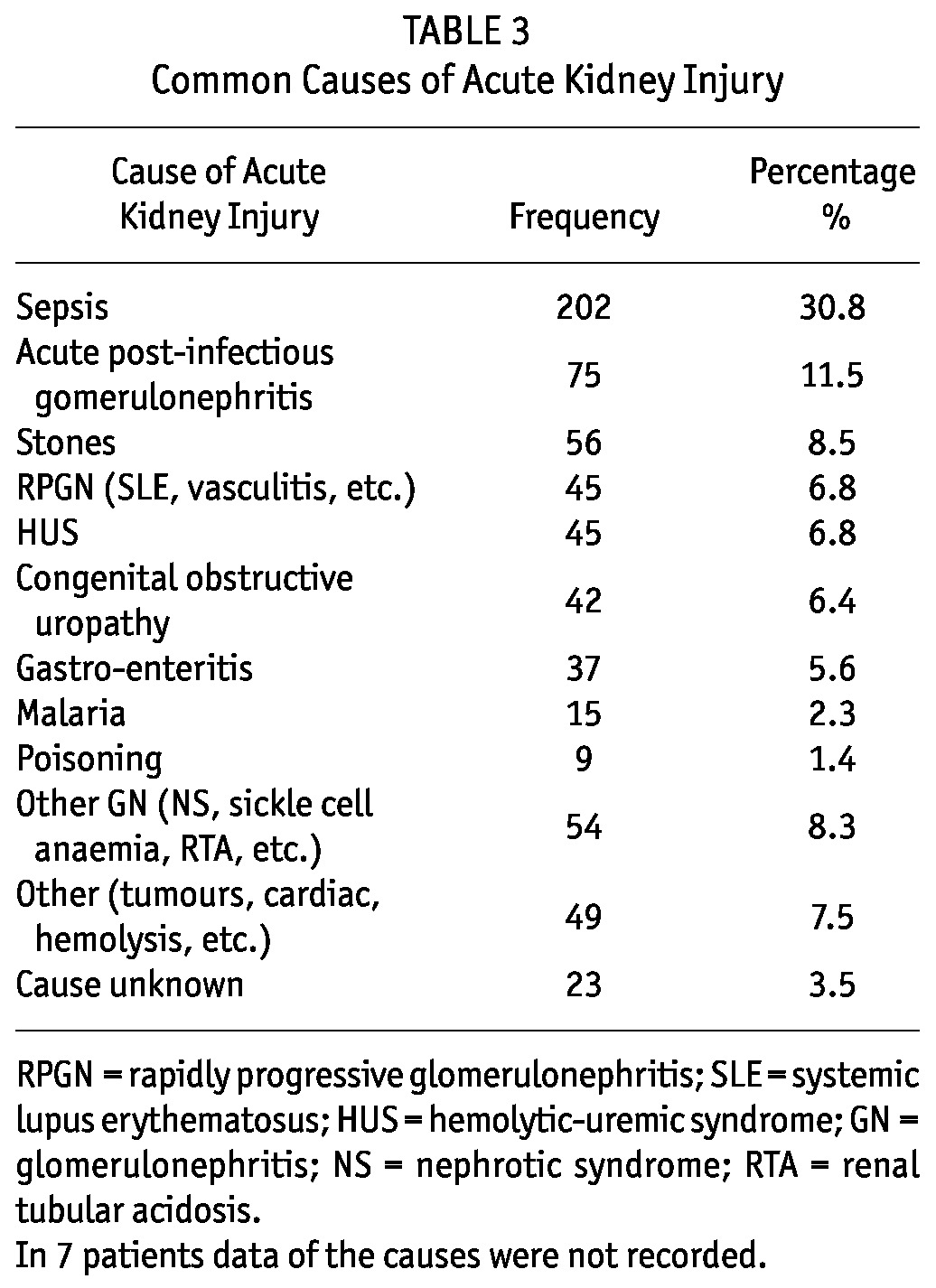
The most common causes of AKI were sepsis: 202 (30.8%); acute glomerulonephritis: 75 (11.5%); and obstructive uropathy due to stones: 56 (8.5%) (Table 3).
TABLE 3.
Common Causes of Acute Kidney Injury
A large number of children, 181 (27.7%), received conservative treatment (fluids, diuretics and antibiotics). The most common dialysis modality used for AKI was PD, 343 (52.4%). Few children received HD, while others received both PD and HD modalities (Figure 2, Table 4).
Figure 2 —
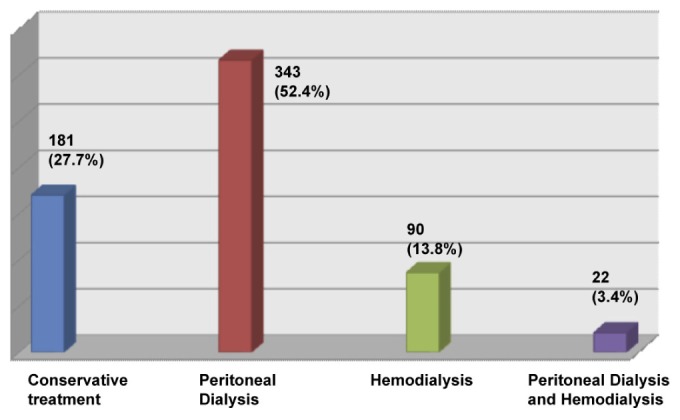
Type of renal replacement therapy for management of AKI. In 23 children, modality of treatment of AKI was not documented. AKI = acute kidney injury.
TABLE 4.
Management of AKI by Age Distribution
On average, PD was continued for 4.5 days (range: 2 - 9 days). When dialysis was needed for a longer period, a soft PD catheter was inserted or the patient was switched to HD. Few children required PD for more than 5 days (37, 10.7%), of whom 17 were shifted to HD (due to catheter malfunction, infection or unresponsiveness), 11 patients recovered (8 patients were using hard catheter and 3 soft catheter) and 9 patients continued PD using soft catheter for 2.5 - 3 months with recovery of 3 patients, 2 children developing chronic kidney disease (CKD) and another 3 children developing end-stage renal disease (ESRD) and continuing on CAPD.
There were few major complications reported: two children had urinary bladder injury which was fixed surgically in one patient and switched to HD, the other patient continued on PD. Six patients developed fresh hemorrhagic effluent and, in 3 patients, a major vessel was found to be injured that required laparotomy. Two patients had small bowel injury which required a switch to HD and conservative treatment with good recovery in one patient and the other patient died because of severe uremia as there was no available HD for infants.
Peritonitis was diagnosed in 53 (15.4 %) patients, of whom 16 patients switched modality due to peritonitis. Exit-site infection was seen in 4 (1.2%) patients. In 7 patients data of the causes were not recorded.
Outcome
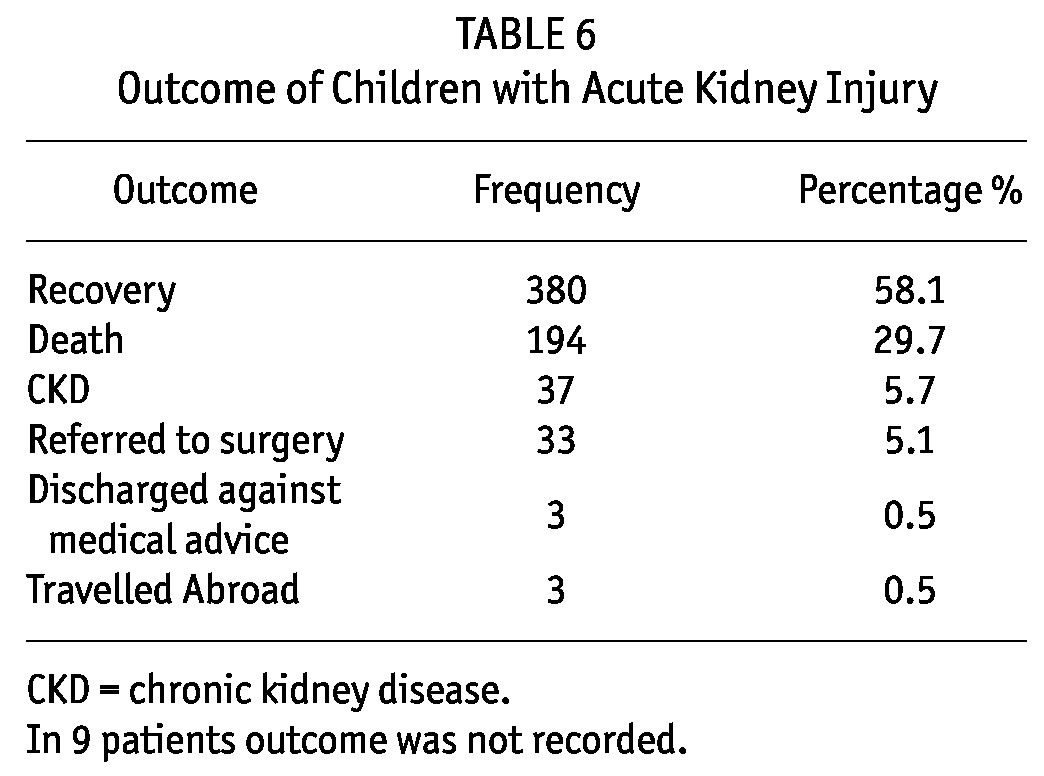
Recovery from AKI was achieved in 450 (68.9%) children. Among them 37 (5.7%) went into CKD and remained under follow-up, 33 (5.1%) were referred to the pediatric surgery for further surgical management (obstructive uropathy due to stones or posterior urethral valves) and 194 (29.7%) died (Table 5). The majority of deaths occurred in neonates and small infants, 35.6% and 27.3% respectively, while children aged 6 -10 years had the least mortalities: 19 (9.8%) (Table 5, 6).
TABLE 5.
Distribution of Mortality Between Age Groups
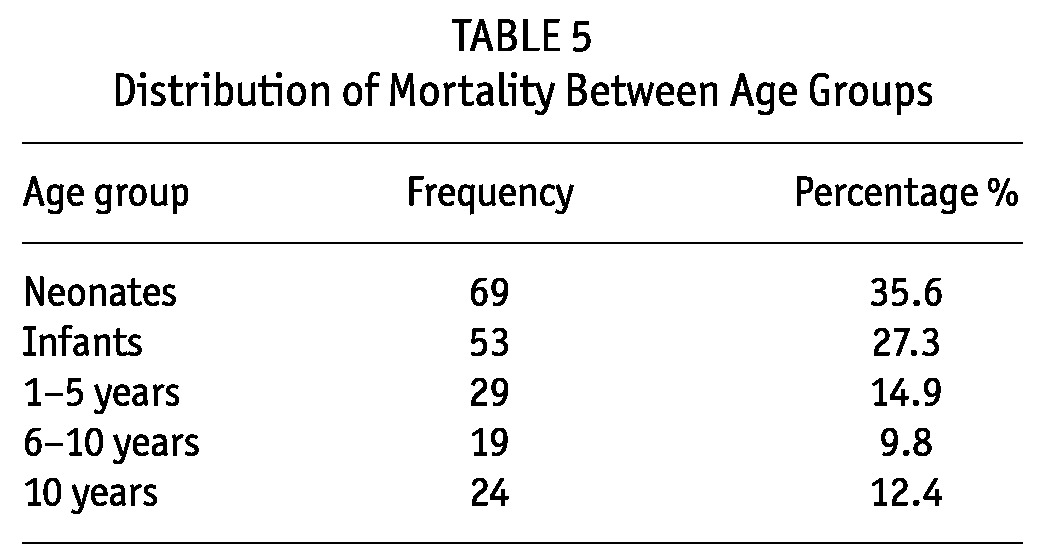
TABLE 6.
Outcome of Children with Acute Kidney Injury
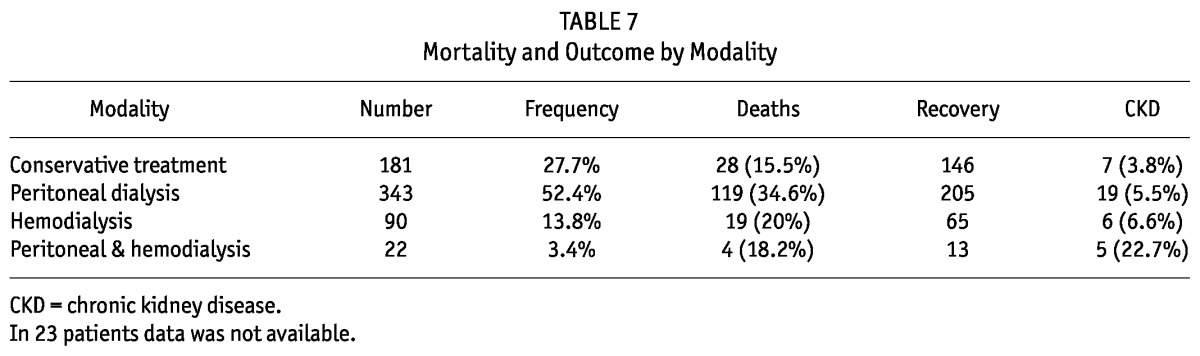
Of the 37 children who went into CKD, 5 (22.7%) were from the group who received both PD and HD modalities and 7 (3.8%) children were from the conservative treatment group (Table 7). Overall, 14 (37.8%) CKD children remained under clinic follow-up, 17 (46%) were lost to follow-up (they were from other states) and 6 (16.2%) went back to dialysis.
TABLE 7.
Mortality and Outcome by Modality
Discussion
In developing countries the epidemiology of AKI is poorly documented. This is in part due to meager resources and lack of trained personnel, lack of a formal infrastructure for epidemiological data collection and the huge variations in the catchment population and methodology (17). Moreover, a large proportion of countries from sub-Saharan Africa do not have any services for AKI management and the renal replacement therapy programs that exist are found in large cities only and are available to those who can afford to pay (6,18,19). The situation in Sudan is no different. However, the newly established pediatric nephrology unit in 2004 by donation, hospital and governmental support and community participation allowed us to serve many children in a tertiary hospital free of charge and to document this experience.
Over a 7-year period, 659 children with AKI were managed in the unit giving rise to an incidence of 9.8 children per million per year. To the best of our knowledge, this is the largest reported pediatric population of AKI from a single center in Africa. Our incidence of AKI is similar to a report from Nigeria of 221 children over 19 years in which Anochie et al. reported an incidence of 11.7 cases per million in a hospital that serves more than 1 million children (6). However, the exact incidence of AKI in children is unknown and estimates vary between African countries and regions. Higher incidence was reported among the adult populations in South Africa (20 per million) compared to a lower incidence among the adult population in Nigeria (20,21).
Children from almost all states of the Sudan are represented in the unit, with the majority, 364 (60.7%), coming from the capital, Khartoum. The change from no renal services to a huge burden of care (PD, CAPD, HD and transplant) gave us an opportunity to build up the unit, develop experience and focus our efforts to improve the service. The staff of the unit were trained very well locally and abroad and became trainers for staff coming from other states that started to develop PD services in big cities.
The most common causes for AKI in our study were sepsis, infections and obstructive uropathy due to stones. This is similar to studies from Sub-Saharan Africa in which the etiology of AKI differs from that of developed countries, with infectious diseases contributing to an enormous proportion of causes, followed by gastroenteritis, acute glomerulonephritis and toxins (4,6). In developed countries, the majority of pediatric AKI cases are related to surgery, toxicity and sepsis, and in very few cases the cause can be attributed to hemolytic uremic syndrome (22). However, the increased number of children with AKI due to stones is due to the fact that our unit is also linked to one of the largest pediatric surgery units in the country, which receives surgical urology cases from different parts of the country.
Of the 659 children referred with the diagnosis of AKI, 181 (27.7%) did not require dialysis and they were managed with fluids, diuretics and/or antibiotics with the least documented mortality among them: 28 (15.5 %). In developing countries, it has been documented that pre-renal causes are common and some would respond to conservative management, unlike those in developed countries, in which intrinsic renal failure is common and more likely to need dialysis (23,24). Moreover, in developing countries, it is always important to re-assess and re-investigate AKI patients referred from rural areas to tertiary hospitals because in many of them dialysis might not be needed. Although prevention is the best approach and fluid and electrolyte repletion is an inexpensive treatment option for dehydration in rural areas, in many circumstances neither practice is carried out due to lack of resources, deficient education and training, and political instability.
PD was done in 343 (52.4%) children compared to HD, 90 (13.8%,) with only 22 (3.5%) children switched from PD to HD due to either PD complications, unresponsiveness to treatment or long stay on PD with hard catheter.
It is well known that neonates and infants with AKI have the highest incidence and mortality among all pediatric age groups (22,25,26). In our study, neonates and infants constituted 27.1% and 17.7 % respectively with the highest reported mortality. It is worth mentioning that most of those children were critically ill, septic and had multiorgan failure. The Pediatric intensive care unit (PICU) and CRRT was not available and the only dialysis modality available for them was PD, which was carried out in 55% of neonates and 75% of infants with a survival rate of 54.7% and 50%, respectively.
Mortalities were less in patients who needed HD, 19 (21 %), compared to PD, 119 (34.6%) patients. However, this would not prove that HD is better than PD because of the effect of bias by indication (most PD patients are small children). When neonates with AKI were excluded, we had a mortality of 18% in all other PD age groups, which is comparable to other dialysis modalities. In a similar environment, PD has been shown to perform comparably to HD. This was demonstrated in a controlled clinical trial which compared the effectiveness and cost of HD with PD using locally made PD fluids in Nigeria. The trial found that the outcomes were comparable, but PD was significantly cheaper (27).
Overall recovery from AKI was achieved in 450 (68.9%) children. Among them 37 (5.7%) went into CKD and remained under follow-up, 33 (5.1%) were referred to pediatric surgery for further surgical management and 194 (29.7%) died. The relatively good clinical outcomes experienced in our study, despite meager resources, suggest a promising future for acute PD in the setting of AKI in Sub-Saharan Africa. Those good outcomes have been achieved despite the challenges of late patient referral, limited supporting resources, and limited availability of diagnostic and microbiologic testing.
Cost, procurement and transport of consumables are major challenges in Sub-Saharan Africa that lead to interruptions in the supply and discontinuation of treatment (17). We feel that full local commitment of the Sudan PD program, non-governmental organization support, availability of professional skills and experience through local and international training, suitable hospital environment and the availability of consumables (range of PD solutions, PD lines, and catheters) have made it possible to sustain our PD program. It would be better if, in the future, dialysis industries manufacture PD solutions and supply chains locally in Africa to reduce costs and expand PD programs.
In conclusion, in the setting of developing countries where AKI is a common cause of morbidity and mortality, reasonably equipped renal units with adequately trained medical staff in conservative management and PD may save many lives. International funding programs of communicable diseases and charity organizations should include AKI management in their programs. It is very important to re-consider PD as the modality of choice for the treatment of AKI in developing countries.
Disclosures
All the authors declared no financial conflicts of interest.
Acknowledgments
This work was supported by Soba University Hospital, the Sudan PD Program and the Sudan National Centre for Kidney Diseases and Transplantation. We would like to acknowledge the staff of the Children’s Renal & Urology Unit, Nottingham University Hospitals, UK for the bidirectional training visits of our staff.
References
- 1. United Nations Development Programme (UNDP) 2012. Sudan; The land and the People. http://www.sd.undp.org/sudan%20overview.htm
- 2. Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 2006; 17:1143–50 [DOI] [PubMed] [Google Scholar]
- 3. Kohli HS, Bhat A, Jairam A, Aravindan AN, Sud K, Jha V, et al. Predictors of mortality in acute renal failure in a developing country: A prospective study. Ren Fail 2007; 29:463–9 [DOI] [PubMed] [Google Scholar]
- 4. Kaballo BG, Khogali MS, Khalifa EH, Khaiii EA, Ei-Hassan AM, Abu-Aisha H. Patterns of “severe acute renal failure” in a referral center in Sudan: excluding intensive care and major surgery patients. Saudi J Kidney Dis Transpl 2007; 18(2):220–5 [PubMed] [Google Scholar]
- 5. Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005; 365:1147–52 [DOI] [PubMed] [Google Scholar]
- 6. Anochie IC, Eke FU. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol 2005; 20(11):1610–4 [DOI] [PubMed] [Google Scholar]
- 7. Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in pa tients with acute kidney injury. Kidney Int Suppl 2008; (108):S87–93 [DOI] [PubMed] [Google Scholar]
- 8. Ellis EN, Pearson D, Belsha CW, Berry PL. Use of pump-assisted hemofiltration in children with acute renal failure. Pediatr Nephrol 1997; 11:196–200 [DOI] [PubMed] [Google Scholar]
- 9. Forni LG, Hilton PJ. Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med 1997; 336:1303–9 [DOI] [PubMed] [Google Scholar]
- 10. Flynn JT, Kershaw DB, Smoyer WE, Brophy PD, McBryde KD, Bunchman TE. Peritoneal dialysis for management of pediatric acute renal failure. Perit Dial Int 2001; 21:390–94 [PubMed] [Google Scholar]
- 11. Watson AR, Abdelraheem M, Ali El-T, Jepson S, Razig SA. Developing pediatric nephrology in a low income country using a sister centre link: The Sudan Experience. Pediatr Nephrol 2010; 25:1569–71 [DOI] [PubMed] [Google Scholar]
- 12. Ali El-T, Abdelraheem M, Mohamed RM, Hassan EG, Watson AR. Chronic renal failure in Sudanese children; Aetiology and outcome. Pediatr Nephrol 2009; 24:349–53 [DOI] [PubMed] [Google Scholar]
- 13. Elhassan E, Kaballo B, Fedail H, Abdelraheem M, Ali T, Medani S, et al. Peritoneal dialysis in the Sudan. Perit Dial Int 2007; (27):503–10 [PubMed] [Google Scholar]
- 14. Maccariello E, Soares M, Valente C, Nogueira L, Valenca RV, Machado JE, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intens Care Med 2007; 33:597–605 [DOI] [PubMed] [Google Scholar]
- 15. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis related infectious complications recommendations: 2005 Update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 16. Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescents. Pediatr Clin North Am 1987; 34:571–90 [DOI] [PubMed] [Google Scholar]
- 17. Carter M, Kilonzo K, Odiit A, Kalyesubula R, Kotanko P, Nathan W, et al. Acute peritoneal dialysis treatment programs for countries of the East African community. Blood Purif 2012; 33:149–52 [DOI] [PubMed] [Google Scholar]
- 18. Yeates K, Cruz DN, Finkelstein FO. Re-examining the role of peritoneal dialysis to treat patients with acute kidney injury. Perit Dial Int 2012. May-Jun; 32(3):238–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ademola AD, Asinobi AO, Ogunkunle OO, Yusuf BN, Ojo AE. Peritoneal dialysis in childhood acute kidney injury: experience in Southwest Nigeria. Perit Dial Int 2012. May-Jun; 32(3):267–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seedat YK, Nathoo BC. Acute renal failure in blacks and Indians in South Africa: Comparison after 10 years. Nephron 1993; 64:198–201 [DOI] [PubMed] [Google Scholar]
- 21. Okoye OC, Unigbe EI, Ojogwu LI. Acute kidney injury in adult Nigerians: a single center experience. (Abstract). NANCONF 2010; ABS-OR-1004:4–5 [Google Scholar]
- 22. Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 2005; 45:96–101 [DOI] [PubMed] [Google Scholar]
- 23. Khakurel S, Satyal PR, Agrawal RK, Chhetri PK, Hada R. Acute renal failure in a tertiary care center in Nepal. JNMA J Nepal Med Assoc 2005; 44:32–5 [PubMed] [Google Scholar]
- 24. Strazdins V, Watson AR, Harvey B. Renal replacement therapy for acute renal failure in children; European guidelines. Pediatr Nephrol 2004; 19:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moghal NE, Brocklebank JT, Meadow SR. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol 1998; 49:91 [PubMed] [Google Scholar]
- 26. Shaheen IS, Watson AR, Harvey B. Acute renal failure in children: etiology, treatment and outcome. Saudi J Kidney Dis Transpl 2006; 17:153–8 [PubMed] [Google Scholar]
- 27. Arogundade FA, et al. An analysis of the effectiveness and benefits of peritoneal dialysis and hemodialysis using Nigerian made PD fluids. Afr J Med Med Sci 2005; 34:227–33 [PubMed] [Google Scholar]


