Editor:
Providencia represents a genus of urease-producing gram-negative bacilli, and the rettgeri species was the first to be isolated in 1904 (1). Providencia species are closely related to Proteus and Morganella species. Providencia species have been cultured predominantly from urine, but also blood, sputum, stool, eye, and skin of humans. Their detection mainly represents colonization, but may sometimes be an infection, most commonly nosocomial or opportunistic in nature. We present the first case of Providencia rettgeri causing peritonitis in a patient on peritoneal dialysis (PD).
A 52-year-old Samoan female with end-stage renal disease with undetermined cause and on PD for 3 years presented to our institution with 2 days’ history of cloudy PD bags and generalized abdominal pain. Her other medical history included hypertension and smoking. In the 6 months prior to her presentation, she had been treated for several relapsing episodes of PD peritonitis. The episodes had cultured Escherichia coli on 2 occasions and Providencia rettgeri on 3 occasions. Three days before this presentation, she had completed her third 2-week course of intra-peritoneal (IP) amikacin therapy for Providencia. She had also received treatment courses with both IP cephazolin and gentamicin in the preceding 6 months.
The patient was admitted and restarted on IP amikacin for presumed relapsed Providencia rettgeri PD peritonitis. Unfortunately, on this occasion she had ongoing fevers and generalized abdominal pain despite therapy with IP amikacin. Therefore, on day 2 of admission her Tenckhoff catheter was surgically removed. The same day a computed tomography (CT) of the abdomen revealed an inflammatory mass around the appendiceal region, reported as a complicated appendicitis (Figure 1). To further investigate the mass, a colonoscopy was undertaken. It demonstrated multiple diverticulae in the sigmoid colon and melanosis in the entire colon. Her PD effluent and blood cultures sent at admission both grew Entercoccus faecalis.
Figure 1 -.
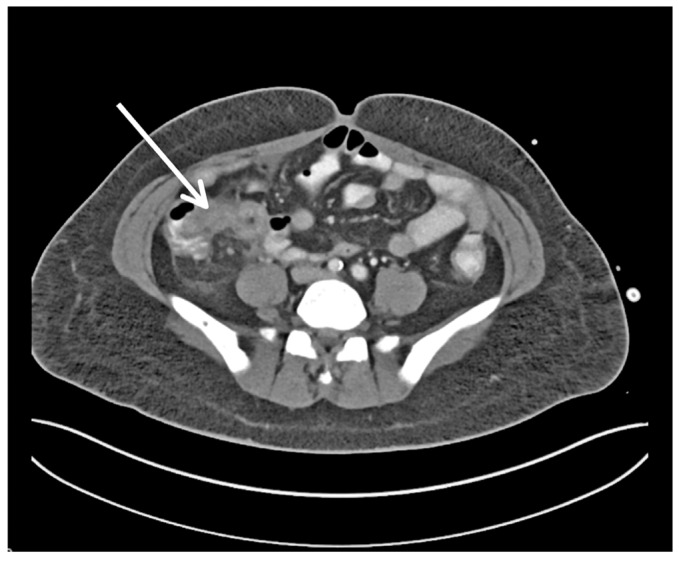
Perforated appendicitis (arrow) seen on computed tomography of abdomen axial section.
The case was discussed with our general surgeons and infectious diseases physicians. A plan was made to treat the IP mass conservatively with a 2-week course of oral augmentin and cotrimoxazole. She was discharged on interim hemodialysis to be followed up with a repeat CT of the abdomen in 1 month. Unfortunately, within the month she re-presented with ongoing symptoms and a repeat CT scan demonstrated a large nearly obliterated appendix with chronic inflammation surrounding, no free pus or perforation. She went forward for appendicectomy, with histology confirming perforated appendiceal diverticulum with supprative appendicitis and serositis. Post-operatively, she made a swift recovery and chose to remain on hemodialysis.
Discussion
The 5 species of Providencia currently recognized, in descending order of prevalence are, Providencia stuartii, Providencia rettgeri, Providencia alcalifaciens, Providencia rustigianii and Providencia heimbachae (1). Outbreaks of Providencia rettgeri were reported several decades ago as nosocomial urinary tract infections in patients with important risk factors such as exposure to multiple antibiotics, indwelling urinary catheters and previous urinary tract pathologies or procedures (2,3). More recently, Providencia rettgeri has been linked to travelers’ diarrhea (4).
One other case of PD peritonitis caused by the Providencia species stuartii has previously been reported in the literature (5). The patient in that case report unfortunately died on day 4 of initial empiric therapy and subsequent, culture-sensitive IP amikacin therapy. Although our first case of Providencia rettgeri peritonitis was not fatal, it was difficult to treat and the patient received multiple courses of antibiotics and had repeated presentations. Providencia species have become increasingly resistant to many antibiotics, including beta-lactams, cephalosporins, tetracyclines, quinolones, and gentamicin but remain sensitive to amikacin (1,3).
It has been suggested that biofilms developing on the surface of indwelling catheters can facilitate bacterial growth and infection, and their urease-producing property allows Providencia rettgeri to thrive in these conditions, causing potentially fatal infections (6). We can therefore hypothesize that the in-situ Tenckhoff catheter in our patient had a biofilm and that this contributed to the development and non-resolution of Providencia peritonitis and possibly subsequent abscess formation. This hypothesis is supported by the fact that once her Tenckhoff catheter was removed and she had received 1 further course of appropriate antibiotics, she had no further recurrence of Providencia peritonitis.
Diagnosis of surgical abdominal pathology in PD patients is difficult. Abdominal imaging frequently has suboptimal sensitivity in this context, and so surgical exploration had been previously recommended, particularly in those refractory to medical therapy (7). In our case, peritoneal fluid at various time-points during our patient’s presentations with PD peritonitis grew Providencia rettgeri, Escherichia coli, and Enterococcus faecalis. The presence of enteric or anaerobic polymicrobial peritonitis can suggest underlying bowel pathology as the cause (6), which in our case was confirmed on CT as a complicated appendicitis. We remain uncertain whether the recurrent PD peritonitis arose from the appendiceal abscess or if the abscess formed as a consequence of the recurrent and refractory Providencia rettgeri PD peritonitis.
In summary, this is the first reported case of PD peritonitis caused by Providencia rettgeri and occurred in the setting of a complicated appendiceal mass. We recommend that when this organism is isolated from PD fluid, a CT scan of the abdomen is always undertaken and a high suspicion of underlying gastrointestinal pathology is maintained. In addition, because of its predilection for biofilms, in order to prevent relapse or recurrence of PD peritonitis, the Tenckhoff catheter will likely need to be removed when this organism is cultured from PD fluid.
Acknowledgments
We would like to thank all the doctors, nurses and multi-disciplinary team of the renal medicine department at Middlemore Hospital, Counties Manukau District Health Board, for their support.
References
- 1. O’Hara CM, Brenner FW, Miller JM. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev 2000; 13:534–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edwards LD, Cross A, Levin S, Landau W. Outbreak of a nosocomial infection with a strain of Proteus rettgeri resistant to many antimicrobials. Am J Clin Pathol 1974; 61:41–6 [DOI] [PubMed] [Google Scholar]
- 3. Kaslow RA, Lindsey JO, Bisno AL, Price A. Nosocomial infection with highly resistant, Proteus rettgeri. Report of an epidemic. Am J Epidemiol 1976; 104:278–86 [DOI] [PubMed] [Google Scholar]
- 4. Yoh M, Matsuyama J, Ohnishi M, Takagi K, Miyagi H, Mori K, et al. Importance of Providencia species as a major cause of travellers’ diarrhoea. J Med Microbiol 2005; 54:1077–82 [DOI] [PubMed] [Google Scholar]
- 5. Unverdi S, Akay H, Ceri M, Inal S, Altay M, Demiroz AP, et al. Peritonitis due to Providencia stuartii. Perit Dial Int 2011; 31:216–7 [DOI] [PubMed] [Google Scholar]
- 6. Broomfield RJ, Morgan SD, Khan A, Stickler DJ. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol 2009; 58:1367–75 [DOI] [PubMed] [Google Scholar]
- 7. Carmeci C, Muldowney W, Mazbar SA, Bloom R. Emergency laparotomy in patients on continuous ambulatory peritoneal dialysis. Am Surg 2001; 67:615–8 [PubMed] [Google Scholar]
- 8. Kiernan L, Finkelstein FO, Kliger AS, Gorban-Brennan N, Juergensen P, Mooraki A, et al. Outcome of polymicrobial peritonitis in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 1995; 25: 461–4 [DOI] [PubMed] [Google Scholar]


