This paper describes the finding that inactivating the exosome in yeast leads to up-regulation of the iron uptake regulon. The up-regulation is caused by increased levels of reactive oxygen species. These observations demonstrate that the exosome in some way affects the physiology of an organism.
Keywords: DIS3, RNA degradation, RRP44, yeast
Abstract
RNA degradation plays important roles for maintaining temporal control and fidelity of gene expression, as well as processing of transcripts. In Saccharomyces cerevisiae the RNA exosome is a major 3′-to-5′ exoribonuclease and also has an endonuclease domain of unknown function. Here we report a physiological role for the exosome in response to a stimulus. We show that inactivating the exoribonuclease active site of Rrp44 up-regulates the iron uptake regulon. This up-regulation is caused by increased levels of reactive oxygen species (ROS) in the mutant. Elevated ROS also causes hypersensitivity to H2O2, which can be reduced by the addition of iron to H2O2 stressed cells. Finally, we show that the previously characterized slow growth phenotype of rrp44-exo− is largely ameliorated during fermentative growth. While the molecular functions of Rrp44 and the RNA exosome have been extensively characterized, our studies characterize how this molecular function affects the physiology of the organism.
INTRODUCTION
The exosome is a major exoribonuclease found in eukaryotes and archaea (Mitchell et al. 1997; Allmang et al. 1999b; Chekanova et al. 2002; Evguenieva-Hackenberg et al. 2003). In eukaryotes, including Saccharomyces cerevisiae, the exosome is a complex of 10 essential proteins, which in addition to its exonuclease activity also has an endonuclease activity (Lebreton et al. 2008; Schaeffer et al. 2009; Schneider et al. 2009). These catalytic activities are carried out by separate domains of the Rrp44 protein. The exosome is known to be located both in the nucleus and in the cytoplasm. In the nucleus it takes part of 3′ processing of the 5.8S rRNA, snoRNAs, snRNAs, and some mRNAs (Mitchell et al. 1996; Allmang et al. 1999a; van Hoof et al. 2000). It also degrades the 5′ external transcribed spacer (ETS) of rRNA, misprocessed RNAs such as hypomodified tRNA, and cryptic unstable transcripts (CUTs) (Kadaba et al. 2004; Milligan et al. 2008). In the cytoplasm, the exosome takes part in the degradation of normal, as well as aberrant, mRNAs (Jacobs Anderson and Parker 1998; van Hoof et al. 2002; Meaux and van Hoof 2006). Most of the activities of the exosome require the exoribonuclease activity of Rrp44, while the function of its endonuclease activity is enigmatic (Lebreton et al. 2008; Schaeffer et al. 2009; Schneider et al. 2009; Schaeffer and van Hoof 2011).
Studies of Rrp44's physiological function are limited. Rrp44 was shown to affect chromosome segregation, cell cycle progressions, and microtubules in Schizosaccharomyces pombe and S. cerevisiae (Murakami et al. 2007; Smith et al. 2011). Rrp44 has also been found to alter the circadian rhythm in Neusporra crassa (Guo et al. 2009). How any of these effects are related to the catalytic activities of Rrp44 is unknown. In addition to studies on Rrp44, it has been shown that the exosome components Rrp4 and Rrp41 have specific roles during early development of Arabidopsis thaliana (Chekanova et al. 2007). Here we characterize the effect of the catalytic activities of the exosome on the physiology of S. cerevisiae and find effects related to reactive oxygen species (ROS), iron uptake, and carbon utilization.
Iron is an essential element that functions in cellular metabolism because of its ability to be easily converted between oxidized and reduced forms, thus being a readily available electron donor or recipient. It is critical to maintain the precise balance of iron concentration inside the cell. Insufficient iron will adversely affect cellular metabolism since iron is a cofactor in many enzymatic reactions. Conversely, too much free iron is toxic to the cell because it takes part in the formation of damaging hydroxyl radical species through the Fenton reaction and leads to oxidative stress. Oxidative stress in turn has been known to damage all cellular components, including lipids, DNA, RNA, and proteins and in humans is related to several diseases, including neurodegenerative diseases such as ALS; Alzheimer's, Huntington's, and Parkinson's disease; and plaque formation in the arteries. It is also a contributing factor in the aging process (Trushina and McMurray 2007; Brieger et al. 2012; Chen and Keaney 2012; Gomez-Cabrera et al. 2012).
When yeast senses that its iron level is insufficient, it up-regulates the master regulator of iron response, Aft1, which then binds to the promoter of genes required for iron uptake and induces their transcription (Crisp et al. 2003; Rutherford et al. 2005). The exact signal that is sensed and transduced to Aft1 is unknown, but it results in relocalization of Aft1. Under iron replete conditions. Aft1 localizes to the cytoplasm, while activation of Aft1 causes its relocalization to the nucleus (Yamaguchi-Iwai et al. 1996, 2002).
As mentioned above, inappropriately high iron level causes production of ROS. Under normal iron levels, respiration is the major contributor to ROS production by electrons leaking from the electron transport chain (Herrero et al. 2008). During growth on glucose, S. cerevisiae suppresses the genes for respiration and grows by fermentation. When glucose is no longer available, it switches to respiration and oxidizes the remaining nonfermentable carbon source.
Here we show that inactivation of the exoribonuclease activity of the exosome results in the reduced ability of the cell to resist oxidative stress and up-regulates the iron uptake response. Moreover, we show that these effects and the significant growth defect of this strain are present during fermentative growth but not during respiratory growth. Overall, these results demonstrate that the molecular function of the exosome affects the physiology of the organism.
RESULTS AND DISCUSSION
Activation of the iron-starvation response is a major physiological consequence of a defect in Rrp44 exonuclease activity
We used microarray analysis to characterize the physiological effects of inactivating either the endoribonuclease or the exoribonuclease activity of the exosome. Specifically, we isolated polyadenylated RNA from quadruplicate cultures of wild type and two rrp44 mutants that have previously been characterized. The rrp44-exo−mutation changes Asp551 of the exonuclease active site to Asn, disrupts the exonuclease activity, and results in many RNA processing and degradation defects (Dziembowski et al. 2007). Importantly, although the exonuclease acts in mRNA decay, it only degrades mRNAs after deadenylation (Decker and Parker 1993; Jacobs Anderson and Parker 1998; Tucker et al. 2001). Therefore, direct mRNA targets of the exonuclease activity should accumulate in the deadenylated form and not be identified by microarray analysis of poly(A)+ RNA. Instead this analysis should reveal the physiological consequences of the rrp44-exo− mutation. The other mutation analyzed, rrp44-endo−, changes Asp171 of the endonuclease active site to Ala and disrupts the endonuclease activity but has no significant effect on known RNA processing and degradation roles of the exosome (Lebreton et al. 2008; Schaeffer et al. 2009; Schneider et al. 2009).
In our analysis we focused on mRNAs that were up-regulated at least twofold in at least three of the four biological replicates. We were surprised that even though the rrp44-exo− mutation has a large effect on cell growth, the effects on gene expression were relatively modest, with 73 mRNAs up-regulated and 63 down-regulated (Table 1; Supplemental Table S1). We found no mRNAs that were affected by the rrp44-endo− mutation. Because the rrp44-endo− mutation has no measurable effect on growth or gene expression, it does not appear to perturb the cell even though the endoribonuclease active site is conserved in most if not all eukaryotes, and the activity has been shown to be conserved for the human ortholog hDIS3 (Tomecki et al. 2010). In addition to these mRNA targets, the rrp44-exo− microarray detected an increased level of polyadenylated ribosomal RNAs, which reflects the known role of the exosome in degradation of polyadenylated aberrant rRNAs (Kuai et al. 2004) and serves as a control to confirm the validity of the microarray approach.
TABLE 1.
Genes up at least twofold in three of four microarrays for rrp44-exo−

We used gene ontology to analyze whether the mRNAs affected by the rrp44-exo− mutation were enriched in specific categories. All of the top gene ontology terms were related to iron uptake, including “iron chelate transport” (P = 7 × 10−11) and “iron ion homeostasis” (P = 1 × 10−9), and 16% of the mRNAs that were up-regulated were annotated with one or more gene ontology terms related to iron uptake (Table 1). To verify that the rrp44-exo− mutation indeed affected iron-starvation response genes, we isolated RNA from a second independently constructed rrp44-exo− mutant strain and analyzed it by Northern blotting with probes for the FIT2 and CTH2/TIS11 mRNAs. As shown in Figure 1A, expression of both genes was also induced in this rrp44-exo− strain. Therefore, we conclude that a major physiological consequence of the rrp44-exo− mutant is the up-regulation of the iron-starvation response.
FIGURE 1.

The Aft1 regulated iron regulon is activated in a rrp44-exo− mutant. (A) Northern blot analysis confirms the microarray results that the expression of FIT2 and TIS11 mRNAs is increased in an rrp44-exo− mutant. To the left of the blot is the fold increase in expression from the microarray analysis. SRP indicates the RNA subunit of the signal recognition particle, which is used as a loading control. (B) The rrp44-exo− mutation increases expression of a lacZ reporter gene that contains a single Aft1 binding site. The graph shows averages and standard deviations of triplicate cultures. As a control, a reporter gene without an Aft1 binding site is very poorly expressed and not affected by rrp44-exo− (see Supplemental Fig. 1). (C) An Aft1-GFP fusion protein was expressed in wild-type and rrp44-exo− mutant strains to localize Aft1. The percentage of cells that showed a nuclear localization of Aft1, reflecting activation of Aft1, is indicated on the right. (D) Western blot analysis of Aft1-GFP in wild-type and rrp44-exo− strains, using anti-GFP antibody. Pgk1 is used for loading control.
The increased abundance of iron uptake-related mRNAs in an RNase mutant could be because that RNase directly degrades these mRNAs or because expression of a common regulator is affected by the RNase. The transcription factor Aft1 is a major contributor to the iron-starvation response and is known to bind to ANTGCACCC (Yamaguchi-Iwai et al. 1996; Zhu et al. 2009). We therefore searched the 6623 intergenic regions of the yeast genome for ANTGCACCC and found 59 occurrences in 52 different intergenic regions. These elements were highly enriched in the intergenic regions just upstream of genes affected by rrp44-exo−. Specifically, 25% of the elements were found in the intergenic regions just upstream of the 73 genes in our data set, including four elements upstream of the most affected gene (FIT2) (Table 1). Several other genes that were affected by rrp44-exo− had ANTGCACCC elements nearby or had imperfect matches. In addition, several genes that were induced less than our twofold threshold also had ANTGCACCC elements. We thus suspected that the rrp44-exo− mutation somehow affected the activity of the transcription factor Aft1, which in turn increased transcription of iron-uptake–related genes.
To test whether Aft1 activity is increased in the rrp44-exo− mutant, we constructed a lacZ reporter gene by cloning the binding site for Aft1 into a construct that contained a minimal promoter and the lacZ ORF. The resulting reporter plasmid was transformed into rrp44-exo− and RRP44 strains, and β-galactosidase activity was measured in three transformants for each strain. Figure 1B shows that β-galactosidase levels were increased 2.3-fold in the rrp44-exo− strain, which matches the effect we see on iron-starvation regulon genes. Expression of β-galactosidase from the parental plasmid that lacks an Aft1 binding site was not affected (Fig. 1B; Supplemental Fig. 1). These data suggest that the Aft1 transcription factor is indeed more active in the rrp44-exo− mutant.
Aft1 regulation is mostly post-translational, such that Aft1 is localized in the cytoplasm under iron replete conditions and becomes relocalized to the nucleus when the cell senses a need for more iron (Yamaguchi-Iwai et al. 2002). We therefore compared the location of an Aft1-GFP fusion protein in the rrp44-exo− mutant and RRP44 strains. Aft1 was observed to be concentrated in the nucleus of 44% of the rrp44-exo− cells and in 15% of the RRP44 cells (Fig. 1C). We also analyzed expression levels of Aft1-GFP by Western blot and noted that they were similar in the wild-type and rrp44-exo− strains (Fig. 1D). The enrichment of ANTGCACCC elements, increase in lacZ expression, and increased nuclear localization of Aft1 all indicate that the rrp44-exo−mutant activates the cellular iron-starvation response by somehow activating and relocalizing this key transcription factor.
Inactivation of the exonuclease activity of Rrp44 increases hydrogen peroxide sensitivity and intracellular ROS levels
Iron is both an essential element because it is a cofactor for many proteins and a toxic element because iron reacts to generate ROS. To further characterize the effect of the increase in Aft1 activity and the resulting up-regulation of the iron-starvation regulon, we analyzed growth under low- and high-iron conditions, as well as oxidative stress. To analyze the effect of iron concentration in the media, we compared four different growth conditions: SC media with the standard iron concentration (0.2 mg/L FeCl3), SC media with 1000-fold increase in iron, SC media with no added iron but with trace iron available from the water or other ingredients, and SC media with no added iron and added iron chelator bathophenanthrolinedisulfonate (BPS). These conditions did not reveal any specific effects on the rrp44-exo− mutant; the rrp44-exo− mutant grows slower than an isogenic RRP44 strain under the first three conditions, and neither the wild-type nor mutant strains grew in the presence of BPS. We conclude that the up-regulation of the iron-starvation regulon we detected in the rrp44-exo− strain does not significantly affect its ability to grow under iron-excess or iron-starvation conditions.
To test the effects of rrp44-exo− mutant further, we tested if this mutant was sensitive to exposure to oxidative stress. As shown in Figure 2A, the rrp44-exo− mutant did not grow on YPD plates supplemented with 2 mM H2O2, indicating increased sensitivity to oxidative stress. Under these same conditions, the wild-type strain did grow, although at a slightly slower rate than in the absence of H2O2. We observed similar effects during growth in liquid media: Addition of 2 mM H2O2 had a modest effect on the growth rate of the RRP44 control strain but completely eliminated growth of the rrp44-exo− mutant (Fig. 2B). This hypersensitivity was specific to H2O2 because the superoxide-generating agent menadione affected the wild type and the rrp44-exo− mutant approximately equally (Fig. 2A). In addition to the low- and high-iron stress. other stresses such as growth at high or low temperature did not specifically affect the growth of the rrp44-exo− strain compared with the wild type, further demonstrating the specificity of the H2O2 sensitivity.
FIGURE 2.
The rrp44-exo− is hypersensitive to H2O2. (A) The rrp44-exo− mutant is hypersensitive to hydrogen peroxide when grown on solid media. Shown are the RRP44 and rrp44-exo− strains grown on solid media either without added reactive oxygen species (ROS) or with added hydrogen peroxide or the superoxide generating compound menadione. (B) Shown are growth curves of the RRP44 and rrp44-exo− strains grown in liquid media in the presence or absence of H2O2. (C) The rrp44-exo− strain hyperaccumulates ROS. Intracellular ROS levels were measured in RRP44 and rrp44-exo− strains grown in the presence or absence of 1 mM H2O2.
Hypersensitivity to oxidative stress could result from inability to repair damage caused by normal levels of ROS, it could be due to increased levels of intracellular ROS, or both. To test whether ROS levels were altered in the rrp44-exo− mutant, we used 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). H2DCFDA is not fluorescent, but upon hydrolysis by intracellular esterases and oxidation by ROS, it is transformed into the fluorescent compound 2′,7′-dichlorofluorescein (DCF). Thus, intracellular ROS levels can be detected by measuring fluorescence after H2DCFDA exposure. By use of this assay, we detected a 2.0-fold higher level of ROS in the rrp44-exo− mutant compared with the mutant during growth in the absence of added ROS and a 2.4-fold higher level after exposure to 1 mM H2O2 (Fig. 2C). We therefore conclude that the rrp44-exo− mutant accumulates increased amounts of ROS.
Altered iron response and sensitivity to oxidative stress in the rrp44-exo− mutant are functionally connected
Knowing the rrp44-exo− mutant has both an up-regulated iron response and increased ROS, we decided to test if these were related. We could envision at least two possible ways these are linked. Aft1 activity is not directly controlled by iron but instead is controlled by the availability of iron-sulfur clusters (Chen et al. 2004; Rutherford et al. 2005). Since hydrogen peroxide damages iron-sulfur clusters, it is possible that the excess ROS in rrp44-exo− decreases the availability of iron-sulfur clusters, which would result in Aft1 induction. Thus, under this scenario the rrp44-exo− mutant responds appropriately to a decreased level of available iron-sulfur clusters that is caused by an increased ROS level. Alternatively, rrp44-exo− may inappropriately up-regulate Aft1 and the resulting increased intracellular Fe levels would cause an increased production of ROS due to the reactivity of iron ions.
To distinguish between these two possibilities, we first exposed the rrp44-exo− mutant to 1 mM H2O2 for 1 h and then removed the H2O2 and added extra iron to the media. This addition of extra iron after H2O2 exposure increased growth of the rrp44-exo− strain. (Fig. 3A, cf. red and green solid lines). In comparison, adding iron to RRP44 cells that were treated with ROS did not significantly change their growth (Fig. 3A, dashed lines). Similarly, adding iron to rrp44-exo− or RRP44 in the absence of H2O2 stress did not significantly affect their growth (Fig. 3B). These data support the first possibility that increased ROS levels lead to a decreased level of available iron-sulfur clusters, which in turn results in Aft1 activation. This possibility suggests that adding H2O2 to RRP44 cells should have a similar effect of Aft1 activation. Figure 3C shows that, indeed, the FIT2 mRNA is up-regulated upon exposure of wild-type cells to H2O2. The effects of H2O2 and rrp44-exo− were not additive; at low concentrations of H2O2, the rrp44-exo− mutant did not show a further increase in FIT2 expression, while at higher concentrations of H2O2, the RRP44 and rrp44-exo− strains responded similarly (Fig. 3C). Taken together, these data suggest that the rrp44-exo− mutant has a higher requirement for iron and responds appropriately to this requirement by up-regulating the iron regulon.
FIGURE 3.
Sensitivity to oxidative stress in the rrp44-exo− mutant is functionally related to the altered iron response. (A) Growth inhibition of the rrp44-exo−after 1 h exposure to 1 mM H2O2 can be suppressed by addition of extra iron to the media. Solid lines indicate growth curves of the rrp44-exo−mutant; dashed lines, growth curves of a RRP44 control strain. Green indicates growth after addition of iron; red, growth in the absence of extra iron. (B) Addition of extra iron has no effect in the absence of prior H2O2 treatment. Colors and solid versus dashed lines as in A. (C) The FIT2 mRNA is induced by exposure to H2O2, and this effect is not additive with the effect of the rrp44-exo− mutation. Shown is a representative Northern blot (left) and its quantitation (right). The SRP RNA served as a loading control as in Figure 1.
The exonuclease activity of the exosome is required for normal growth rate during fermentative conditions but not during respiratory conditions
When yeast is grown under standard laboratory conditions in YEP + 2% glucose, it initially ferments the glucose to ethanol and, only after glucose is depleted, respires the ethanol to CO2. The above experiments and most published experiments with exosome mutants use log phase cells that almost exclusively ferment. Under these circumstances, ROS production is relatively low, and iron needs are likely to be low as well, since a number of mitochondrial proteins required for respiration contain Fe-S clusters. Therefore, to extend our understanding of the physiological effects of the rrp44-exo− mutant beyond the fermentative conditions, we compared its growth rate to the wild-type strain under conditions where yeast can only respire. Specifically, we compared growth rates of wild-type cells and the rrp44-exo− mutant on media with glycerol or ethanol instead of glucose as a carbon source. Glycerol and ethanol are nonfermentable and require the cells to grow by respiration. Figure 4A shows that wild-type and rrp44-exo− strains grow with similar rates when grown on YEP plates with 2% glycerol or 2% ethanol. Growth in liquid culture revealed that the rrp44-exo− mutant grew slightly slower than the wild type, but the difference was much less pronounced than during fermentative growth (Fig. 4B). To test whether expression of Rrp44 was different during fermentative and respiratory growth, we performed Western blot on an epitope-tagged strain. Figure 4C shows that Rrp44 levels during growth on YPD and YEP + 2% glycerol were similar. Thus, the less-pronounced growth defect of Rrp44-exo− during respiratory growth is not correlated with reduced expression of the protein.
FIGURE 4.
Lack of the Rrp44 exonuclease activity results in slow growth under fermentative but not respiratory growth. (A) Shown are the RRP44 and rrp44-exo− strains grown on solid media either with fermentable glucose or with nonfermentable glycerol or ethanol as the carbon source. (B) Shown are growth curves of the RRP44 and rrp44-exo− strains grown in liquid media with glycerol as the carbon source (cf. with the growth in medium with glucose in Fig. 2A). (C) Western blot analysis of TAP-tagged Rrp44 levels during fermentative (glucose) and respiratory (glycerol) growth. Pgk1 is used as a loading control. (D) The rrp44-exo− strain does not hyperaccumulate intracellular ROS when grown in media with glycerol as the carbon source (cf. with ROS accumulation when grown in media with glucose in Fig. 2C). (E) The rrp44-exo− mutant grows slowly in media containing glucose but is not inefficient in converting glucose to biomass. The mutant and wild-type strains were grown in YPD media, and growth (OD600) and glucose levels were monitored every 2 h and plotted.
Our observations that the rrp44-exo− strain is hypersensitive to ROS and has a slow growth phenotype specifically during fermentative growth appear paradoxical, since ROS production should be low during fermentation. To resolve this puzzle, we measured ROS levels during respiratory growth and found that under these conditions the rrp44-exo− mutant had levels of ROS comparable to wild type (Fig. 4C), presumably because mechanisms to detoxify ROS are also up-regulated during respiratory growth. Thus, these observations further strengthen the correlation between the growth defect of rrp44-exo− and ROS accumulation.
Because the rrp44-exo− mutant has a growth defect during fermentative growth on glucose but has much less of a growth defect during respiratory growth, we also analyzed whether it was inefficient in converting glucose to biomass. Specifically, we simultaneously measured biomass accumulation (by measuring optical density) and glucose depletion during growth in YEP + 2% glucose. As shown in Figure 4D the wild type and rrp44-exo− mutant converted glucose to biomass with very similar efficiency. Therefore, the slow growth during fermentation is not due to inefficient conversion of glucose to biomass.
The exonuclease activity of the exosome is required for RNA processing and degradation during fermentative and respiratory conditions
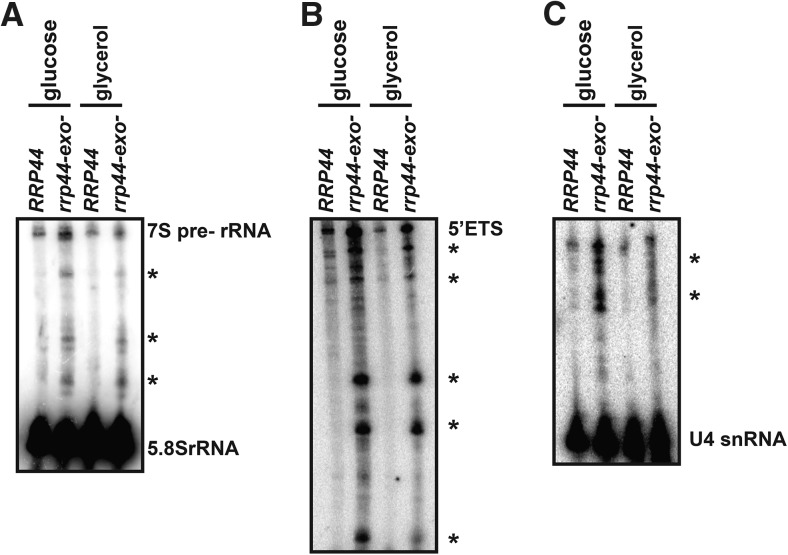
Yeast growth is typically coordinated with ribosome production (Waldron and Lacroute 1975; Mager and Planta 1991). This suggests the possibility that the slow growth phenotype of the rrp44-exo− strain is related to its known defect in ribosomal RNA maturation. Thus, to characterize the relation between slow growth and this rRNA processing defect, we isolated RNA from the wild-type and rrp44-exo− mutant strains growing in fermentative or respiratory conditions and analyzed it by Northern blotting. Figure 5A shows that the aberrant 5.8S rRNA processing intermediates present in the rrp44-exo− strain during growth on glucose are also present during growth on glycerol (marked with an asterisk in Fig. 5). Similarly, the known defects of the rrp44-exo− mutant in 5′ ETS degradation (Fig. 5B) and U4 snRNA (Fig. 5C) processing were present, even when grown on glycerol. Therefore, we conclude that the growth defect exhibited by rrp44-exo− during fermentative growth is independent of these specific processing and degradation defects and that the exonuclease activity of the exosome is required for RNA processing and degradation during fermentative and respiratory conditions.
FIGURE 5.
Although the growth defect of rrp44-exo− is specific for fermentative growth, the known RNA processing defects are seen during both fermentative and respiratory growth. The RRP44 and rrp44-exo− strains were grown in YEP + glucose or YEP + glycerol as indicated. RNA was isolated and analyzed by Northern blotting with probes specific for 5.8S rRNA (A), the 5′ external transcribed spacer of the pre-rRNA (5′ ETS; B), or the U4 snRNA (C). RNA species that accumulate in the rrp44-exo− mutant are indicated with asterisks.
CONCLUSIONS
Our results suggest that the major physiological consequence of inactivating the exonuclease of Rrp44 is an increase in ROS accumulation, which results in an increased need for iron and a decreased growth rate under fermentative conditions. Although the RNA exosome has been extensively studied at the molecular level and mutants that reduce RNA exosome activity cause slow growth, how the RNA exosome affects the physiological state of the cell was previously largely unknown. To investigate this, we used a microarray approach to identify genes that were up- or down-regulated in response to inactivating the active site residues of the main catalytic subunit of the exosome. Despite the very slow growth of the rrp44-exo− strain, the number of genes affected was surprisingly low and dominated by genes in the iron-uptake regulon. The main transcription factor for this regulon is Aft1, and we show that expression of a lacZ reporter gene that incorporates a single binding site for Aft1 is increased in the rrp44-exo− mutant. We further confirmed that Aft1 is activated by showing that a GFP fusion is translocated into the nucleus. Aft1 activation and nuclear import have been shown to be directed by a pair of glutaredoxins (Grx3 and Grx4), which by an unknown mechanism respond to the level of available iron-sulfur clusters (Ojeda et al. 2006; Pujol-Carrion et al. 2006).
Strikingly, three different RNases and RNA degradation pathways have previously been implicated in the regulation of the iron response. First, RRP6 encodes a nuclear cofactor of the exosome, and iron regulon RNAs were shown to increase in rrp6Δ strains (Lee et al. 2005; Houalla et al. 2006; Ciais et al. 2008). The molecular mechanism behind this up-regulation was not fully explored, but it was suggested that iron regulon mRNAs were likely direct targets of the nuclear exosome (Lee et al. 2005; Houalla et al. 2006; Ciais et al. 2008). In contrast, our findings of up-regulation in rrp44-exo− can be fully explained by activation of the Aft1 transcription factor, and we do not suspect that these mRNAs are direct Rrp44 targets. A further difference is that the increased CTH2 mRNA level in rrp6Δ is mostly due to accumulation of 3′ extended mRNAs (Ciais et al. 2008), which we did not detect in rrp44-exo−. Thus rrp6Δ and rrp44-exo− appear to have different effects on iron regulon mRNAs, which likely reflect two different mechanisms of control. The second RNase previously implicated in expression of iron-uptake–related mRNAs is the endonuclease Rnt1 (Lee et al. 2005). Rnt1 and Rrp44 also appear to function in separate pathways because genes up-regulated in rnt1Δ were not enriched for Aft1 targets, rnt1Δ resulted in decreased expression of lacZ reporter under Aft1 control while rrp44-exo− resulted in an increased expression, and the rrp44-exo− mutation increases expression of FIT2 mRNA while rnt1Δ decreases its expression. Finally, CTH2, one of the Aft1 targets that we find up-regulated in rrp44-exo−, encodes an RNA binding protein that controls decapping of specific mRNAs by Dcp2 followed by Xrn1-mediated degradation (Puig et al. 2005; Pedro-Segura et al. 2008), but the target mRNAs of Cth2 differ from the Aft1 targets that we find up-regulated in rrp44-exo−. Recently, a second RNA binding protein, Air2, has also been implicated in the regulation of the iron regulon (Schmidt et al. 2012). Air2 is a cofactor of the exosome and Schmidt et al. (2012) reported that the FIT2 mRNA level is reduced in an rrp6Δ strain but increased in an rrp6Δ air2Δ double mutant. This suggests that Air2p acts in an Rrp6-independent pathway to affect FIT2 mRNA. Whether this Air2-dependent pathway is the same as the pathway we describe here remains to be determined. Overall, these results indicate that iron homeostasis is regulated by at least four different RNases—Rrp44, Rrp6, Rnt1, and Dcp2/Xrn1, and the available evidence indicates that each of these RNases acts in different pathways.
We also show that the rrp44-exo− mutant strain accumulates ROS. Because ROS are especially reactive with iron-sulfur clusters, we hypothesize that this increased ROS causes a reduction in the level of iron-sulfur clusters, which turns on the Aft1 regulon. Our observations that the hydrogen peroxide sensitivity of rrp44-exo− can be suppressed by adding iron and that the iron regulon is induced upon addition of hydrogen peroxide to wild-type cells are consistent with this possibility. The molecular mechanism of how the exonuclease activity of the RNA exosome affects this signaling pathway remains unknown. However, the exosome has been shown to be required for the degradation of long noncoding RNAs (Lebreton et al. 2008), which can affect gene expression. For example, the expression of SER3 in response to serine starvation is regulated by the noncoding RNA SRG1 (Martens et al. 2004, 2005). One possibility is that a similar noncoding RNA affects expression of a key regulator that controls ROS levels.
Although the exosome has many known functions, it is not known which of these functions is most critical for normal growth or whether multiple important aspects of cell physiology are perturbed in exosome mutants. Here we show that the growth defect of rrp44-exo− is much more severe under fermentative conditions than under respiratory conditions. In addition, under conditions where rrp44-exo− grows slowly, this strain hyperaccumulates ROS, whereas under conditions where it does not hyperaccumulate ROS, growth is much more similar to that of the wild type. In contrast, the 5.8S rRNA and U4snRNA processing and 5′ ETS degradation defects seen in the mutant do not correlate with a defect in growth rate. Thus, our results suggest the possibility that the ROS hyperaccumulation we observe is a major contributor to the slow growth seen in this and possibly other exosome mutants.
MATERIALS AND METHODS
Yeast strains
All strains were derived from the heterozygous diploid RRP44/rrp44Δ::NEO strain obtained from Open Biosystems using standard yeast genetics techniques as previously described (Schaeffer et al. 2009). The microarray analysis was done on quadruplicate cultures of yAV1129, yAV1131, and yAV1135. Follow-up experiments were done with these same strains as well as a second independent isolate of rrp44-exo−, yAV1284. All experiments were done at least in duplicate. To generate a second independent isolate of rrp44-exo−, first the ORF for NEO resistance was replaced by an ORF encoding Nourseothricin resistance (NAT) by transforming the RRP44/rrp44Δ::NEO strain obtained from Open Biosystems with p4339 (Tong and Boone 2006) obtained from Dr. Charlie Boone's laboratory. Next the rrp44-exo− plasmid was introduced as previously described (Schaeffer et al. 2009). The genotypes of the strains used are as follows:
yAV1129 (wild-type strain): Matα, leu2-Δ0, ura3-Δ0, his3-Δ1, rrp44Δ::NEO [RRP44, LEU2];
yAV1131 (rrp44-exo−): Matα, leu2-Δ0, ura3-Δ0, his3-Δ1, rrp44Δ::NEO [rrp44D551N, LEU2];
yAV1135 (rrp44-endo−): Matα, leu2-Δ0, ura3-Δ0, his3-Δ1, rrp44Δ::NEO [rrp44D171A, LEU2]; and
yAV1284 (rrp44-exo−): leu2-Δ0, ura3-Δ0, his3-Δ1, lys2-Δ0, rrp44Δ::NAT [rrp44D551N, LEU2].
Plasmids
The RRP44, rrp44-exo−, and rrp44-endo− plasmids have previously been described (Schaeffer et al. 2009). The Aft1-GFP plasmid has also been described (Crisp et al. 2003) and was kindly provided by Dr. Jerry Kaplan (University of Utah). The lacZ control plasmid pCM64 was kindly provided by Dr. Kevin Morano (University of Texas Health Science Center Houston). pCM64 is a derivative of pLG669-Z (Guarente and Ptashne 1981) with the 434-bp XhoI fragment replaced with a linker introducing a unique BglII site. The Aft1-lacZ reporter plasmid pAV804 is a derivative of PCM64 with the Aft1 binding site form the FET3 promoter inserted into the unique BglII site. pAV804 was generated by cloning oAV886 (GATCTCTTCAAAAGTGCACCCATTTGCAGGTGCTCC) and oAV887 (TCGAGGAGCACCTGCAAATGGGTGCACTTTTGAAGA) into the BglII site of pCM64.
Media and growth conditions
All growth assays were repeated at least three times. Unless otherwise indicated, all cultures were started overnight in 5 mL YPD and then transferred the following day to the specified media. Growth was at 30°C unless otherwise noted. YPD (YEP + glucose), YEP + glycerol, YEP + ethanol, and SC media were prepared as previously described (Burke et al. 2000). Yeast nitrogen base (catalog no. 1501-250), yeast nitrogen base lacking iron (catalog no. 1551-100), and the amino acids mixes were from Sunrise Science. The iron chelator BPS was from Sigma-Aldrich (catalog no. 146617-1G). For the experiments in Figure 3, ammonium iron(III) sulfate dodecahydrate was from Sigma Aldrich (catalog no. 221260-25G). Glucose levels were measured using a glucose (HK) assay kit (Sigma-Aldrich catalog no. GAHK20-1KT) according to the manufacturer's instructions.
For growth assays on solid media, fivefold serial dilutions of S. cerevisiae cells were made in a 96-well plate starting with OD600= 0.6. These were then spotted on the indicated media and incubated at 30°C (unless otherwise indicated).
Liquid growth assays were performed either in 96-well plates in a Synergy MX automated microplate reader or in test tubes.
Microscopy
Cells were transformed with the Aft1-GFP plasmid and grown in selective media. Before microscopy, cells were stained with Hoechst 33342 stain for 10 min, washed with H2O, and observed with an Olympus IX81 microscope.
β-galactosidase assay
Strains containing either pCM64 or pAV804 were grown to OD600 = 0.6, and β-galactosidase activity was measured using the Beta-Glo reagent (Promega) according to the manufacturer's instructions using a Biotech Synergy MX plate reader. Briefly, 50 μL of culture was mixed with 50 μL of β-glo reagent in a 96-well microtiter plate, incubated for 30 min, and light production measured and normalized to OD600. Similar results were obtained when β-galactosidase activity was measured using ortho-nitrophenyl-β-galactoside (ONPG).
ROS detection
Cultures were either treated with the indicated concentrations of H2O2 or left untreated. After 1 h treatment, 1 mL of culture was centrifuged and the cells washed in phosphate buffered saline (PBS) and resuspended in 1 mL PBS containing 25 μM H2DCFDA. Fluorescence was detected at 525 nm with excitation at 495 nm. Fluorescence was normalized to OD600.
RNA isolation and analysis
Liquid cultures (5 mL) were grown overnight at 30°C. The cultures were then diluted into 20 mL of fresh media to an OD600 of 0.125 and grown at 30°C to an OD600 of 0.6. The culture was briefly centrifuged, and the cell pellet was collected, washed once with ddH2O, and either used immediately or frozen at −80°C prior to RNA extraction. RNA was extracted as previously described (He et al. 2008), separated on 6% polyacrylamide or 1.3% agarose gels, and blotted onto Zeta-Probe membrane (Biorad). The blots were probed with 5′P32-labeled oligonucleotides for FIT2 mRNA (CGACGGCTTGAGTGACGGTC), TIS11 mRNA (GGGAGTTTCCTGCACTTGGC), 5.8S rRNA (TTTCGCTGCGTTCTTCATC), 5′ ETS (CGAACGACAAGCCTACTG), U4 snRNA (CGGACGAATCCTCACTGATA), and the RNA subunit of the signal recognition particle (SRP; GTCTAGCCGCGAGGAAGG) as a loading control.
For the microarray experiments, quadruplicate cultures of yAV1129, yAV1131, and yAV1135 were grown in YPD, and RNA was isolated and processed as described previously (He et al. 2003). Fragmented cRNA was prepared using the Affymetrix 3′IVT express kit and was hybridized to Affymetrix Yeast Genome 2.0 arrays. The relative abundance of each transcript was determined using Affymetrix Microarray Suite 5.0.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank Drs. Kevin Morano, Charlie Boone, and Jerry Kaplan for plasmids, as well as Kevin Morano for use of the 96-well plate reader. This work was supported by grants from the NIH (5R01GM099790) and The Welch Foundation (AU-1773) to A.v.H. and from the NIH (R37GM27757) to A.J.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.043257.113.
REFERENCES
- Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D 1999a. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J 18: 5399–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C, Petfalski E, Podtelejnikov A, Mann M, Tollervey D, Mitchell P 1999b. The yeast exosome and human PM–Scl are related complexes of 3′→5′ exonucleases. Genes Dev 13: 2148–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ Jr, Krause KH 2012. Reactive oxygen species: from health to disease. Swiss Med Wkly 142: w13659. [DOI] [PubMed] [Google Scholar]
- Burke D, Dawson D, Stearns T 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Chekanova JA, Dutko JA, Mian IS, Belostotsky DA 2002. Arabidopsis thaliana exosome subunit AtRrp4p is a hydrolytic 3′→5′ exonuclease containing S1 and KH RNA-binding domains. Nucleic Acids Res 30: 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekanova JA, Gregory BD, Reverdatto SV, Chen H, Kumar R, Hooker T, Yazaki J, Li P, Skiba N, Peng Q, et al. 2007. Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell 131: 1340–1353 [DOI] [PubMed] [Google Scholar]
- Chen K, Keaney JF Jr. 2012. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr Atheroscler Rep 14: 476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, Kaplan J 2004. Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J Biol Chem 279: 29513–29518 [DOI] [PubMed] [Google Scholar]
- Ciais D, Bohnsack MT, Tollervey D 2008. The mRNA encoding the yeast ARE-binding protein Cth2 is generated by a novel 3′ processing pathway. Nucleic Acids Res 36: 3075–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp RJ, Pollington A, Galea C, Jaron S, Yamaguchi-Iwai Y, Kaplan J 2003. Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast. J Biol Chem 278: 45499–45506 [DOI] [PubMed] [Google Scholar]
- Decker CJ, Parker R 1993. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev 7: 1632–1643 [DOI] [PubMed] [Google Scholar]
- Dziembowski A, Lorentzen E, Conti E, Seraphin B 2007. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat Struct Mol Biol 14: 15–22 [DOI] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg E, Walter P, Hochleitner E, Lottspeich F, Klug G 2003. An exosome-like complex in Sulfolobus solfataricus. EMBO Rep 4: 889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Vina J 2012. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med 50: 1287–1295 [DOI] [PubMed] [Google Scholar]
- Guarente L, Ptashne M 1981. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci 78: 2199–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Cheng P, Yuan H, Liu Y 2009. The exosome regulates circadian gene expression in a posttranscriptional negative feedback loop. Cell 138: 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Li X, Spatrick P, Casillo R, Dong S, Jacobson A 2003. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol Cell 12: 1439–1452 [DOI] [PubMed] [Google Scholar]
- He F, Amrani N, Johansson MJ, Jacobson A 2008. Chapter 6. Qualitative and quantitative assessment of the activity of the yeast nonsense-mediated mRNA decay pathway. Methods Enzymol 449: 127–147 [DOI] [PubMed] [Google Scholar]
- Herrero E, Ros J, Belli G, Cabiscol E 2008. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta 1780: 1217–1235 [DOI] [PubMed] [Google Scholar]
- Houalla R, Devaux F, Fatica A, Kufel J, Barrass D, Torchet C, Tollervey D 2006. Microarray detection of novel nuclear RNA substrates for the exosome. Yeast 23: 439–454 [DOI] [PubMed] [Google Scholar]
- Jacobs Anderson JS, Parker RP 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J 17: 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev 18: 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai L, Fang F, Butler JS, Sherman F 2004. Polyadenylation of rRNA in Saccharomyces cerevisiae. Proc Natl Acad Sci 101: 8581–8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Tomecki R, Dziembowski A, Seraphin B 2008. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456: 993–996 [DOI] [PubMed] [Google Scholar]
- Lee A, Henras AK, Chanfreau G 2005. Multiple RNA surveillance pathways limit aberrant expression of iron uptake mRNAs and prevent iron toxicity in S. cerevisiae. Mol Cell 19: 39–51 [DOI] [PubMed] [Google Scholar]
- Mager WH, Planta RJ 1991. Coordinate expression of ribosomal protein genes in yeast as a function of cellular growth rate. Mol Cell Biochem 104: 181–187 [DOI] [PubMed] [Google Scholar]
- Martens JA, Laprade L, Winston F 2004. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429: 571–574 [DOI] [PubMed] [Google Scholar]
- Martens JA, Wu PY, Winston F 2005. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev 19: 2695–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaux S, van Hoof A 2006. Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay. RNA 12: 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan L, Decourty L, Saveanu C, Rappsilber J, Ceulemans H, Jacquier A, Tollervey D 2008. A yeast exosome cofactor, Mpp6, functions in RNA surveillance and in the degradation of noncoding RNA transcripts. Mol Cell Biol 28: 5446–5457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Tollervey D 1996. The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev 10: 502–513 [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D 1997. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell 91: 457–466 [DOI] [PubMed] [Google Scholar]
- Murakami H, Goto DB, Toda T, Chen ES, Grewal SI, Martienssen RA, Yanagida M 2007. Ribonuclease activity of Dis3 is required for mitotic progression and provides a possible link between heterochromatin and kinetochore function. PLoS One 2: e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda L, Keller G, Muhlenhoff U, Rutherford JC, Lill R, Winge DR 2006. Role of glutaredoxin-3 and glutaredoxin-4 in the iron regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae. J Biol Chem 281: 17661–17669 [DOI] [PubMed] [Google Scholar]
- Pedro-Segura E, Vergara SV, Rodriguez-Navarro S, Parker R, Thiele DJ, Puig S 2008. The Cth2 ARE-binding protein recruits the Dhh1 helicase to promote the decay of succinate dehydrogenase SDH4 mRNA in response to iron deficiency. J Biol Chem 283: 28527–28535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Askeland E, Thiele DJ 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110 [DOI] [PubMed] [Google Scholar]
- Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA 2006. Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J Cell Sci 119(Pt 21): 4554–4564 [DOI] [PubMed] [Google Scholar]
- Rutherford JC, Ojeda L, Balk J, Muhlenhoff U, Lill R, Winge DR 2005. Activation of the iron regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial but not cytosolic iron-sulfur protein biogenesis. J Biol Chem 280: 10135–10140 [DOI] [PubMed] [Google Scholar]
- Schaeffer D, van Hoof A 2011. Different nuclease requirements for exosome-mediated degradation of normal and nonstop mRNAs. Proc Natl Acad Sci 108: 2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D, Tsanova B, Barbas A, Reis FP, Dastidar EG, Sanchez-Rotunno M, Arraiano CM, van Hoof A 2009. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol 16: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Xu Z, Mathews DH, Butler JS 2012. Air proteins control differential TRAMP substrate specificity for nuclear RNA surveillance. RNA 18: 1934–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Leung E, Brown J, Tollervey D 2009. The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res 37: 1127–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Kiss DL, Turk E, Tartakoff AM, Andrulis ED 2011. Pronounced and extensive microtubule defects in a Saccharomyces cerevisiae DIS3 mutant. Yeast 28: 755–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomecki R, Kristiansen MS, Lykke-Andersen S, Chlebowski A, Larsen KM, Szczesny RJ, Drazkowska K, Pastula A, Andersen JS, Stepien PP, et al. 2010. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J 29: 2342–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Boone C 2006. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 313: 171–192 [DOI] [PubMed] [Google Scholar]
- Trushina E, McMurray CT 2007. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145: 1233–1248 [DOI] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, Parker R 2001. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104: 377–386 [DOI] [PubMed] [Google Scholar]
- van Hoof A, Lennertz P, Parker R 2000. Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol Cell Biol 20: 441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A, Frischmeyer PA, Dietz HC, Parker R 2002. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295: 2262–2264 [DOI] [PubMed] [Google Scholar]
- Waldron C, Lacroute F 1975. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J Bacteriol 122: 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J 15: 3377–3384 [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Ueta R, Fukunaka A, Sasaki R 2002. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J Biol Chem 277: 18914–18918 [DOI] [PubMed] [Google Scholar]
- Zhu C, Byers KJ, McCord RP, Shi Z, Berger MF, Newburger DE, Saulrieta K, Smith Z, Shah MV, Radhakrishnan M, et al. 2009. High-resolution DNA-binding specificity analysis of yeast transcription factors. Genome Res 19: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]