Acute kidney injury (AKI) is a common disorder that affects approximately 30% of patients in intensive care units (ICU) (1). Recent studies have shown that peritoneal dialysis (PD) can be an option for select AKI patients for promoting adequate metabolic and fluid control with a mortality rate similar to that of other dialysis modalities (2-7).
However, PD can increase intra-abdominal pressure (IAP) after dialysate infusion leading to impaired diaphragm mobilization, decreasing inspiration and expiration pressure, total pulmonary capacity and functional residual capacity, which may cause or worsen respiratory failure (8-12).
Despite the important association between AKI and injured lungs in the prognosis of patients, there are no studies on the influence of PD on respiratory mechanics of AKI patients. Thus, this study aimed to evaluate the respiratory mechanics, oxygenation and IAP in mechanically ventilated AKI patients undergoing high volume PD.
Methods
This was a prospective cohort study that evaluated respiratory mechanics in 44 high-volume PD sessions performed in 20 AKI patients undergoing mechanical ventilation and admitted to the Clinical Hospital of Botucatu School of Medicine over 18 consecutive months. This study was approved by the medical ethics committee for local research and informed consent was obtained from all participants or their legal representatives.
Inclusion criteria were patients older than 18 years, with a clinical diagnosis of AKI according to Acute Kidney Injury Network (AKIN) criteria (13) caused by ischemic, nephrotoxic or mixed acute tubular necrosis, treated with PD for at least one session and undergoing mechanical ventilation.
Exclusion criteria were patients with pre- and post-renal AKI etiology, severe hemodynamic instability (systolic blood pressure below 80 mmHg or using norepinephrine at a dose exceeding 1 ucg/kg/min), absolute contraindications for PD use, early mechanical complications related to PD (occurring within 24 hours), pregnancy, severe chronic renal disease (baseline serum creatinine > 4 mg/dl), kidney transplant, patients undergoing tracheostomy, in which it was not possible to perform measurements of respiratory mechanics appropriately due to lack of deep sedation and patients on alveolar recruitment (with positive end-expiratory pressure (PEEP) > 10 cm H2O).
Acute kidney injury patients were treated with continuous high-volume PD modality which is designed to achieve higher small-solute clearances. It is performed using automated cyclers, a flexible catheter, and a high volume of dialysis fluid as described in previous studies. Each session of high-volume PD lasts 24 hours, and sessions are repeated daily, 7 times per week. Prescribed Kt /V was 0.60 per session and the total dialysate volume per session ranged from 36 L to 44 L (2 L per cycle) (2-7). Peritoneal access was established by a nephrology team through the percutaneous Tenckhoff catheter using a trocate. The dialysate used was Dianeal (Baxter Healthcare Corporation, Deerfield, IL, USA)(Na = 132 mEq/L, Ca = 3.5 mEq/L, K = 0 mEq/L = 1.5 mEq Mg/L = 40 lactate mEq/L, glucose = 1.5, 2.5 or 4.25%) and the exchanges were performed using a HomeChoice cycler (Baxter Healthcare Corporation, Deerfield, IL, USA).
We evaluated IAP, respiratory mechanics and oxygenation. Respiratory mechanics and IAP were evaluated at 5 moments during 3 days of dialysis. On the first dialysis day, patients were evaluated at 3 moments: M0 (pre-dialysis and dry state), M1 (post-infusion of dialysate: filled state) and M2 at the end of dialysis (dry state). On the second and third dialysis days, patients were evaluated only at the end of the dialysis session (M3 and M4: dry state) (Figure 1).
Figure 1 —
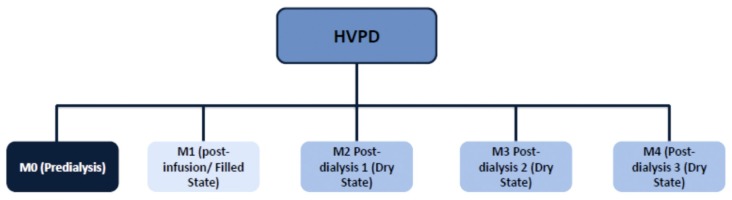
Moments that respiratory mechanics and intra-abdominal pressure were evaluated. HVPD = high-volume peritoneal dialysis.
The estimate of the compliance (Psc) and respiratory system resistance (Rsr) was based on the technique of end-inspiratory occlusion (14). Such measures require the paralysis of the patient to eliminate spontaneous inspiratory effort (15). Thus, the patients who were not previously sedated were temporarily sedated according to physician evaluation and prescription, until they reached the level of deep sedation (-5 on the Richmond Agitation-Sedation Scale) (16) and absence of spontaneous inspiratory effort. If it was necessary, patients were paralyzed.
Patients were ventilated using a constant flow (square wave) volume controlled mode with an inspiratory pause of at least 2 seconds, PEEP of 5 cm H2O and a tidal volume of 6 mL/kg (ideal weight). At each assessment, 5 consecutive values of respiratory pressure peak (PIP), plateau pressure (PLATP), tidal volume (Vt), inspiratory flow (V’) and auto-PEEP were collected and the average value was used for the calculations (16).
The efficiency of gas exchange was assessed pre- and post-dialysis daily by an oxygenation index that corresponds to the ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) (17), and also by the fraction of inspired oxygen (FiO2), expressed as a percentage.
Intra-abdominal pressure was measured by indirect technique using a 3-way intravesical catheter, based on the original method of Kron (18). Twenty-five mL of saline were instilled into the bladder and the patient was levelled in order to be in complete supine position with zero reference point at the mid-axillary line, as determined by consensus intra-abdominal hypertension/abdominal compartment syndrome (IAH/ACS) (19). After saline infusion, it was expected that the pressure monitor would be stabilized at a fixed value, so as to ensure its reliability (26).
Data analysis was performed using SAS for Windows, version 9.2 (SAS Institute Inc., Cary, NC, USA). Variables with normal distribution were described as mean ± standard deviation and the variables with non-normal distribution, as median and interquartile range.
For the analysis of variables Rsr and PaO2/FiO2, repeated measures ANOVA and multiple comparisons adjusted by Tukey were used. Comparisons of variables Psc, IAP and FiO2 were performed using repeated measures model and asymmetric distribution (Gama) using the GENMOD procedure. Multiple comparison tests were performed by the same procedure DIFF option. The same type of corrected adjustment was used for chronic obstructive pulmonary disease (COPD) patients, pulmonary infection, and invasive mechanical ventilation (IMV) time.
In all tests, the significance level considered was 5%.
Results
During the study period, a total of 106 patients were treated by dialysis: 26 by PD (24.5 %) and 80 by hemodialysis (HD) (75.5%). Absolute contraindication for PD occurred in 61 patients (57.5%) and they were: recent abdominal surgery (< 1 month), multiple abdominal surgeries (> 3), severe hyperkalemia with electrocardiogram changes, severe respiratory failure (FiO2 > 70%) and severe fluid overload. Six patients treated with PD were excluded (23%); 3 patients had mechanical complications related to the peritoneal catheter in the first 24 h of dialysis, and 3 had tracheostomy.
Twenty AKI patients who underwent 44 sessions of high-volume PD were evaluated. Eleven patients had 3 PD sessions analyzed, 8 patients had 2 PD sessions and 1 patient had only 1 session analyzed. The number of patients included on the final day was 11.
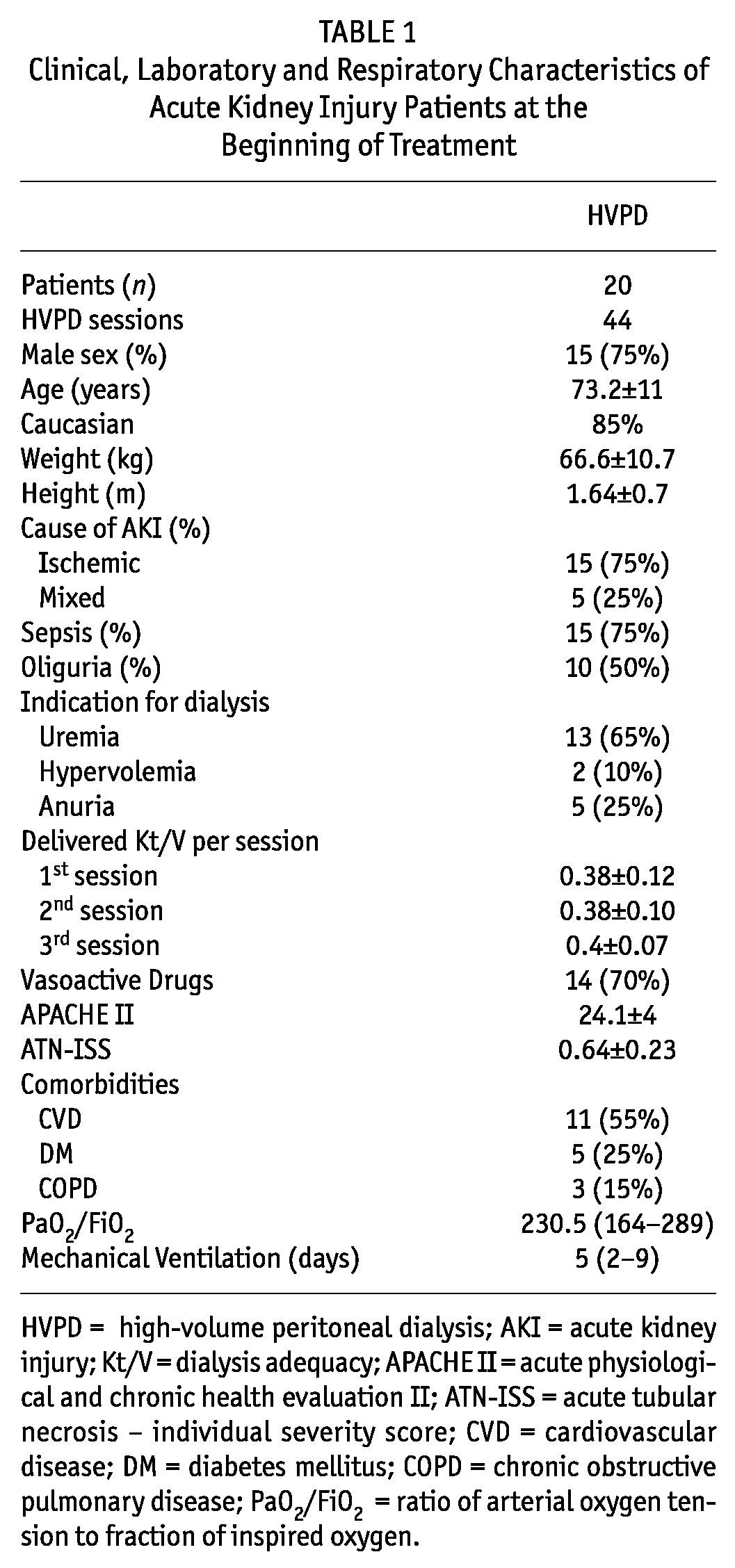
Table 1 shows the clinical, laboratory, and dialysis characteristics of AKI patients undergoing high-volume PD.
TABLE 1.
Clinical, Laboratory and Respiratory Characteristics of Acute Kidney Injury Patients at the Beginning of Treatment
Evaluation of respiratory mechanics and IAP are shown in Table 2. The estimate of the compliance improved significantly after 3 sessions of high-volume PD and Rsr remained stable, without significant changes. Intra-abdominal pressure increased significantly after the first dialysate infusion. However, after subsequent drainages these values decreased, reaching values close to baseline after the third PD session. Regarding oxygenation parameters, FiO2 did not change during the first and the second PD sessions and decreased after 2 sessions. PaO2/FiO2 increased progressively after 1 dialysis session.
TABLE 2.
Respiratory Mechanics and Intra-Abdominal Pressure of Acute Kidney Injury Patients Treated with Peritoneal Dialysis
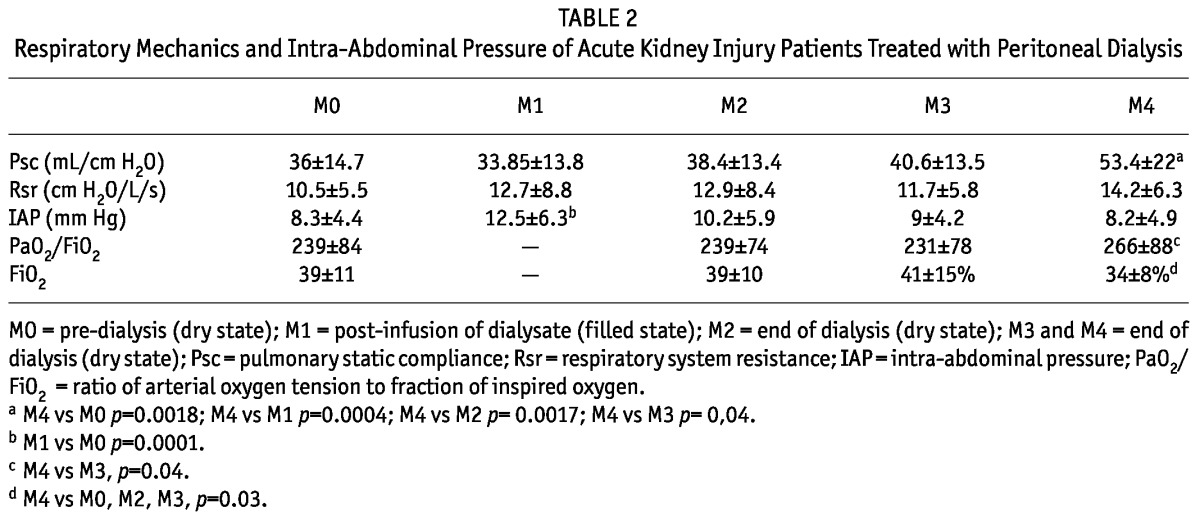
After adjustment for the presence or absence of COPD, lung infection and IMV time, there were no changes in results obtained on mechanical ventilation, oxygenation and IAP.
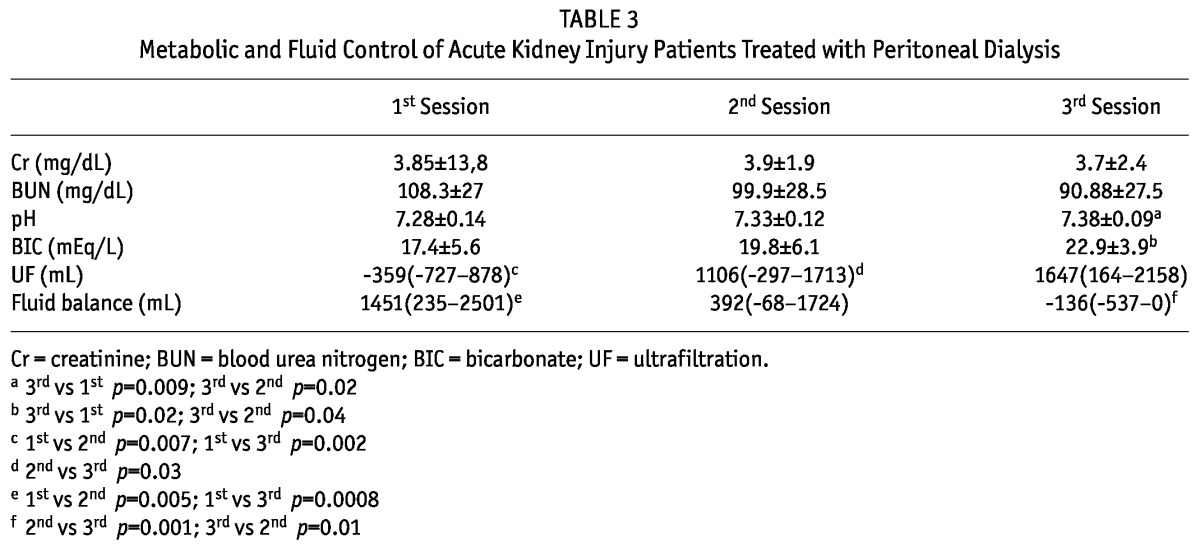
The metabolic and fluid control of AKI patients treated with high-volume PD is shown in Table 3. After the second session, serum creatinine stabilized. Ultrafiltration increased progressively during the treatment, while fluid balance decreased with the treatment.
TABLE 3.
Metabolic and Fluid Control of Acute Kidney Injury Patients Treated with Peritoneal Dialysis
Discussion
This prospective study aimed to evaluate the effects of PD on respiratory mechanics in AKI patients undergoing mechanical ventilation. There are few previous studies on PD in AKI and its respiratory implications were not evaluated in the majority of them.
Peritoneal dialysis can be associated with a number of complications that are directly related to hydrostatic pressure changes in the abdominal cavity or changes in the intraperitoneal volume that occur during PD, which are further complicated by a rise in the IAP. The rise in IAP is reported to be proportional to the volume infused. A rise in IAP leads to the serious complication of IAH and ACS in patients admitted to the critical care department. Furthermore, the increase in abdominal pressure causes diaphragmatic pleural pressure changes leading to decreased pulmonary compliance and total pulmonary capacity (15-19).
In this study, 45% of patients had increased IAP (≥ 7 mm Hg) at initial evaluation (M0). Malbraim et al. (20) reported that about 30% of critically ill patients have increased IAP. These results are most likely a consequence of the severity of the patients’ illness and high-volume replacement in sepsis. According to Pelosi et al. (11), IAP of 12 mm Hg is sufficient to cause significant decrease in pulmonary compliance. In an experimental model of acute lung injury, pulmonary edema increased twice after IAP had been increased to 15 mm Hg (21).
However, in this study there was a progressive increase in Psc, showing improvement in respiratory mechanics in patients undergoing high-volume PD. This finding can be explained, at least partially, by IAP levels kept stable. In fact, after infusion of dialysate (filled state), there was significant increase in IAP without reaching critical levels and without worsening respiratory mechanics. Furthermore, IAP increase was not maintained after drainage (dry state).
Reduction in fluid balance due to an increase in ultrafiltration may have contributed to decrease in pulmonary edema and consequently an improvement in Psc. Similar results were obtained by Werner et al. (22), who evaluated 32 AKI children undergoing PD. The authors found a reduction in airway pressure, suggesting improvement in respiratory mechanics. Clinical data show that positive fluid balance and oliguria can contribute negatively to pulmonary function, leading to increased time of IMV (23,24).
Some authors suggest that PD may lead to a reduction in lung volumes, including functional residual capacity. Low functional residual capacity can cause collapse of small airways, which can lead to a decrease in ventilation, perfusion matching and consequently arterial hypoxemia (25). In this study, in AKI patients treated with PD, hypoxemia did not worsen and PaO2/FiO2 increased progressively. These findings are consistent with Sagy and Silver (26) who found increased PaO2/FiO2 in AKI children undergoing PD.
Removal of uremic toxins by PD is another hypothesis that can be considered for the improvement of Psc in mechanically ventilated AKI patients. Harper et al. (27,28) found a significant increase in pulmonary vascular permeability to water and protein in uremic patients. The increase in vascular permeability can lead to edema and this can affect lung expansion. In this study, after 3 high-volume PD sessions, urea and creatinine levels were stable.
Regarding the evaluation of Rsr, the present study found values higher than expected for most patients before PD treatment (M0). These findings may be related to factors such as mucosal edema of airways, bronchospasm, the presence of secretions, and condensation of liquids in the system’s mechanical respirator. However, there was no significant difference in Rsr after PD treatment. Respiratory system resistance remained stable and did not decline with fluid removal.
The present study has some limitations, such as having been performed in a small number of sessions per patient, with differences in numbers of patients at different moments of evaluation and at a single center. Besides, only 1 evaluation was performed at filled status, which may have an influence on the interpretation of respiratory mechanics in relation to IAP. Other possible limiting factors, such as the presence or absence of COPD, infectious pulmonary disease, and time of IMV, were eliminated after statistical adjustment. Despite showing some limitations, this is the first study to describe the effects of PD on respiratory mechanics in mechanically ventilated AKI patients. Furthermore, results suggested that PD does not appear to worsen respiratory mechanics despite a modest increase in IAP.
Disclosures
The authors have no financial conflicts of interest to declare.
References
- 1. Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med 2010; 38:268–75 [DOI] [PubMed] [Google Scholar]
- 2. Ponce D, Caramori JCT, Balbi AL, Barretti P. Peritoneal dialysis in acute kidney injury: Brazilian experience. Perit Dial Int 2012; 32:242–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponce D, Berbel MN, Regina de Goes C, Almeida CTP, Balbi AL. High-volume peritoneal dialysis in acute kidney injury: indications and limitations. Clin J Am Soc Nephrol 2012; 7(6):887–94 [DOI] [PubMed] [Google Scholar]
- 4. Gabriel DP, Balbi AL, Amerling R. Advances in peritoneal dialysis in acute kidney injury. Blood Purif 2012; 34:107–16 [DOI] [PubMed] [Google Scholar]
- 5. Ponce D, Berbel MN, Abrão JMG, Goes CR, Balbi AL. A randomized clinical trial of high-volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. Int Urol Nephrol 2012; 1:1–10 [DOI] [PubMed] [Google Scholar]
- 6. Ponce D, Balbi AL. Peritoneal dialysis in acute kidney injury: a viable alternative. Perit Dial Int 2011; 31:387–9 [DOI] [PubMed] [Google Scholar]
- 7. Goes CR, Berbel MN, Balbi AL, Ponce D. Metabolic implications of peritoneal dialysis in acute kidney injury patients. Perit Dial Int 2013; accepted for publication [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gavelli G, Zompatori Thoracic complications in uremic patients and in patients undergoing dialytic treatment: state of the art. Eur Radiol 1997; 7:708–17 [DOI] [PubMed] [Google Scholar]
- 9. Hughes GC, Ketchersid TL, Lenzen JM, Lowe JE. Thoracic complications of peritoneal dialysis. Ann Thorac Surg 1999; 67:1518–22 [DOI] [PubMed] [Google Scholar]
- 10. Mahale AS, Katyal A, Khanna R. Complications of peritoneal dialysis related to increased intra-abdominal pressure. Adv Perit Dial 2003; 19:130–5 [PubMed] [Google Scholar]
- 11. Pelosi P, Quintel M, Malbrain ML. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg 2007; 62(1):78–88 [DOI] [PubMed] [Google Scholar]
- 12. Toens CH, Schachtrupp A, Hoer J, Junge K, Klosterhalfen B, Schumpelick V. Porcine model of abdominal compartment syndrome. Shock 2002; 18:37–45 [DOI] [PubMed] [Google Scholar]
- 13. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network (AKIN): report of an intitiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levy P, Similowski T, Corbeil C, Albala M, Pariente R, Milic-Emili J, et al. A method for studying the static volume-pressure curves of the respiratory system during mechanical ventilation. J Crit Care 1989; 4:83–9 [Google Scholar]
- 15. Grinnan DC, Truwit JD. Clinical review: respiratory mechanics in spontaneous and assisted ventilation. Crit Care 2005; 9:472–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients - reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289:2983–91 [DOI] [PubMed] [Google Scholar]
- 17. Gilbert R, Keighley JF. The arterial/alveolar oxygen tension ratio. An index of gas exchange applicable to varying inspired oxygen concentrations. Am Rev Respir Dis 1974; 109:142 [DOI] [PubMed] [Google Scholar]
- 18. Kron IL, Harman PK, Nolan SP. Measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg 1984; 199:28–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med 2007; 33:951–62 [DOI] [PubMed] [Google Scholar]
- 20. Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intra-abdominal hypertension in a mixed population of critically ill patients. A multiple-center epidemiology study. Crit Care Med 2005; 33(2):315–22 [DOI] [PubMed] [Google Scholar]
- 21. Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, et al. An increase of abdominal pressure increases pulmonary oedema in oleic acid induced lung injury. Am J Respir Crit Care Med 2004; 169(4):534–41 [DOI] [PubMed] [Google Scholar]
- 22. Werner HA, Wensley DF, Lirenman DS, LeBlanc JG. Peritoneal dialysis in children after cardiopulmonary bypass. J Thorac Cardiovasc Surg 1997; 113:64–70 [DOI] [PubMed] [Google Scholar]
- 23. Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol 2010; 6:107–15 [DOI] [PubMed] [Google Scholar]
- 24. Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL; Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 2008; 12:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahale AS, Katyal A, Khanna R. Complications of peritoneal dialysis related to increased intra-abdominal pressure. Adv Perit Dial 2003; 19:130–5 [PubMed] [Google Scholar]
- 26. Sagy M, Silver P. Continuous flow peritoneal dialysis as a method to treat severe anasarca in children with acute respiratory distress syndrome. Crit Care Med 1999; 27(11): 2532–6 [DOI] [PubMed] [Google Scholar]
- 27. Harper SJ, Bates DO. Endothelial permeability in uremia. Kidney Int 2003; 63(84):S41–4 [DOI] [PubMed] [Google Scholar]
- 28. Harper SJ, Tomson CRV, Bates DO. Human uremic plasma increases microvascular permeability to water and protein in vivo. Kidney Int 2002; 61:1416–22 [DOI] [PubMed] [Google Scholar]


