Abstract
Background
Accumulating evidence suggests that low concentrations of serum 25(OH)D is coupled with increased risks of hypertension, obesity, and cardiovascular disease. However, this relationship has not been established in populations with very low levels of 25(OH)D. Therefore, the aim of our study was to clarify the associations between 25(OH)D and blood pressure, obesity, sex, and lipid profiles in the Kazak ethnic population, who have an extremely low level of 25(OH)D.
Material/Methods
A multistage-cluster sampling survey was carried out for residents with Kazak ethnicity in Xinjiang, China. Anthropometric measurements of each participant were taken and the concentrations of 25(OH)D, calcium, alkaline phosphatase, and lipid profiles were measured. Individuals were classified into different groups in terms of vitamin D status, degree of adiposity, presence of hypertension, and other comorbidities.
Results
The madian concentration of 25(OH)D was 16.2 (11.8–20.5) ng/mL and the prevalence of vitamin D deficiency was 72.4% in this Kazak population (n=928, 59.0% women). Females had a lower 25(OH)D concentration than males – 14.6 (10.5–19.4) ng/mL vs. 17.7 (14.8–22.5) ng/mL, P<0.001. The subjects were classified into 3 groups according to their vitamin D status. There were significant differences in BMI (P=0.046), waist circumference (P=0.037), hip circumference (P=0.003), systolic BP (P=0.035), and LDL cholesterol (P=0.008) among the groups after adjustment for sex and age. On the other hand, there was no significant difference in vitamin D levels between groups with or without hypertension (P=0.586), and groups with or without obesity (P=0.639). A multifactor-regression analysis revealed that every increment of 1mg/dL in LDL cholesterol was associated with a 1.0 ng/mL decline in serum 25(OH)D.
Conclusions
The insufficiency of vitamin D is highly prevalent in Kazaks. Sex, LDL cholesterol, and hip circumference are 3 variables strongly associated with serum 25(OH)D concentration. In a population with low levels of 25(OH)D, the negative relationship between obesity and serum 25(OH)D, a common finding from most previous studies, could not be established.
MeSH Keywords: Blood Pressure, Calcifediol, Gender Identity, Obesity
Background
Vitamin D plays essential roles in mineral metabolism and its deficiency is a worldwide health issue. Recent evidence, mainly focusing on vitamin D and metabolism, shows that low vitamin D levels are associated with increased risks for numerous diseases, especially for hypertension, cardiovascular diseases [1], diabetes, [2] and certain cancers [3]. The epidemiological data [4–6], including a meta-analysis (1) containing 3 cohort studies conducted in Europe and North America, has shown that lower levels of 25(OH) vitamin D is associated with an increased risk of hypertension.
Obesity is another serious health problem. A growing body of epidemiological evidence [7–11] suggests that a lower level of vitamin D is not only associated with increased obesity, but also with a greater risk for weight gain over time. In US whiten [12], Hispanic, and African-American [13] populations, a negative relationship between adiposity and serum 25(OH)D concentration has been established.
The incidences of vitamin D insufficiency, hypertension, and obesity vary among different ethnic groups [14–21]. In a population with extremely low vitamin D levels, the relationship between vitamin D and obesity has not been investigated. Xinjiang province in north-western China is located at 34° to 48° N latitude and has a long winter. There are 148.3 million ethnic Kazaks in Xinjiang. The Kazak people love food made from wheat flour and meat from cattle and horses. Milk tea is their favorite drink. Because of the cold weather, the Kazak people wear long trousers and long sleeves, which reduces their sunlight exposure. The Kazak ethnic population has a higher prevalence of hypertension (48.9% in adults) and obesity (overweight/obesity 67.2% in adults) [22–26] compared to other ethnic populations living in Xinjiang. They also have low vitamin D levels. Therefore, clarification of the relationship between vitamin D and hypertension and obesity in the Kazak population may reveal more valuable information on vitamin D and material metabolism.
In this study, we set out to investigate the relationship between vitamin D status and blood pressure and obesity in a Kazak population in Xinjiang, China. Our hypothesis was that lower serum 25(OH)D is associated with hypertension, obesity, and detrimental atherogenic dyslipidemia in the Kazak population.
Material and Methods
Study population
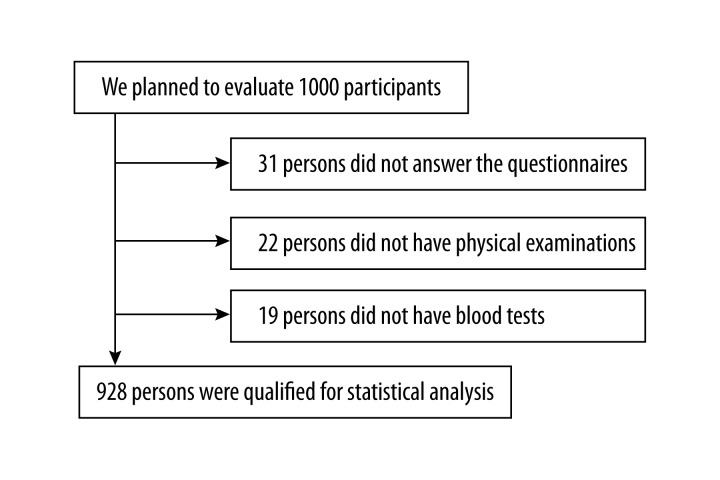
This cross-sectional study was conducted in a population of ethnic Kazak adults. A multistage-cluster sampling survey was conducted in Fukang, Xinjiang province, China, between October 2013 and December 2013. According to distribution of the population in the units, all samples were recruited by postal address. We planned to evaluate 1000 participants, but only 928 (92.8%) completed the questionnaire, physical examinations, and blood tests (Figure 1). The criteria for inclusion were Kazak ethnic adults, residing in Xinjiang, and 30–75 years of age. The exclusive criteria were any 1 of the following: medical histories of bariatric surgery, diabetes and other chronic systemic diseases; drug and alcohol abuse; hyperthyroidism, Cushing syndrome, or hyperparathyroidism; myocardial infarction or stroke within 6 months; cancers in the previous 5 years; or taking vitamin D or calcium supplements in the previous 3 months. The study was conducted in agreement with the 1990 Declaration of Helsinki and subsequent amendments. The study protocol was approved by the Ethics Committee Board in the first affiliated hospital of Xinjiang Medical University. Written informed consent was obtained from all participants.
Figure 1.
Flow diagram of participant enrollment in our study.
The questionnaire contained many items, including age, sex, and medical history of diabetes mellitus and hypertension. The survey was performed by professionally trained investigators.
Anthropometric measurements
Anthropometric measurements were obtained after overnight fasting and the subjects wore light clothes and no shoes. Height was determined by a fixed wall scale and was measured to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg by using an electronic scale that was calibrated prior to each measurement. Waist circumference was measured to the nearest 0.1 cm in a horizontal plane at the level of the umbilicus at the end of normal expiration. Hip circumference was measured at the maximum extension of the buttocks and recorded to the nearest 0.1 cm. BMI was calculated by a standard formula (weight in kilograms/height in meters squared). Blood pressure was manually measured with a mercury sphygmomanometer after the subject had been seated for at least 15 minutes. Measurements were recorded to the nearest 2 mmHg.
Blood tests
Blood samples were obtained after an overnight fast. Total serum 25(OH)D, including D2 and D3, was measured by a ROCHE MODULAR ANALYTICS E170 with commercially available kits. The measurable range was 3.0–70.0 ng/mL, with an inter- and intra- assay variable coefficient of 9–15% and <10%, respectively. Biochemical parameters, such as fasting blood glucose, calcium, phosphate, alkaline phosphatase, urea nitrogen, serum creatinine, and lipid profiles (including triglycerides, total cholesterol, HDL cholesterol and LDL cholesterol), were measured by an auto-analyzer (ADVIA1650; Siemens, NY, USA) with commercially available kits. Bioelectrical impedance analysis (BIA) was performed using a Body Fat Analyzer model HBF-206IT (Omron Institute of Life Sciences, Vernon Hills, IL) in a fasting state. The body composition was measured by standard procedures according to operation manuals. The individual stood on the metal foot pads with bare feet, and the fat percentage, the skeletal muscle percentage and visceral fat index were determined as described previously [27].
Definitions
Obesity was defined by the World Health Organization. Normal: 18.5≤ BMI <25 kg/m2; overweight: 25≤ BMI <30 Kg/m2; obesity: 25≤ BMI <30 kg/m2.
Hypertension was identified from the self-reported questionnaire and the clinical data measured by the investigators. The diagnosis met at least 1 of 3 criteria: systolic BP (SBP) ≥140 mmHg; diastolic BP (DBP) ≥90 mmHg; using anti-hypertension medications.
In this study, the participants were classified into 3 groups in terms of vitamin D concentrations: (1) Sufficiency: concentration of 25(OH)D above 30 ng/ml; (2) insufficiency: 25(OH)D level between 20 and 30 ng/ml; and (3) Deficiency: 25(OH)D level below 20 ng/ml.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software, version 17.0 (Chicago, IL, USA). Data are given as median (interquartile range) for continuous variables or percentages (%) for categorical variables. Analysis of covariance (ANCOVA) was used to compare the differences among the subgroups with different levels of vitamin D, BMI, and blood pressure. Post hoc Bonferroni correction was used for multiple comparisons. Statistical comparisons for categorical variables were performed using the chi-square or Fisher’s exact tests. Univariate and multivariate logistic regression analyses were used to explore the relationship between 25(OH)D and other variables, such as age, sex, BMI, systolic BP, diastolic BP and lipid levels. Age, BMI, systolic BP, diastolic BP, lipid levels, and serum 25(OH)D (log-transformed) were used as continuous variables for the purpose of regression analysis. A 2-sided probability value ≤0.05 was considered statistically significant.
Results
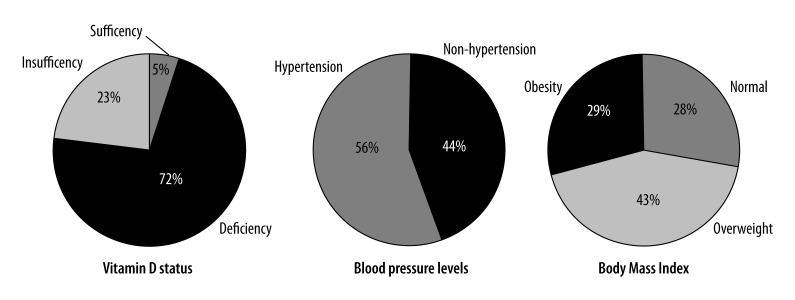
The anthropometric and biochemical characteristics of the study population (n=928) are listed in Table 1 and Figure 2. The concentration of vitamin 25(OH)D was 16.2 (11.8–20.5) ng/mL (from 4.0 to 67.5 ng/mL). The incidence of vitamin D deficiency and insufficiency was 72.4% and 22.7%, respectively, according to the definition. Only 4.8% of the population had vitamin D level in the normal range. Women (n=548) had significantly lower 25(OH) D concentrations than men (n=380), 14.6 (10.5–19.4) vs. 17.7 (14.8–22.5) ng/mL (P<0.001) and a higher incidence of vitamin D deficiency (77.0% vs. 65.8%; P<0.001).
Table 1.
Demographic and clinical characteristics for Kazak men and women.
| Variable | Total (n=928) | Women (n=548) | Men (n=380) | P value* | P value** |
|---|---|---|---|---|---|
| Clinical and anthropometric measures | |||||
| Age (years) | 47.0 (39.0–56.4) | 46.0 (38.0–55.1) | 49.1 (41.0–58.1) | 0.001 | |
| BMI (kg/m2) | 27.5 (24.4–30.6) | 27.3 (24.1–30.9) | 27.7 (25.0–30.3) | 0.540 | <0.001 |
| Waist circumference (cm) | 99.0 (88.0–107.0) | 95.0 (85.3–105.0) | 102.0 (91.3–102.0) | <0.001 | <0.001 |
| Hip circumference (cm) | 100.0 (92.0–105.0) | 98.0 (90.0–105.0) | 100.0 (95.0–106.0) | 0.001 | <0.001 |
| Systolic blood pressure (mmHg) | 139.0 (125.0 –160.0) | 136.0 (122.0 –156.0) | 142.0 (130.0 –163.0) | <0.001 | <0.001 |
| Diastolic blood pressure (mmHg) | 80.0 (71.0–90.0) | 80.0 (70.0–90.0) | 81.5 (72.0–92.0) | 0.120 | <0.001 |
| Hypertension (%) | 56.1 | 52.4 | 61.6 | 0.005 | |
| Biochemical measures | |||||
| 25(OH)D (ng/dL) | 16.2 (11.8–20.5) | 14.6 (10.5–19.4) | 17.7 (14.8–22.5) | <0.01 | |
| 25(OH)D deficiency (%) | 72.4 | 77.0 | 65.8 | <0.01 | |
| 25(OH)D insufficient (%) | 22.7 | 18.2 | 29.2 | <0.01 | |
| 25(OH)D sufficient (%) | 4.8 | 4.7 | 5.0 | <0.01 | |
| Calcium (mg/dL) | 9.2 (9.2–9.6) | 9.2 (9.2–9.6) | 9.2 (9.2–9.6) | 0.120 | 0.190 |
| Phosphate (mg/dL) | 4.2 (3.7–4.5) | 4.3 (4.0–4.5) | 4.0 (3.7–4.3) | <0.001 | <0.001 |
| Alkaline phosphatase (U/L) | 86.0 (71.0–105.0) | 81.0 (65.0–102.0) | 91.0 (78.0–107.0) | <0.001 | <0.001 |
| Fasting plasma glucose (mg/dL) | 88.2 (81.0–95.4) | 86.4 (81.0–95.4) | 90.0 (82.8–97.2) | 0.002 | <0.001 |
| Total cholesterol (mg/dL) | 200.8 (173.7 –223.9) | 196.9 (169.9 –220.1) | 208.5 (181.5 –231.7) | <0.001 | <0.001 |
| HDL cholesterol (mg/dL) | 46.3 (38.6–57.9) | 50.2 (42.5–57.9) | 42.4 (34.7–50.2) | <0.001 | <0.001 |
| LDL cholesterol (mg/dL) | 81.1 (65.6–95.5) | 73.4 (57.9–88.8) | 88.8 (73.4–104.2) | <0.001 | <0.001 |
| Triglycerides (mg/dL) | 88.5 (61.9–132.7) | 79.6 (53.1–123.9) | 106.2 (70.8–159.3) | <0.001 | <0.001 |
| Total cholesterol to HDL ratio | 4.20 (3.45–5.18) | 3.82 (3.23–4.64) | 4.92 (4.01–5.83) | <0.001 | <0.001 |
| Urea nitrogen (mg/dL) | 11.5 (9.8–14.3) | 10.9 (9.2–13.2) | 12.6 (10.6–15.4) | <0.001 | <0.001 |
| Serum creatinine (mg/dL) | 0.7 (0.6–0.8) | 0.6 (0.6–0.7) | 0.9 (0.8–0.9) | <0.001 | <0.001 |
| Body fat percentage | 33.1 (29.0–39.2) | 38.2 (34.1–41.5) | 28.8 (27.9–31.4) | <0.001 | <0.001 |
| Body skeletal muscle percentage | 26.1 (23.0–29.2) | 23.5 (21.8–25.3) | 29.4 (27.9–31.4) | <0.001 | <0.001 |
| Visceral fat index | 12.0 (8.0–17.0) | 10.0 (6.0–15.0) | 16.0 (11.3–20.0) | <0.001 | <0.001 |
| Basal metabolic rate (Kcal) | 1506.0 (1322.0 –1694.0) | 1368.0 (1241.0 –1489.0) | 1723.0 (1580.0 –1861.0) | <0.001 | <0.001 |
Data are shown by median (interquartile range) for continuous variables and percentages (%) for categorical variables.
P value indicates one-way analysis of variance (ANOVA) for continuous variables, and chi-square test for categorical variables, between men and women;
P value indicates after adjusted for age by ANOVA, Bonferroni post hoc test, between men and women.
BMI – body mass index; 25(OH) D – 25-hydroxyvitamin D; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
Figure 2.
Distribution of vitamin D status, blood pressure, and obesity in a Kazak ethnic population.
To examine if the levels of plasma vitamin D are associated with changes in metabolic parameters, we categorized the subjects into 3 groups based on the concentrations of plasma vitamin D, as described in the methods section: vitamin D deficient group (n=672), insufficient group (n=211), and sufficient group (n=45). Significant difference were detected in BMI (P=0.046), waist circumference (P=0.037), hip circumference (P=0.003), systolic BP (P=0.035), and LDL cholesterol (P=0.008) among the 3 groups after sex and age were adjusted for. The vitamin D deficient group had the highest body fat percentage (P=0.009) and lowest body skeletal muscle percentage (P=0.026) after adjustment for sex and age among the 3 groups. There were no significant differences in diastolic BP, calcium, phosphate, fasting blood glucose, triglycerides, HDL cholesterol, total cholesterol to HDL cholesterol ratio, visceral fat index, and basal metabolic rate among the 3 groups (Table 2).
Table 2.
Comparison among the 3 groups with different vitamin D status.
| Variable | Vitamin D deficiency (n=672) | Vitamin D insufficiency (n=211) | Vitamin D sufficiency (n=45) | P value* | P value** |
|---|---|---|---|---|---|
| Women (%) | 62.8 | 47.4 | 57.8 | <0.001 | |
| Age (years) | 47.5 (39.0–63.0) | 45.0 (38.1–54.0) | 58.0 (48.0–61.7) | <0.001 | |
| BMI (Kg/m2) | 27.3 (24.3–30.4) | 28.2 (25.0–31.1) | 27.6 (24.3–30.7) | 0.155 | 0.046 |
| Waist circumference (cm) | 99.0 (88.0–107.0) | 100.0 (88.0–108.0) | 96.0 (84.0–104.0) | 0.256 | 0.037 |
| Hip circumference (cm) | 98.0 (90.0–104.5) | 102.0 (94.0–107.5) | 100.0 (95.0–108.0) | <0.001 | 0.003 |
| Systolic BP (mmHg)a | 130.0 (120.0–140.0) | 125.0 (117.0–138.0) | 144.0 (121.0–169.0) | 0.004 | 0.009 |
| Diastolic BP (mmHg)a | 75.0 (68.0–82.0) | 73.0 (66.0–80.0) | 75.0 (67.0–89.0) | 0.700 | 0.146 |
| Hypertension | 54.5 | 56.9 | 77.8 | 0.009 | |
| Fasting glucose (mg/dL) | 88.2 (81.0–95.4) | 90.0 (82.8–97.2) | 91.8 (86.4–97.2) | 0.160 | 0.969 |
| Total cholesterol (mg/dL) | 200.8 (172.6–227.8) | 201.8 (173.7–220.1) | 196.9 (181.5–223.9) | 0.933 | 0.168 |
| HDL cholesterol (mg/dL) | 46.3 (37.6–57.9) | 42.5 (38.6–54.1) | 46.4 (37.9–61.8) | 0.042 | 0.423 |
| LDL cholesterol (mg/dL) | 81.8 (65.6–96.5) | 81.0 (57.9–92.7) | 73.4 (65.6–93.6) | 0.384 | 0.008 |
| Triglycerides (mg/dL) | 88.5 (61.9–132.7) | 88.4 (62.0–141.6) | 88.3 (61.7–132.9) | 0.727 | 0.491 |
| Total cholesterol to HDL ratio | 4.10 (3.43–5.11) | 4.52 (3.50–5.56) | 4.53 (3.28–4.98) | 0.039 | 0.209 |
| Body fat percentage | 33.8 (29.1–39.4) | 32.4 (28.8–37.6) | 32.7 (28.0–39.3) | 0.214 | 0.009 |
| Body skeletal muscle percentage | 25.3 (22.9–28.9) | 27.1 (23.7–29.4) | 27.3 (23.0–30.0) | 0.026 | 0.026 |
| Visceral fat index | 12.0 (8.0–17.0) | 14.0 (8.0–19.0) | 14.0 (9.0–18.0) | 0.017 | 0.116 |
| Basal metabolic rate (Kcal) | 1481.0 (1315.0 –1663.0) | 1563.0 (1368.0 –1797.0) | 1556.0 (1321.0 –1651.0) | 0.001 | 0.151 |
Data are expressed by median (interquartile range) for continuous variables and percentages (%) for categorical variables.
P-value indicates one-way analysis of variance (ANOVA) for continuous variables, and chi-square test for categorical variables, among the 3 groups;
P-value indicates after adjusted for age and sex by ANOVA, Bonferroni post hoc test, among the 3 groups;
participants who were not on antihypertensive drugs (n=559).
25 (OH)D concentration – Sufficiency (>30 ng/ml), insufficiency (20–30 ng/ml), Deficiency (<20 ng/ml). BMI – body mass index; BP – blood pressure; 25(OH)D – 25-hydroxyvitamin D; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
To further investigate the relationship between plasma vitamin D levels and blood pressure, we divided the participants into 2 groups: hypertension (n=521) and non-hypertension (n=407). There were significant differences in BMI, waist circumference, hip circumference, lipid levels, body fat percentage, and body skeletal muscle percentage between the 2 groups. However, there was no significant difference in vitamin D levels between the 2 groups (P=0.586) (Table 3). To determine if plasma vitamin D levels were associated with blood pressure, we chose those participants who were not on antihypertensive drugs (non-hypertension n=407, hypertension without drugs n=152, total n=559), and divided them into 4 groups according to their systolic BP (SBP) levels: SBP less than 120 mmHg; SBP 120–140 mmHg; SBP 140–160 mmHg; and SBP above 160 mmHg. There was still no difference in vitamin D levels among these 4 groups.
Table 3.
Characteristics of participants with or without hypertension.
| Variable | No hypertension (n=407) | Hypertension (n=521) | P value * | P value** |
|---|---|---|---|---|
| Female (%) | 64.1 | 55.1 | 0.005 | |
| Age (years) | 41.0 (34.9–48.0) | 52.2 (45.0–60.0) | <0.001 | |
| BMI (Kg/m2) | 26.1 (23.4–28.9) | 28.5 (25.7–31.3) | <0.001 | |
| Waist circumference (cm) | 94.0 (84.0–104.0) | 101.0 (92.0–108.0) | <0.001 | 0.528 |
| Hip circumference (cm) | 97.0 (89.0–103.0) | 101.0 (92.0–108.0) | <0.001 | 0.696 |
| 25(OH)D (ng/mL) | 16.4 (12.4–19.9) | 15.8 (11.2–21.0) | 0.586 | 0.959 |
| 25(OH)D deficiency (%) | 75.2 | 70.2 | 0.009 | |
| 25(OH)D insufficiency (%) | 22.4 | 24.0 | 0.009 | |
| 25(OH)D sufficiency (%) | 2.5 | 6.7 | 0.009 | |
| Fasting glucose (mg/dL) | 86.4 (79.2–93.6) | 90.0 (84.6–99) | <0.001 | 0.035 |
| Total cholesterol (mgl/dL) | 189.2 (166.0–212.4) | 208.5 (181.5–231.7) | <0.001 | 0.007 |
| HDL cholesterol (mg/dL) | 45.9 (38.6–57.9) | 46.3 (38.6–54.1) | <0.001 | 0.332 |
| LDL cholesterol (mg/dL) | 73.4 (57.9–88.8) | 84.9 (69.5–100.3) | 0.061 | 0.049 |
| Triglycerides (mg/dL) | 30.9 (23.2–46.3) | 46.3 (30.9–65.6) | <0.001 | <0.001 |
| Total cholesterol to HDL ratio | 3.83 (3.15–4.76) | 4.45 (3.69–5.40) | <0.001 | <0.001 |
| Body fat percentage | 32.4 (28.3–36.9) | 34.2 (29.4–40.4) | <0.001 | 0.367 |
| Body skeletal muscle percentage | 26.8 (23.9–29.7) | 25.2 (22.3–29.1) | <0.001 | 0.329 |
| Visceral fat index | 10.0 (6.8–15.0) | 14.0 (10.0–19.0) | <0.001 | 0.753 |
| Basal metabolic rate (Kcal) | 1464.0 (1254.0–1638.3) | 1560.0 (1370.0–1737.0) | <0.001 | 0.269 |
Data are shown by median (interquartile range) for continuous variables and percentages (%) for categorical variables. P value * indicates one-way analysis of variance (ANOVA) for continuous variables, and chi-square test for categorical variables, between groups with or without hypertension; P value ** indicates after adjusted for age and BMI by ANOVA, Bonferroni post hoc test, between groups with or without hypertension. BMI – body mass index; BP – blood pressure; 25(OH)D – 25-hydroxyvitamin D; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
To determine if the levels of plasma vitamin D were associated with changes in overall body status, we categorized the subjects into 3 groups based on BMI: normal weight (n=267), overweight (n=393), and obese (n=268). The prevalence of overweight and obesity in the whole population was 42.4% and 28.9%, respectively. Significant differences in systolic BP, diastolic BP, triglycerides, HDL cholesterol, LDL cholesterol, total cholesterol to HDL cholesterol ratio, fasting blood glucose, body fat percentage, and skeletal muscle percentage were detected among the 3 groups (Table 4). However, participants with different BMI had similar vitamin D levels (Table 4).
Table 4.
Comparison of clinical features among 3 groups with different body mass index (Kg/m2).
| Variable | BMI <25 (n=261) | 25 ≤BMI <30 (n=393) | BMI ≥30 (n=268) | P value* | P value** |
|---|---|---|---|---|---|
| Female (%) | 64.8 | 55.2 | 59.7 | 0.051 | |
| Age (years) | 43.0 (34.5–49.0) | 48.9 (40.9–58.0) | 49.0 (42.0–58.5) | <0.001 | |
| Waist circumference (cm) | 86.0 (78.0–93.0) | 99.0 (90.0–104.0) | 110.0 (104.0–116.0) | <0.001 | <0.001 |
| Hip circumference (cm) | 90.0 (82.0–96.0) | 100.0 (93.0–104.0) | 109.0 (104.0–115.0) | <0.001 | <0.001 |
| Systolic BP (mmHg) | 130.0 (120–144.0) | 140.0 (127.0–160.5) | 147.0 (130.0–170.0) | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 74.0 (68.0–82.0) | 72.0 (80.0–91.0) | 84.5 (77.0–94.0) | <0.001 | <0.001 |
| Hypertension (%) | 37.2 | 60.1 | 69.4 | <0.001 | |
| 25(OH)D (ng/mL) | 16.5 (12.3–17.9) | 16.2 (11.3–20.4) | 16.1 (11.5–22.5) | 0.639 | 0.402 |
| 25(OH)D deficiency (%) | 74.7 | 74.0 | 67.2 | 0.140 | |
| 25(OH)D insufficiency (%) | 19.9 | 21.6 | 27.6 | 0.140 | |
| 25(OH)D sufficiency (%) | 5.4 | 4.3 | 5.2 | 0.140 | |
| Fasting glucose (mg/dL) | 86.4 (79.2–95.4) | 88.2 (82.8–96.1) | 90.0 (81.9–100.8) | 0.005 | 0.005 |
| Total cholesterol (mg/dL) | 185.3 (148.3–208.9) | 204.6 (177.7–235.5) | 208.5 (181.5–227.8) | <0.001 | <0.001 |
| HDL cholesterol (mg/dL) | 54.1 (42.5–61.8) | 46.3 (38.6–45.3) | 42.7 (34.7–73.4) | <0.002 | <0.001 |
| LDL cholesterol (mg/dL) | 65.6 (54.1–81.1) | 84.9 (69.5–100.4) | 85.1 (73.4–112.0) | <0.003 | <0.001 |
| Triglycerides (mg/dL) | 61.9 (44.2–88.5) | 87.9 (70.9–132.7) | 123.9 (79.6–168.1) | <0.004 | <0.001 |
| Total cholesterol to HDL ratio | 3.51 (2.94–4.18) | 4.28 (3.58–5.17) | 4.93 (4.11–5.90) | <0.001 | <0.001 |
| Body fat percentage | 29.8 (23.8–32.2) | 35.3 (29.1–39.0) | 40.1 (32.0–43.1) | <0.005 | <0.001 |
| Body skeletal muscle percentage | 27.4 (25.7–32.3) | 24.9 (23.2–29.7) | 22.0 (21.0–27.4) | <0.006 | <0.001 |
| Visceral fat index | 6.0 (5.0–9.0) | 12.0 (10.0–15.0) | 20.0 (17.0–23.0) | <0.007 | <0.001 |
| Basal metabolic rate (Kcal) | 1301.0 (1183.5 –1522.0) | 1489.0 (1362.0 –1703.5) | 1659.0 (1536.5 –1875.0) | <0.008 | <0.001 |
Data are shown by median (interquartile range) for continuous variables and percentages (%) for categorical variables. P value * indicates one-way analysis of variance (ANOVA) for continuous variables, and chi-square test for categorical variables, among t3 groups with different BMI ranges; P value ** indicates adjusted for age by ANOVA, Bonferroni post hoc test among three groups with different BMI ranges. BMI – body mass index; BP – blood pressure; 25(OH)D – 25-hydroxyvitamin D; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
A univariate linear regression analysis, to identify risk factors for serum 25(OH)D concentration, revealed that women had a significantly lower 25(OH)D level than men (Table 5). Although there was a negative association between vitamin D and body fat percentage, the clinical value was limited due to a small B coefficient (Table 5). There were no significant associations between 25(OH)D and systolic or diastolic BP.
Table 5.
Univariate linear regression analysis with serum 25(OH)D as a dependent variable (n=928).
| Variable | B Coefficient | Standard Error | 95%CI | P value |
|---|---|---|---|---|
| Age (years) | −0.002 | 0.001 | (−0.004, 0.001) | 0.208 |
| Gender (male as reference) | −0.201 | 0.029 | (−0.257, −0.144) | <0.001 |
| HBP (non-HBP as reference) | −0.018 | 0.029 | (−0.075, 0.039) | 0.532 |
| Systolic BP (mmHg)* | 0 | 0.002 | (−0.004, 0.002) | 0.928 |
| Diastolic BP (mmHg)* | 0 | 0.002 | (−0.004, 0.002) | 0.596 |
| BMI (Kg/m2) | 0 | 0.003 | (−0.007, 0.005) | 0.757 |
| Waist circumference (cm) | 0 | 0.001 | (−0.003, 0.002) | 0.648 |
| Hip circumference (cm) | 0.003 | 0.001 | (0.000, 0.005) | 0.029 |
| Body fat percentage | −0.011 | 0.002 | (−0.015, −0.007) | <0.001 |
| Visceral fat index | 0.005 | 0.002 | (0.001, 0.01) | 0.009 |
| Body skeletal muscle percentage | 0.02 | 0.003 | (0.014, 0.027) | <0.001 |
| Total cholesterol (mg/dL) | 0.000 | 0.000 | (−0.001, 0.000) | 0.037 |
| Triglycerides (mg/dL) | 0.000 | 0.000 | (0.000, 0.000) | 0.140 |
| HDL cholesterol (mg/dL) | −0.002 | 0.001 | (−0.004, 0.000) | 0.027 |
| LDL cholesterol (mg/dL) | −0.002 | 0.001 | (−0.003, −0.000) | 0.010 |
| Total cholesterol to HDL ratio | 0.014 | 0.011 | (−0.007, 0.035) | 0.186 |
Participants who were not on antihypertensive drugs (n=559).
BMI – body mass index; BP – blood pressure; 25(OH)D – 25-hydroxyvitamin D; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
In a multivariate regression analysis, sex, hip circumference, body fat percentage, body skeletal muscle percentage, visceral fat index, total cholesterol, HDL cholesterol, and LDL cholesterol were put into analysis as possible influence factors for serum vitamin D. It was shown that sex, LDL cholesterol, and hip circumference were independent factors for vitamin D. After sex and hip circumference were adjusted, every increment of 1mg/dL in LDL cholesterol was associated with a 1.0-ng/mL decline in serum 25(OD)D (Table 6).
Table 6.
Multivariate linear regression analysis with serum 25(OH)D as a dependent variable (n=928).
| Variable | B Coefficient | Standard Error | 95% CI | P value |
|---|---|---|---|---|
| Gender (male as reference) | −0.234 | 0.029 | (−0.292, −0.177) | <0.001 |
| LDL cholesterol (mg/dL) | −0.003 | 0.001 | (−0.004, −0.002) | <0.001 |
| Hip circumference (cm) | 0.003 | 0.001 | (0.001, 0.005) | 0.012 |
25(OH)D – 25-hydroxyvitamin D; LDL – low-density lipoprotein.
Discussion
Our study shows that the incidence of vitamin D insufficiency is astonishingly high in ethnic Kazak adults residing in Xinjiang, China. The mean 25(OH)D concentration is as low as 17.1 ng/mL and the prevalence of vitamin D deficiency, insufficiency, and sufficiency is 72.4%, 22.7%, and 4.8%, respectively. The serum 25(OH)D level is negatively coupled with LDL cholesterol and is lower in women.
Vitamin D level in Kazaks is much lower than in other ethnic populations [28–30]. In ethnic Chinese Han, the average concentration of 25(OH) D is 26.9ng/mL and the incidence of vitamin D deficiency is 28.6%, significantly higher than that in Kazaks [31]. The difference could be due to several reasons. First, Kazaks geographically reside in areas with low sunlight levels. Fukang city, locating in north-western China, is at near 44° N latitude and has approximately 66 sunny days per year. Also, low vitamin D content in food and little use of dietary supplements might exacerbate the situation.
The prevalence of hypertension in our study was 56.1%, and no association was found between serum 25(OH)D and blood pressure. This is not consistent with the results of most studies in Hispanic and non-Hispanic populations, in which the serum vitamin D level is inversely related with blood pressure [1,4,5]. Three such studies were conducted in Chinese populations. One study confirmed an inverse association between 25(OH)D and diastolic BP [29], and another found that the inverse association only existed in men [28]. The third study found no significant associations. Therefore, a conclusive association between vitamin D level and blood pressure in Chinese populations has not been determined.
There is a consistent finding that serum vitamin D is inversely coupled with adipose deposit [32–34]. Increased sequestration of fat-soluble vitamin D in adipocytes is a supposed mechanism for this phenomenon. However, in our study, such a relationship could not be confirmed. This may be because vitamin D levels in the Kazak ethnic population are too low to establish the relationship with BMI. The mean concentration of 25(OH)D was 17.1 ng/mL in our study, and it was 25.9–35.7 ng/mL in some studies focusing on obese participants [35–37]. Our findings suggest that in a population with severe vitamin D deficiency, the inverse relationship between vitamin D and obesity, a common finding in the most previous studies, may disappear. More studies, focusing on populations with very low levels of vitamin D, are required to confirm this hypothesis.
Our study revealed that women had lower 25(OH)D than men. Compared to men, women have more fat deposits in the gluteal-femoral regions [38], which may sequestrate more vitamin D in these areas. In fact, the relationship between vitamin D and sex is not well established. Some studies showed a higher prevalence of vitamin D deficiency in men than in women [39,40], while another study showed the opposite results [41].
Interestingly, a significantly negative association between serum LDL cholesterol and 25(OH)D was found in our study. After being adjusted for sex and hip circumference, every 1-mg/dL increment in LDL cholesterol was associated with a 1.0-ng/mL decline in serum 25(OH)D. A similar inverse relationship between 25(OH)D and total cholesterol and LDL cholesterol and triglycerides has been reported [42,43]. Furthermore, a positive association between vitamin D and HDL cholesterol and apolipoprotein A1 was also found [44,45]. All these results point to a consistent tendency that a higher 25(OH)D level is associated with a beneficial lipid profile, but a cause-and-effective relationship could not be established due to the limitation of cross-sectional designed studies.
The underlying mechanism between hypovitaminosis D and cholesterol metabolism is not yet fully understood. One theory is that when exposed to sunlight, squalene in epidermal cells is converted into 7-dehydrocholesterol and vitamin D; otherwise, it may shift its metabolic pathway to the formation of cholesterol [46]. Another possible explanation is that LDL cholesterol may be the precursor for vitamin D [47]. Without sunlight, LDL cholesterol in epidermal cells cannot be transformed to vitamin D and would be accumulated. Notably, vitamin D synthesis occurs mainly in the deeper epidermal layers, possibly due to the higher expression of LDL receptors in the basal layers than in the superficial layers (keratinocytes) [48].
A strong relationship between low 25(OH)D concentration and increased cardiovascular events, possibly mediated by atherogenic dyslipidemia, was revealed by some meta-analyses [1,49,50]. However, supplementation of vitamin D, in an attempt to improve lipid profiles and reduce cardiovascular risks, demonstrated no beneficial effects [51–53]. Heterogeneity of participants and insufficient vitamin D supplementation in these clinical trials may impair the effects of vitamin D supplementation [51–53]. Therefore, more well-designed and prospective clinical studies are needed to clarify the beneficial effect of vitamin D supplementation on lipid profile and cardiovascular risks.
Although our study revealed a severe vitamin D deficiency in the Kazak population, especially in women, and established the relationship between plasma vitamin D levels with parameters of metabolic syndrome, this study also has some limitations. First, the factors influencing vitamin D metabolism, such as sunlight exposure, sunscreen use, dietary vitamin D intake, and physical activity, were not assessed. Second, in our study, serum 25(OH)D was measured at the end of the long winter season in the Xinjiang region of China. However, our findings strongly suggest that we likely underestimating the vitamin D deficiency in the Kazak population. Third, patients with cardiovascular disease or cancer were not included in our study, and the relationship between vitamin D and these diseases needs further investigation. Despite these limitations, our study has several strengths. The study uncovered a severe vitamin D deficiency in the Kazak ethnic population, which has a high prevalence of obesity and hypertension and lives in places without sufficient sunlight exposure. Furthermore, we revealed, for the first time, that in a population with severe vitamin D deficiency, the inverse relationship between vitamin D and blood pressure and obesity may disappear.
Conclusions
Deficiency of vitamin D is highly prevalent in the Kazak population and the negative association between serum vitamin D and obesity disappeared in this population. Low level of 25 (OH)D was independently coupled with higher LDL cholesterol, which, if oxidized, may induce higher risks of cardiovascular events. Supplementary vitamin D may benefit patients with cardiovascular diseases by improving atherogenic dyslipidemia.
Abbreviations
- 25(OH)D
25-hydroxy vitamin D
- BMI
body mass index
- CI
confidence interval
- BP
blood pressure
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: The study was supported by Natural Science Foundation of China (No: 81160038)
References
- 1.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–29. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 5.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–19. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Scragg RK, Camargo CJ, Simpson RU. Relation of serum 25-hydroxyvitamin D to heart rate and cardiac work (from the National Health and Nutrition Examination Surveys) Am J Cardiol. 2010;105:122–28. doi: 10.1016/j.amjcard.2009.08.661. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun MA, Boueiz A, Shroff MR, et al. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. J Clin Endocrinol Metab. 2010;95:3814–27. doi: 10.1210/jc.2010-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock K, Huang WY, Fraser DR, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121:462–66. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herranz AS, Garcia MMC, Alvarez DFV. [Vitamin D deficiency in morbidly obese patients. A case-control study]. Endocrinol Nutr. 2010;57:256–61. doi: 10.1016/j.endonu.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Valina-Toth AL, Lai Z, Yoo W, et al. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clin Endocrinol (Oxf) 2010;72:595–603. doi: 10.1111/j.1365-2265.2009.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LK, Hofso D, Aasheim ET, et al. Impact of gender on vitamin D deficiency in morbidly obese patients: a cross-sectional study. Eur J Clin Nutr. 2012;66:83–90. doi: 10.1038/ejcn.2011.140. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–48. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–13. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley-Lewis R, Powe C, Ankers E, et al. Effect of Race/Ethnicity on Hypertension Risk Subsequent to Gestational Diabetes Mellitus. Am J Cardiol. 2014;113(8):1364–70. doi: 10.1016/j.amjcard.2014.01.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki Y, Yoon SS, Chong Y, Carroll MD. NCHS Data Brief. 2014. Hypertension, abnormal cholesterol, and high body mass index among non-Hispanic Asian adults: United States, 2011–2012; pp. 1–8. [PubMed] [Google Scholar]
- 16.Dolezsar CM, McGrath JJ, Herzig AJ, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34. doi: 10.1037/a0033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavicchia PP, Liu J, Adams SA, et al. Proportion of Gestational Diabetes Mellitus Attributable to Overweight and Obesity Among Non-Hispanic Black, Non-Hispanic White, and Hispanic Women in South Carolina. Matern Child Health J. 2014 doi: 10.1007/s10995-014-1437-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincoln KD, Abdou CM, Lloyd D. Race and Socioeconomic Differences in Obesity and Depression among Black and Non-Hispanic White Americans. J Health Care Poor Underserved. 2014;25:257–75. doi: 10.1353/hpu.2014.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K, Wen M, Henry KA. Residential racial composition and black-white obesity risks: differential effects of neighborhood social and built environment. Int J Environ Res Public Health. 2014;11:626–42. doi: 10.3390/ijerph110100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiscella K, Winters P, Tancredi D, Franks P. Racial Disparity in Blood Pressure: is Vitamin D a Factor? J Gen Intern Med. 2011;26:1105–11. doi: 10.1007/s11606-011-1707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sulistyoningrum DC, Green TJ, Lear SA, Devlin AM. Ethnic-specific differences in vitamin D status is associated with adiposity. PLoS One. 2012;7:e43159. doi: 10.1371/journal.pone.0043159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Ma YT, Xie X, et al. [Prevalence and associated factors of diabetes mellitus in children of Han, Uigurs and Kazaks ethnicities in Xinjiang]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:1130–32. [PubMed] [Google Scholar]
- 23.Jiang S, Xie Z. Comparison Study of Metabolic Syndrome’s Differences and Diagnostic Criteria’s Applicability among Xingjiang Uighur, Kazak and Han Population. Int J Endocrinol. 2012;2012:212383. doi: 10.1155/2012/212383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang YN, Xie X, Ma YT, et al. Type 2 diabetes in Xinjiang Uygur autonomous region, China. PLoS One. 2012;7:e35270. doi: 10.1371/journal.pone.0035270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HW, Jiang S, Xu YC. [A cross-sectional study on serum uric acid level and the distribution of metabolic syndrome among Uigur, Han and Kazak prediabetic groups in Xinjiang]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34:958–60. [PubMed] [Google Scholar]
- 26.Luo JY, Ma YT, Yu ZX, et al. Prevalence, awareness, treatment and control of dyslipidemia among adults in northwestern China: the cardiovascular risk survey. Lipids Health Dis. 2014;13:4. doi: 10.1186/1476-511X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosy-Westphal A, Later W, Hitze B, et al. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes Facts. 2008;1:319–24. doi: 10.1159/000176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorjgochoo T, Ou SX, Xiang YB, et al. Circulating 25-hydroxyvitamin D levels in relation to blood pressure parameters and hypertension in the Shanghai Women’s and Men’s Health Studies. Br J Nutr. 2012;108:449–58. doi: 10.1017/S0007114511005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu L, Yu Z, Pan A, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–83. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samefors M, Ostgren CJ, Molstad S, et al. Vitamin D Deficiency in Elderly People in Swedish Nursing Homes is Associated with Increased Mortality. Eur J Endocrinol. 2014;170(5):667–75. doi: 10.1530/EJE-13-0855. [DOI] [PubMed] [Google Scholar]
- 31.Yin X, Sun Q, Zhang X, et al. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutr J. 2012;11:68. doi: 10.1186/1475-2891-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–99. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 33.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obes Rev. 2013;14:393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 34.Konradsen S, Ag H, Lindberg F, et al. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47:87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 35.Palacios C, Gil K, Perez CM, Joshipura K. Determinants of vitamin D status among overweight and obese Puerto Rican adults. Ann Nutr Metab. 2012;60:35–43. doi: 10.1159/000335282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilarrasa N, Maravall J, Estepa A, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–58. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 37.Bertrand KA, Giovannucci E, Liu Y, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012;108:1889–96. doi: 10.1017/S0007114511007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofso D, Jenssen T, Bollerslev J, et al. Anthropometric characteristics and type 2 diabetes in extremely obese Caucasian subjects: a cross-sectional study. Diabetes Res Clin Pract. 2009;86:e9–11. doi: 10.1016/j.diabres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Aasheim ET, Hofso D, Hjelmesaeth J, et al. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr. 2008;87:362–69. doi: 10.1093/ajcn/87.2.362. [DOI] [PubMed] [Google Scholar]
- 40.Lagunova Z, Porojnicu AC, Lindberg F, et al. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–20. [PubMed] [Google Scholar]
- 41.Majumdar V, Nagaraja D, Christopher R. Vitamin D status and metabolic syndrome in Asian Indians. Int J Obes (Lond) 2011;35:1131–34. doi: 10.1038/ijo.2010.232. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Bailo B, Da CL, Arora P, et al. Plasma vitamin D and biomarkers of cardiometabolic disease risk in adult Canadians, 2007–2009. Prev Chronic Dis. 2013;10:E91. doi: 10.5888/pcd10.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagnon C, Lu ZX, Magliano DJ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97:1953–61. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 44.Pacifico L, Anania C, Osborn JF, et al. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165:603–11. doi: 10.1530/EJE-11-0545. [DOI] [PubMed] [Google Scholar]
- 45.Osmancevic A, Landin-Wilhelmsen K, Larko O, Krogstad AL. Vitamin D status in psoriasis patients during different treatments with phototherapy. J Photochem Photobiol B. 2010;101:117–23. doi: 10.1016/j.jphotobiol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. QJM. 1996;89:579–89. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 47.Bogh MK, Schmedes AV, Philipsen PA, et al. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol. 2010;130:546–53. doi: 10.1038/jid.2009.323. [DOI] [PubMed] [Google Scholar]
- 48.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 49.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–33. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Sokol SI, Tsang P, Aggarwal V, et al. Vitamin D status and risk of cardiovascular events: lessons learned via systematic review and meta-analysis. Cardiol Rev. 2011;19:192–201. doi: 10.1097/CRD.0b013e31821da9a5. [DOI] [PubMed] [Google Scholar]
- 51.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–12. doi: 10.1016/j.plipres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Elamin MB, Abu EN, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]