Abstract
The molecular link between proteinuria and hyperlipidemia in nephrotic syndrome is not known. We show in the present study that plasma angiopoietin-like 4 (Angptl4) links proteinuria with hypertriglyceridemia through two negative feedback loops. In previous studies in a rat model that mimics human minimal change disease, we observed localized secretion by podocytes of hyposialylated Angptl4, a pro-proteinuric form of the protein. But in this study we noted high serum levels of Angptl4 (presumably normosialylated based on a neutral isoelectric point) in other glomerular diseases as well. Circulating Angptl4 was secreted by extrarenal organs in response to an elevated plasma ratio of free fatty acids (FFAs) to albumin when proteinuria reached nephrotic range. In a systemic feedback loop, these circulating pools of Angptl4 reduced proteinuria by interacting with glomerular endothelial αvβ5 integrin. Blocking the Angptl4–β5 integrin interaction or global knockout of Angptl4 or β5 integrin delayed recovery from peak proteinuria in animal models. But at the same time, in a local feedback loop, the elevated extrarenal pools of Angptl4 reduced tissue FFA uptake in skeletal muscle, heart and adipose tissue, subsequently resulting in hypertriglyceridemia, by inhibiting lipoprotein lipase (LPL)-mediated hydrolysis of plasma triglycerides to FFAs. Injecting recombinant human ANGPTL4 modified at a key LPL interacting site into nephrotic Buffalo Mna and Zucker Diabetic Fatty rats reduced proteinuria through the systemic loop but, by bypassing the local loop, without increasing plasma triglyceride levels. These data show that increases in circulating Angptl4 in response to nephrotic-range proteinuria reduces the degree of this pathology, but at the cost of inducing hypertriglyceridemia, while also suggesting a possible therapy to treat these linked pathologies.
Molecular pathways that link proteinuria with hyperlipidemia, two key hallmarks of nephrotic syndrome, are not known. Hyperlipidemia has two components: hypercholesterolemia and hypertriglyceridemia1. In the past, hypercholesterolemia has been attributed to increased hepatic synthesis of lipoproteins in response to proteinuria and hypoalbuminemia2. However, the precise molecular link between proteinuria and increased hepatic lipoprotein synthesis remains unknown. The development of hypertriglyceridemia has received much less attention. A major determinant of plasma triglyceride levels is the activity of endothelium-bound LPL, as it hydrolyzes triglycerides to release FFAs3, which promotes their tissue uptake. Mice that lack LPL develop very high triglyceride levels and die soon after birth4. LPL is expressed predominantly in skeletal muscle, heart and adipose tissue, and prior studies have shown that the activity and expression of LPL protein, but not mRNA, are reduced in nephrotic syndrome5. The molecular basis of this reduction in LPL protein activity and expression and its relationship to proteinuria in nephrotic syndrome has not been determined. Other studies have shown that urine albumin in patients with nephrotic syndrome has markedly lower FFA content than plasma albumin from these patients6. A link of these observations with hyperlipidemia has not been explored.
A prior study from our laboratory showed increased expression of Angptl4 in podocytes and in circulation in human and experimental minimal change disease (MCD)7,8, the most common cause of nephrotic syndrome in children. In this disease, podocytes secrete two distinct forms of Angptl4: a high-isoelectric point (pI) pro-proteinuric form that is hyposialylated and noted only in the glomerulus and urine and a neutral-pI form that is properly sialylated7,8. To study the biological role of podocyte-secreted Angptl4, we generated NPHS2 (also called podocin)-Angptl4 transgenic rats, which selectively overexpress Angptl4 within the glomerulus from podocytes and develop massive albuminuria without increasing circulating Angptl4 levels7. Treatment with the sialic acid precursor N-acetyl-D-mannosamine (ManNAc) converts high-pI glomerular Angptl4 to neutral-pI Angptl4 in vivo and significantly reduces albuminuria and proteinuria7. To study whether circulating Angptl4 can induce proteinuria, we generated aP2-Angptl4 transgenic rats, which selectively overproduce and secrete Angptl4 from adipose tissue. These rats develop high circulating Angptl4 levels but do not have proteinuria. In the present study, we used the aP2-Angptl4 transgenic rats to explore the biological role of circulating Angptl4 in nephrotic syndrome.
Angptl4 is known to inactivate LPL9 and block its activity10, which reduces triglyceride conversion to FFA and results in hypertriglyceridemia. Population-based sequencing studies of the human ANGPTL4 gene revealed low plasma triglyceride levels in about 3% of the European-American population that has an E40K variant11. Subsequent studies showed that recombinant Angptl4 with the E40K variant is unable to inhibit LPL activity in vitro12. Angptl4 in circulation is cleaved into an N-terminal fragment (which contains an LPL-inhibiting region and a coiled-coil domain and forms oligomers) and a C-terminal fragment (which contains a fibrinogen-like domain and remains monomeric), and mutating the Angptl4 cleavage region between amino acids 161 and 164 improves the stability of the full-length protein12. We used these properties of Angptl4 to develop mutants of potential therapeutic value.
In the present study we investigated the biological role of circulating Angptl4 in nephrotic syndrome. We showed that elevated circulating Angptl4 had neutral or below-neutral pI and was essential for the development of hypertriglyceridemia in nephrotic syndrome. Circulating Angptl4 was secreted mostly from skeletal muscle, adipose tissue, heart and liver in response to an elevated plasma ratio of FFA to albumin after moderate to severe proteinuria had developed. In MCD, some circulating Angptl4 also originated from podocytes. Elevated circulating Angptl4 protein levels, whether caused by tissue upregulation, transgenic expression or injection of recombinant protein, participated in two feedback loops. In a systemic feedback loop, Angptl4 with a more neutral pI reduced proteinuria in nephrotic rodents by binding to glomerular endothelial αvβ5 integrin. Concurrently, in a local feedback loop, Angptl4 also reduced the uptake of FFAs in skeletal muscle, heart and adipose tissue by inhibiting LPL activity and reducing the hydrolysis of triglycerides to FFA, thus resulting in hypertriglyceridemia. Angptl4 is therefore a direct molecular link between proteinuria and hypertriglyceridemia in nephrotic syndrome.
RESULTS
Circulating Angptl4 induces hypertriglyceridemia
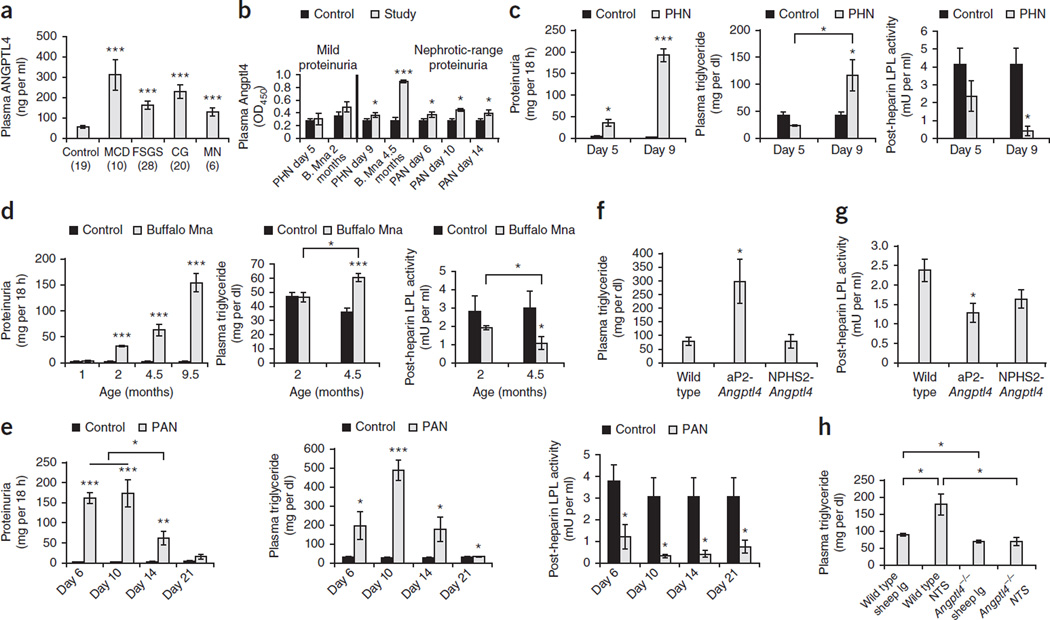
When compared with normal healthy volunteers, we noted significantly higher fasting plasma ANGPTL4 levels (Fig. 1a and Supplementary Table 1) with neutral or below-neutral pI (Supplementary Fig. 1a,b) in untreated individuals with nephrotic syndrome due to MCD, focal and segmental glomerulosclerosis (FSGS), non-HIV collapsing glomerulopathy (CG) or membranous nephropathy (MN). To determine whether high plasma ANGPTL4 levels induced hypertriglyceridemia by inhibiting LPL activity in nephrotic syndrome, we measured plasma Angptl4 and triglyceride levels and post-heparin LPL activity in rats with passive Heymann nephritis (PHN, a model of MN)13,14, Buffalo Mna rats, which spontaneously develop FSGS15,16, and rats with puromycin aminonucleoside nephrosis (PAN, a model of MCD)13. Fasting plasma Angptl4 levels were increased in these models after, but not before, the development of moderate to severe proteinuria (Fig. 1b). In PHN and Buffalo Mna rats, we noted significant hypertriglyceridemia when plasma Angptl4 levels were increased and plasma LPL activity was reduced (Fig. 1c,d). In PAN, hypertriglyceridemia was present throughout proteinuria, persisted after proteinuria had normalized and was accompanied by a decrease in LPL activity (Fig. 1e).
Figure 1.
Elevated circulating Angptl4 levels are required for the development of hypertriglyceridemia in nephrotic syndrome. (a) Plasma ANGPTL4 levels measured by ELISA in subjects with nephrotic syndrome due to primary glomerular disease. The numbers of subjects analyzed in each group are shown in parentheses. (b) Plasma Angptl4 levels measured by ELISA at prenephrotic and nephrotic stages in PHN (n = 4 rats per group), Buffalo Mna (B. Mna; n = 9 rats per group) and single-dose intravenous PAN (n = 4 rats per group). OD450, optical density at 450 nm. The timing of Angptl4 levels is days after disease induction in PHN and PAN and age in months in B. Mna rats. (c) Proteinuria (left), plasma triglyceride levels (middle) and post-heparin LPL activity (right) in PHN. The timing of the results is days after disease induction. (d) Proteinuria (left), plasma triglyceride levels (middle) and post-heparin LPL activity (right) in Buffalo Mna rats. (e) Proteinuria (left), plasma triglyceride levels (middle) and post-heparin LPL activity (right) in PAN rats. (f) Plasma triglyceride levels in wild-type Sprague Dawley rats (n = 6 rats), adipose tissue–specific Angptl4-overexpressing rats (aP2-Angptl4; n = 6 rats) and 3-month-old podocyte-specific Angptl4-overexpressing rats (NPHS2-Angptl4; n = 6 rats). (g) Post-heparin LPL activity in wild-type, aP2-Angptl4 transgenic and NPHS2-Angptl4 transgenic rats. (h) Plasma triglyceride levels in wild-type and Angptl4−/− mice (n = 4 mice per reading) 48 h after the induction of nephrotic syndrome using NTS. All error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 determined by two-way t test.
Overexpression of Angptl4 from adipose tissue in aP2-Angptl4 transgenic rats also induced hypertriglyceridemia and reduced LPL activity (Fig. 1f,g). By contrast, 3-month-old NPHS2-Angptl4 transgenic rats did not develop increased triglyceride levels or reduced LPL activity. To study the relative importance of Angptl4 in the development of hypertriglyceridemia in nephrotic syndrome, we induced severe heterologous-phase complement- and leukocyte-independent proteinuria in wild-type and Angptl4−/− mice using γ2-nephrotoxic serum (NTS) (Fig. 1h). When compared with wild-type mice, hypertriglyceridemia was absent in Angptl4−/− mice injected with NTS, despite these mice having significant (P < 0.001) proteinuria (Supplementary Fig. 1c). These studies show that circulating Angptl4 is a critical mediator of hypertriglyceridemia in nephrotic syndrome.
The origins of elevated circulating Angptl4
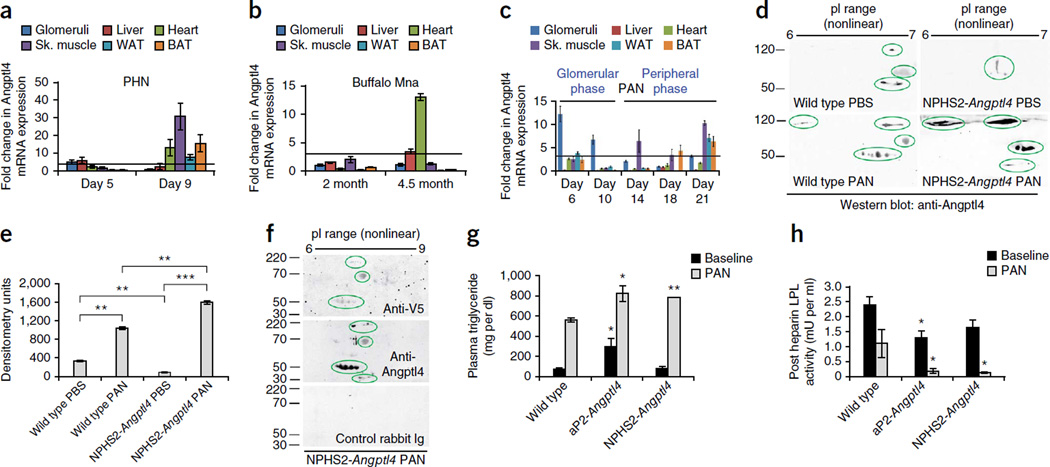
To determine the origins of the increased levels of circulating Angptl4, we conducted multiorgan Angptl4 mRNA expression in rat models of nephrotic syndrome. On PHN day 9 (Fig. 2a), which corresponds with elevated circulating Angptl4 levels (Fig. 1b) and heavy proteinuria (Fig. 1c), we noted prominent upregulation in the heart, skeletal muscle, white adipose tissue (WAT) and brown adipose tissue (BAT). We found that transient mild upregulation in the glomeruli and liver on day 5 of PHN had subsided on day 9. In 4.5-month-old Buffalo Mna rats (Fig. 2b) with increased Angptl4 levels (Fig. 1b) and moderate to severe proteinuria (Fig. 1d), we observed upregulation in the heart and liver, whereas 2-month-old rats with mild proteinuria had no peripheral Angptl4 upregulation. We did not find glomerular upregulation of Angptl4 in this model. In PAN, a self-limiting acute model of nephrotic syndrome, elevated circulating Angptl4 levels occurred throughout disease (Fig. 1b). Prominent upregulation of glomerular Angptl4 during the crescendo phase of proteinuria (days 6 and 10) (Fig. 2c) was also described previously7. This ‘glomerular’ phase of Angptl4 upregulation (days 6 and 10) was followed by a ‘peripheral’ phase (days 14–21) during which glomerular upregulation was absent and prominent upregulation was present in skeletal muscle, WAT and BAT.
Figure 2.
The source of circulating Angptl4 in nephrotic syndrome. (a–c) Multiorgan Angptl4 mRNA expression relative to control (n = 6 templates per organ per time point) in PHN (a), Buffalo Mna rats (b) and PAN (c). Sk., skeletal. (d) Representative two-dimensional gel electrophoresis and western blot of plasma showing circulating Angptl4 levels in wild-type Sprague Dawley and proteinuric NPHS2-Angptl4 transgenic rats before and after the induction of low-dose PAN. (e) Densitometry analysis of the two-dimensional gels (n = 3 readings per value) in d. (f) Two-dimensional gel electrophoresis and western blot of plasma from NPHS2-Angptl4 transgenic rats with PAN to look for V5-tagged transgene-expressed Angptl4 in the circulation. (g) Plasma triglyceride levels 6 d after the induction of PAN in wild-type Sprague Dawley, aP2-Angptl4 transgenic and NPHS2-Angptl4 transgenic rats (n = 4 readings per group). (h) Post-heparin LPL activity corresponding to the plasma triglyceride levels shown in g. In g and h, the black bars correspond to the data from Figure 1f,g, which is included for comparison. All error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 determined by two-way t test. In g and h, statistical significance is shown for the difference between transgenic rats and the corresponding wild-type controls. P < 0.001 for each rat type before and after induction of PAN. In a–c, the threshold for significance was a threefold change (horizontal line).
As monogenic overexpression of Angptl4 from podocytes in NPHS2-Angptl4 transgenic rats does not increase circulating Angptl4 levels7, we performed two-dimensional gel western blot studies of rat plasma after we induced low-dose PAN and noted significantly higher circulating levels of Angptl4 during the glomerular phase in NPHS2-Angptl4 transgenic rats with PAN than in wild-type rats with PAN (Fig. 2d,e and Supplementary Fig. 1d). This circulating protein was reactive with the V5-specific antibody (Fig. 2f), thereby confirming its podocyte origin, as the transgene-expressed protein is tagged by V5 at its C-terminal end. As a result of increased levels of circulating Angptl4 overexpressed by the transgene, aP2-Angptl4 and NPHS2-Angptl4 transgenic rats with PAN had higher plasma triglyceride levels and lower LPL activity than wild-type rats with PAN (Fig. 2g,h). Although aP2-Angptl4 transgenic rats were able to respond to elevated circulating Angptl4 levels by upregulating LPL mRNA expression in heart and skeletal muscle (Supplementary Fig. 2a), nephrotic rats with PAN were unable to do so until proteinuria had subsided to low levels (Supplementary Fig. 2b). Consequently, LPL protein expression was reduced, especially in the skeletal muscle, WAT and BAT (Supplementary Fig. 2c–e), during the peripheral phase of Angptl4 expression in PAN.
Unlike normal Sprague Dawley rats, nephrotic rats lost Angptl4 in the urine (Supplementary Fig. 3a), suggesting that circulating Angptl4 levels underestimated the total Angptl4 production and effect in nephrotic syndrome. Whereas normal rats degrade Angptl4-released LPL by hepatic uptake from the circulation17, we observed additional loss in the urine in nephrotic syndrome (Supplementary Fig. 3b,c). Reactivity with 5D2 (Supplementary Fig. 3d,e), a monoclonal antibody that selectively binds active dimeric LPL18, suggested that active LPL was also lost in the urine during heavy proteinuria. These factors may contribute to the maintenance of Angptl4-mediated hypertriglyceridemia in nephrotic syndrome.
Factors behind Angptl4 upregulation in peripheral tissues
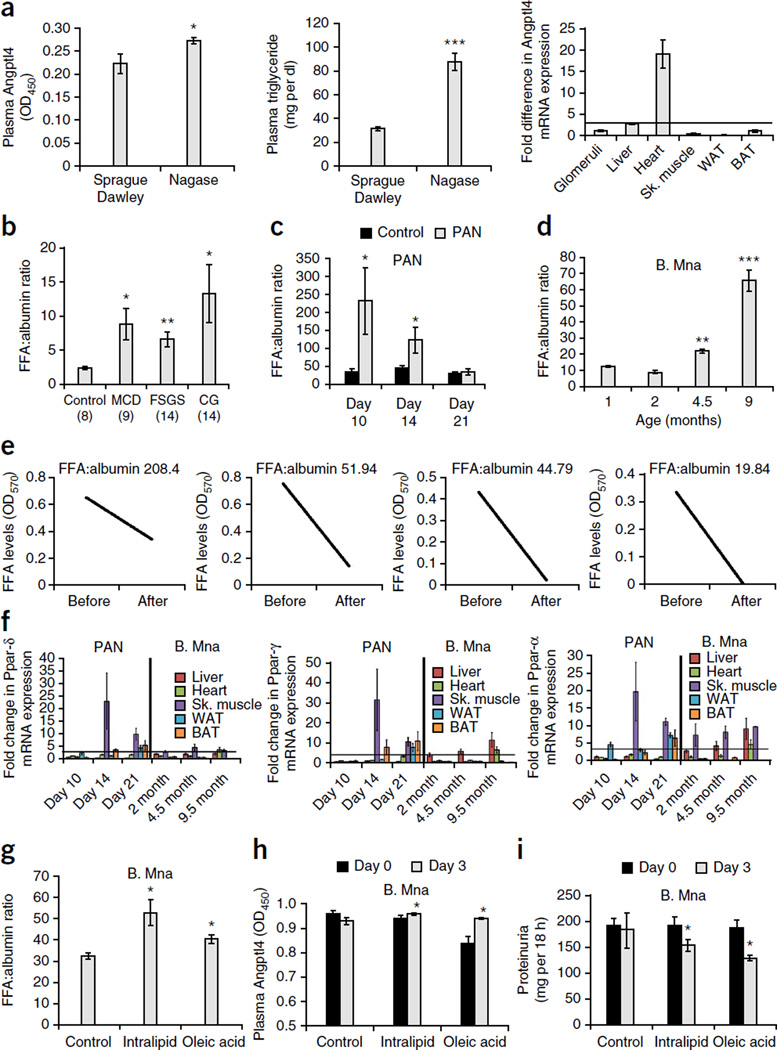
To test whether lack of circulating albumin independently induces increased peripheral Angptl4 upregulation, we studied tissue and blood samples from Nagase analbuminemic rats19, a strain that serves as a functional albumin knockout. These rats, which are known to have hypertriglyceridemia20, had increased circulating Angptl4 levels and confirmed plasma hypertriglyceridemia (Fig. 3a). We observed increased expression of Angptl4 mRNA in the heart and liver (Fig. 3a). This finding suggests that at least some peripheral Angptl4 upregulation could occur as a general compensatory response to the lack of plasma albumin.
Figure 3.
Mechanisms of Angptl4 upregulation in peripheral organs in nephrotic syndrome. (a) Angptl4 levels (n = 5 rats per group; left), plasma triglyceride levels (n = 5 rats per group; middle) and peripheral organ Angptl4 mRNA expression (n = 6 templates per reading; right) in Normal Sprague Dawley and Nagase analbuminemic rats. (b) Plasma ratio of FFAs to albumin in a subset of individuals with MCD, FSGS or CG and age- and sex-matched control subjects. The numbers of subjects analyzed are indicated in parentheses. (c) Plasma ratio of FFAs to albumin in Sprague Dawley rats with puromycin nephrosis (n = 4 rats per group). (d) Plasma ratio of FFAs to albumin in Buffalo Mna rats (1 month, n = 5 rats; 2 months, n = 5 rats; 4.5 months, n = 14 rats; 9 months, n = 9 rats) rats per time point). (e) Plasma FFA levels before and after affinity absorption of albumin from two patients with MCD (far left and right) and two patients with CG (middle). (f) Changes in the mRNA expression (n = 4 templates per organ per time point) of Ppar-δ (left), Ppar-γ (middle) and Ppar-α (right) in liver, heart, skeletal muscle, WAT and BAT during the peripheral phase of PAN and in Buffalo Mna rats. (g) Plasma ratio of FFAs to albumin in Buffalo Mna rats (n = 4 rats per group) 5 h after the first dose of normal saline, Intralipid or oleic acid. (h) Change in plasma Angptl4 levels after 3 d of treatment. (i) Proteinuria at baseline and after 3 d of treatment. All error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 determined by two-way t test. In f, the threshold for significance was a threefold change (horizontal line).
Because analbuminemia is rare in human disease, we explored additional factors that could contribute toward increased Angptl4 upregulation in peripheral organs. As circulating FFAs are non-covalently bound to albumin and induce ANGPTL4 upregulation in skeletal muscle21,22 and heart23 through peroxisome proliferator-activated receptor (PPAR) activation, we measured and found a high plasma ratio of FFAs to albumin in subjects with MCD, FSGS and CG compared to healthy control subjects (Fig. 3b) and in animal models of MCD and FSGS (Fig. 3c,d). The study in Buffalo Mna rats (Fig. 3d) showed that the plasma ratio of FFAs to albumin was only significantly increased when proteinuria became severe (4.5 months of age). There was a time lag between the increase in the ratio of FFAs to albumin and peripheral Angptl4 upregulation (Figs. 3c and 2c) in PAN. A prior study6 showed that this high plasma ratio of FFAs to albumin results from selective loss in the urine of albumin with relatively low FFA content in patients with MCD and other causes of nephrotic syndrome. We confirmed the presence of a relatively low urine ratio of FFAs to albumin in a subset of individuals with MCD and FSGS in which we obtained plasma and urine samples simultaneously (Supplementary Fig. 4a). We also noted a low urinary ratio of FFAs to albumin compared to the ratio in plasma in rats with PAN, with the difference narrowing as the proteinuria subsided (Supplementary Fig. 4b). In Buffalo Mna rats, a low urinary ratio of FFAs to albumin as compared to the ratio in plasma preceded the development of nephrotic-range proteinuria and decreased further as proteinuria increased (Supplementary Fig. 4c).
Next we explored whether a major albumin-independent fraction existed in nephrotic individuals by absorbing out albumin from human plasma using affinity columns (Supplementary Fig. 5a) and measuring FFA levels in the column-unbound fraction (Fig. 3e). Albumin-independent FFA was restricted to individuals with a very high ratio of FFAs to albumin and was virtually undetectable once the ratio declined to around 20. Because Angptl4 is a Ppar target gene and knockout mouse studies23 have shown that FFA-induced Angptl4 upregulation is mediated by members of the Ppar family, we studied the expression of Ppar-δ, Ppar-γ and Ppar-α in peripheral tissues in rats with PAN and Buffalo Mna rats (Fig. 3f). In rats with PAN, Ppar expression in the peripheral tissues was higher than that in control rats during the peripheral phase (days 14 and 21) and was relatively unchanged at the end of the glomerular phase (day 10). In Buffalo Mna rats, Ppar expression was high in nephrotic rats (4.5 and 9.5 months of age) and was normal or near normal in 2-month-old rats with subnephrotic proteinuria.
To test the effect of raising plasma FFA levels on nephrotic syndrome using exogenous-administered FFAs, we treated nephrotic Buffalo Mna rats with oleic acid, a long-chain FFA, Intralipid, a parenteral lipid supplement that is rich in FFAs and has been approved for human use, or control normal saline daily for 3 days. Five hours after the first dose of FFAs or Intralipid, the plasma ratio of FFAs to albumin was higher in both treatment groups compared to the control group (Fig. 3g). At the end of the 3-day study, both treatment groups had higher mRNA expression of Angptl4 in peripheral tissues (Supplementary Fig. 5b), higher circulating Angptl4 levels despite severe proteinuria (Fig. 3h) and a mild, though significant, reduction in proteinuria (Fig. 3i) when compared with the control group.
High circulating ANGPTL4 levels reduce proteinuria
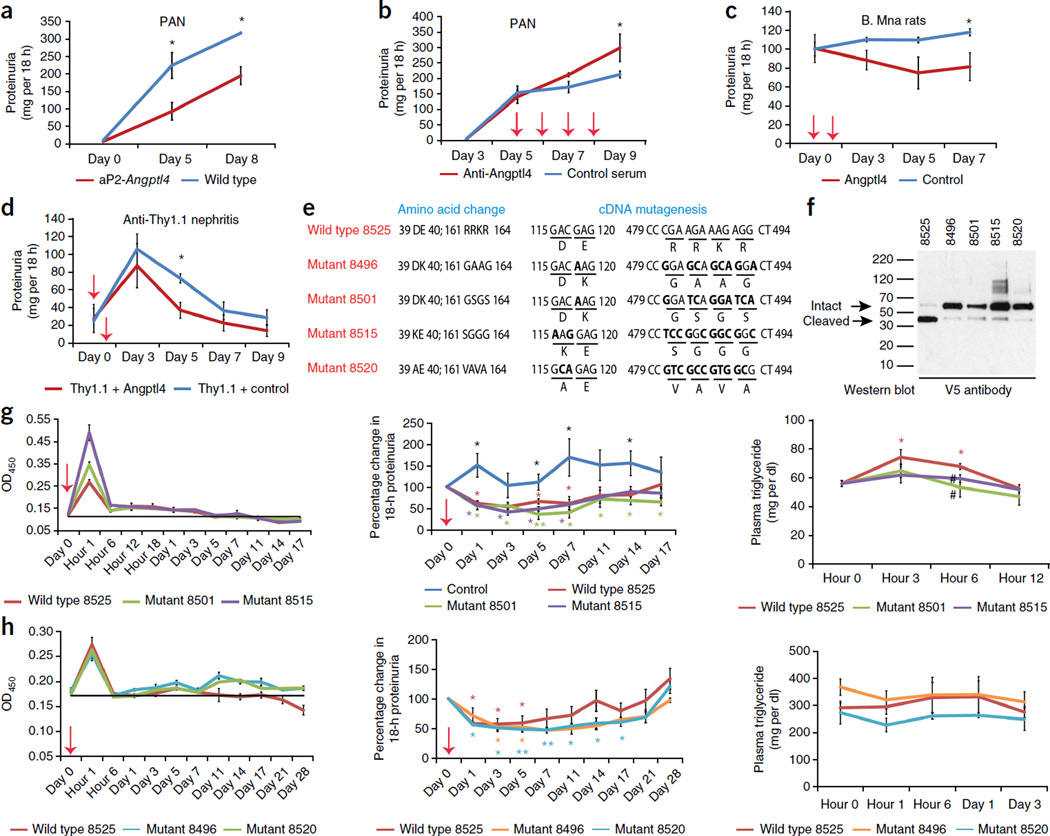
Induction of PAN in aP2-Angptl4 transgenic and wild-type Sprague Dawley rats revealed significantly lower proteinuria in the transgenic rats (Fig. 4a), suggesting that circulating Angptl4 had an anti-proteinuric effect. To test whether this anti-proteinuric effect was induced specifically by circulating Angptl4, we injected wild-type PAN rats with a previously characterized rabbit anti-rat Angptl4 antibody7 after the onset of proteinuria to partially deplete circulating Angptl4 levels and noted increased proteinuria (Fig. 4b). Recombinant normosialylated rat Angptl4 injected into Buffalo Mna rats (Fig. 4c) and rats with anti-Thy1.1 nephritis, a model of mesangial injury (Fig. 4d), also caused significant reduction of proteinuria.
Figure 4.
Effect of circulating Angptl4 on proteinuria. Red arrows indicate the time points at which an antibody or recombinant protein was injected. (a) Proteinuria after induction of high-dose PAN in wild-type Sprague Dawley and aP2-Angptl4 transgenic rats (n = 4 rats per group). (b) The effect of depleting circulating Angptl4 using an Angptl4-specifc antibody on proteinuria in Sprague Dawley rats with intermediate-dose PAN (n = 3 rats per group). (c) Proteinuria in Buffalo Mna rats after injecting recombinant rat Angptl4 or supernatant from a control cell line (n = 4 rats per group). (d) Proteinuria in severe anti-Thy1.1 nephritis with injection of recombinant rat Angptl4 or supernatant from a control cell line (n = 4 rats per group). (e) Schematic representation of wild-type and mutant human ANGPTL4 proteins showing mutations in areas that are important for LPL interaction (amino acid 40 and the adjacent amino acid 39) and protein cleavage (amino acids 161–164). (f) Western blot of recombinant tagged proteins using mouse V5-specific antibody to demonstrate the expected size of the intact protein and reduced cleavage in the mutant proteins (arrows). (g) Effect of injecting 55 µg of wild-type (8525) or mutant (8501 and 8515) human ANGPTL4 in Buffalo Mna rats (n = 3 rats per group) on plasma levels of the recombinant protein (left), proteinuria (middle) and plasma triglyceride levels (right). (h) Effect of injecting a lower dose (15 µg) of wild-type (8525) or mutant (8496 and 8520) human ANGPTLl4 in ZDF rats (n = 4 rats per group) on plasma levels of the recombinant protein (left), proteinuria (middle) and plasma triglyceride levels (right). All error bars, s.e.m. *P < 0.05, **P < 0.01 determined by two-way t test, except in b and c, in which one-way t test was used. In g (middle), black asterisks are shown where all three study groups were individually different from the control injected rats. In g and h, colored asterisks are shown where individual values were significantly different from the corresponding baseline values. The pound symbol in g (right) indicates P < 0.05 in the rats injected with mutant ANGPTL4 compared to the group injected with wild-type protein.
To dissociate the effects of Angptl4 on LPL activity and proteinuria, we induced mutations at two sites in human ANGPTL4 (Fig. 4e). One set of mutations was at12 or near a site that is known to be important for inhibiting LPL activity (amino acid 40 or 39). Another set of mutations was made in a region that is known to be involved in the cleavage of full-length ANGPTL4 (amino acids 161–164)12. Next we developed HEK293-based stable cell lines for each of the mutant and wild-type plasmids. Assessment of recombinant wild-type and mutant human ANGPTL4 by western blotting using the V5-specific antibody showed migration of the mutant proteins at the appropriate size with reduced cleavage (Fig. 4f). After single intravenous injection of recombinant human ANGPTL4 into proteinuric Buffalo Mna rats, we noted higher peak levels for the mutant proteins (Fig. 4g). There was significant reduction in proteinuria compared to baseline levels that lasted for 2 weeks in rats injected with either wild-type or mutant ANGPTL4 (wild-type 8525, 53.8 ± 6.3 (mean ± s.e.m. of the nadir as a percentage of baseline); mutant 8501, 35.9 ± 12.1; mutant 8515, 41.2 ± 7.2) (Fig. 4g). Plasma triglyceride levels were significantly higher compared to baseline in rats injected with wild-type but not mutant ANGPTL4 at 3 and 6 h after injection (Fig. 4g). Plasma triglyceride levels were significantly lower in rats injected with mutant protein compared to those injected with wild-type protein after 6 h (Fig. 4g). Fasting plasma triglyceride levels after 12 h were indistinguishable between the rats injected with wild-type and mutant ANGPTL4 (data not shown). We also noted increased circulating levels of human ANGPTL4 and a reduction in proteinuria in Zucker Diabetic Fatty (ZDF) rats injected with a lower dose of wild-type ANGPTL4 and in those injected with two different ANGPTL4 mutants (Fig. 4h). Because of the lower dose used, the rise in plasma triglyceride levels induced by wild-type ANGPTL4 was not statistically significant (Fig. 4h).
Angptl4 interaction with αvβ5 integrin reduces proteinuria
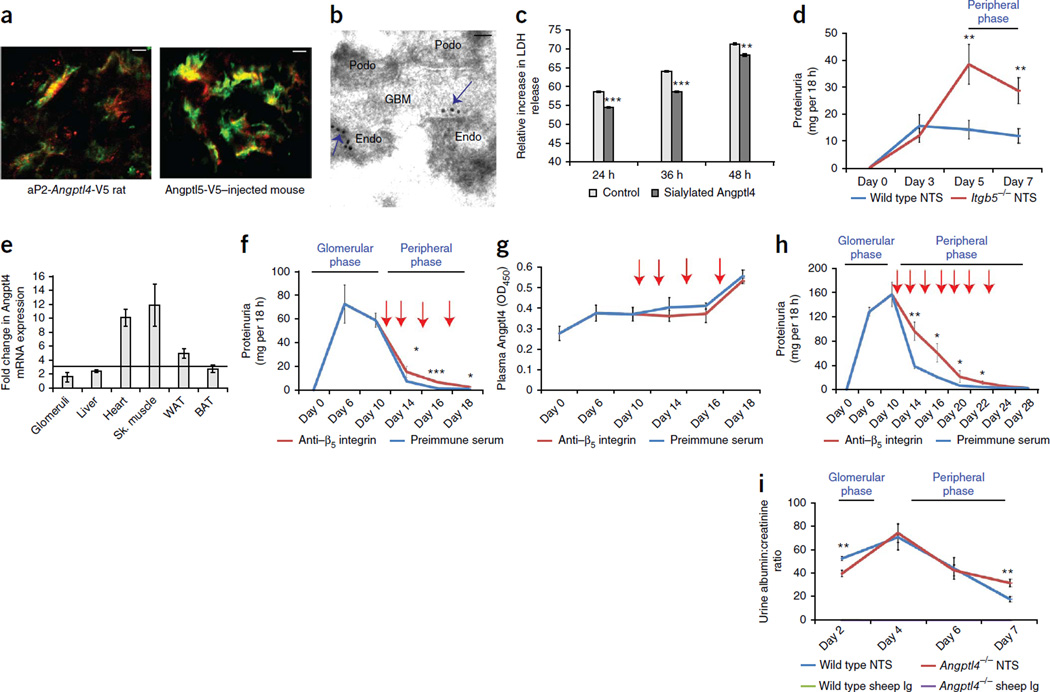
Using confocal imaging with a V5-specific antibody, we colocalized transgene-expressed Angptl4-V5 in aP2-Angptl4 transgenic rats and recombinant V5-tagged rat Angptl4 injected into wild-type mice with glomerular endothelium (Fig. 5a). Immunogold electron microscopy revealed that Angptl4-V5 bound prominently to the endothelial cell surface at its interface with the glomerular basement membrane (Fig. 5b). Recombinant normosialylated rat Angptl4, which mimics circulating Angptl4 in nephrotic states, could protect cultured rat glomerular endothelial cells from oxidative injury (Fig. 5c), whereas hyposialylated Angptl4, the key mediator of proteinuria that is secreted by podocytes in MCD, increased the effects of oxidative injury (Supplementary Fig. 5c).
Figure 5.
Circulating Angptl4 reduces proteinuria through its interaction with glomerular endothelial αvβ5 integrin. Red arrows indicate the time points at which β5 integrin–specific antibody or preimmune serum was injected. (a) Confocal images of glomeruli from an aP2-Angptl4 transgenic rat (left) and a wild-type mouse injected with recombinant rat Angptl4 (right) to demonstrate co localization of adipose tissue–secreted (left) or injected (right) Angptl4-V5 (V5-specific antibody; red) with glomerular endothelium (von Willebrand factor–specific antibody; green). (b) Immunogold electron micrograph of a glomerulus from an aP2-Angptl4 transgenic rat using V5-specific antibody to show glomerular endothelial cell surface localization (arrows) of adipose tissue–secreted Angptl4-V5. Podo, podocyte; GBM, glomerular basement membrane; Endo, endothelial cell. (c) Effect of normosialylated recombinant rat Angptl4 on apoptosis induced in cultured rat glomerular endothelial cells using H2O2-induced oxidative stress (n = 3 readings per group). (d) Proteinuria during the peripheral phase of Angptl4 secretion after injection of NTS in wild-type and Itgb5−/− mice (n = 5 mice per group). (e) Multiorgan mRNA expression profile for Angptl4 7 d after injection of NTS (n = 6 templates per organ) in the wild-type mice in d. The threshold for significance was a threefold change (horizontal line). (f) Recovery from peak proteinuria in Sprague Dawley rats with low-dose PAN (n = 4 rats per group) after blocking the endothelial β5 integrin–Angptl4 interaction using a β5 integrin–specific antibody. (g) Plasma Angptl4 levels in the rats in f. (h) Recovery from peak proteinuria in aP2-Angptl4 transgenic rats with full-dose PAN (n = 4 rats per group) after blocking the endothelial β5 integrin–Angptl4 interaction using a β5 integrin–specific antibody. (i) Recovery from peak proteinuria in wild-type and Angptl4−/− mice injected with NTS (n = 7 mice per group). All error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 determined by two-way t test. Scale bars, 8 µm (a); 0.2 µm (b).
Because Angptl4 binds β5 integrin24, we tested whether the anti-proteinuric effects of circulating Angptl4 could be mediated through its binding to αvβ5 integrin that is present in glomerular endothelium. We confirmed this protein-protein interaction and noted a strong dose-dependent binding of recombinant rat Angptl4 with purified human αvβ5 integrin (Supplementary Fig. 5d). Confocal imaging of wild-type and β5 integrin-knockout (Itgb5−/−) mice injected with recombinant rat Angptl4-V5 showed strong staining in glomeruli in wild-type mice and faint staining in Itgb5−/− mice (Supplementary Fig. 5e). Induction of nephrotic syndrome using NTS in wild-type and Itgb5−/− mice revealed significantly higher levels of proteinuria in the knockout mice during the recovery phase (days 5 and 7) (Fig. 5d), which corresponds with the peripheral phase of circulating Angptl4 production from skeletal muscle, heart and adipose tissue in this model (Fig. 5e and Supplementary Fig. 5f). This finding suggests that the decline from peak proteinuria was influenced by the presence of circulating proteins such as Angptl4 that exert anti-proteinuric effects by binding αvβ5 integrin. To block the αvβ5 integrin-Angptl4 interaction, we induced PAN in Sprague Dawley rats and injected a β5 integrin-specific antibody during the peripheral phase of Angptl4 expression (Fig. 5f). We observed a significant delay in recovery from peak proteinuria (Fig. 5f) in the presence of rising plasma Angptl4 levels in both groups (Fig. 5g). We observed a similar, although more pronounced, delay in recovery from peak proteinuria in aP2-Angptl4 transgenic rats with PAN (Fig. 5h), as these rats have constantly elevated circulating Angptl4 levels7. Angptl4−/− mice injected with NTS had a significant delay in recovery from peak proteinuria (day 7) that was attributable to the absence of a peripheral Angptl4 phase (Fig. 5i). Lower proteinuria in NTS-injected Angptl4−/− mice during the glomerular phase is consistent with the role of podocyte-secreted hyposialylated Angptl4 as one of several causes of proteinuria in this model7 (Supplementary Fig. 1c).
DISCUSSION
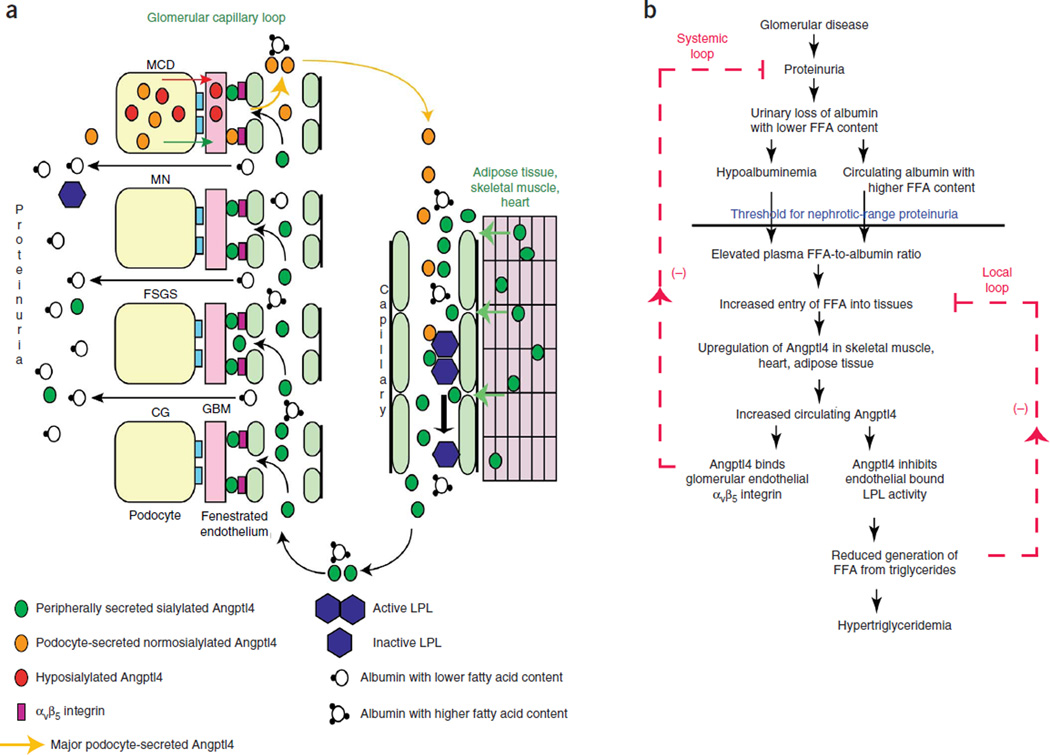
This study shows that circulating Angptl4 is a key molecular mediator in nephrotic syndrome. It describes how two major components of this syndrome, proteinuria and hypertriglyceridemia, are linked at a molecular level. The glomerulus is central to the development of proteinuria through mechanisms that vary among different diseases (Fig. 6a). Using animal models of MN and FSGS, two conditions that are commonly associated with subacute onset of proteinuria and edema, we demonstrate that peripheral organs, especially skeletal muscle, adipose tissue, heart and liver, respond to increasing proteinuria and a rising plasma ratio of FFAs to albumin by upregulating Angptl4 expression, likely through FFA-induced activation of Ppar proteins. Circulating Angptl4 has two potent effects in nephrotic syndrome. First, it binds to αvβ5 integrin in the glomerular endothelium and reduces proteinuria, suggesting a major systemic feedback response against rising proteinuria (Fig. 6b). Second, it inhibits the activity of LPL, which is expressed mostly in the same organs that produce Angptl4, thereby reducing triglyceride hydrolysis and the uptake of FFAs into tissues, suggesting a local feedback response to its own upregulation (Fig. 6b). The additional consequence of this local feedback loop is the development of hypertriglyceridemia.
Figure 6.
Pathobiology of circulating Angptl4 in nephrotic syndrome. (a) Diagram representation of the production of circulating Angptl4 protein and its biological effects. The circulating, normosialylated form of Angptl4 is secreted from peripheral organs (mostly skeletal muscle, heart and adipose tissue) in MCD, MN, FSGS and CG. In addition, podocytes in MCD secrete a hyposialylated form of the protein that remains restricted to the kidney and induces proteinuria7 and a normosialylated form that enters the circulation. Circulating Angptl4 binds to glomerular endothelial αvβ5 integrin to reduce proteinuria or inactivates endothelium-bound LPL in skeletal muscle, heart and adipose tissue to reduce the hydrolysis of plasma triglycerides to FFA, resulting in hypertriglyceridemia. Some Angptl4 and LPL are lost in the urine. (b) Schematic illustration of negative feedback loops in the link between proteinuria, hypoalbuminemia and hypertriglyceridemia that are mediated by Angptl4 and FFA (unesterified fatty acids with a free carboxylate group). Plasma FFAs are noncovalently bound to albumin, and because of the preferential loss of albumin with low FFA content during proteinuria, albumin with higher FFA content is retained in circulation. As glomerular disease progresses and proteinuria increases, hypoalbuminemia develops, and the combination of high albumin-FFA content and lower plasma albumin levels increases the plasma ratio of FFAs to albumin. This increased available FFA enters the skeletal muscle, heart and adipose tissue to induce upregulation of Angptl4, mediated at least in part by Ppar proteins. Angptl4 secreted from these organs participates in two feedback loops. In the systemic loop, it binds to glomerular endothelial αvβ5 integrin and reduces proteinuria. In a local loop, it inhibits LPL activity in the same organs from which it is secreted to reduce the uptake of FFAs, thereby curtailing the stimulus for its own upregulation.
It is likely that the local feedback loop limits the rise in circulating Angptl4 levels and, consequently, its ability to reduce proteinuria through the systemic feedback loop. The significant decline in proteinuria in the absence of a statistically significant increase in plasma triglyceride levels in ZDF rats injected with small doses of recombinant human wild-type ANGPTL4 protein suggests that the systemic loop is the dominant of the two negative feedback loops.
The cause of the selective loss in urine of albumin with low FFA content, which initiates the feedback loops, remains unknown and needs to be explored in the future. However, the presence of a lower ratio of FFAs to albumin in urine than in plasma in Buffalo Mna rats with mild proteinuria confirms that the partitioning of plasma and urinary FFAs is an early event during the pathogenesis of nephrotic syndrome. The lag between the onset of proteinuria and the development of hypertriglyceridemia in human nephrotic syndrome is also explained by peripheral production of ANGPTL4, as it requires moderate to severe proteinuria (at least 3.5 g per 24 h in the human context) to substantially elevate the plasma ratio of FFAs to albumin and induce peripheral organ ANGPTL4 upregulation. Early, mild proteinuria in the Buffalo Mna and PHN animal models was not associated with Angptl4 upregulation in peripheral organs or increased plasma Angptl4 and triglyceride levels. Because increased Angptl4 secretion from peripheral tissues is also a physiological response to fasting25, it is possible that some of the increased peripheral production of Angptl4 is part of a fasting-like response that is used by the body to curb excessive urinary protein loss in nephrotic syndrome.
Additional interesting lessons can be learned from rats with PAN, in which onset of proteinuria is acute. Prior studies from our lab7 have shown that podocytes in MCD produce two distinct forms of Angptl4: a high-pI, hyposialylated form that induces proteinuria and remains within the glomerulus and a neutral-pI normosialylated form that is identical to circulating Angptl4. Podocyte Angptl4 upregulation is absent in FSGS and is only mild and transient in MN7. Circulating normosialylated Angptl4 levels remain elevated throughout the duration of the PAN model, and the source of this Angptl4 is the glomerulus during the initial stage (i.e., glomerular phase) and the skeletal muscle and adipose tissue during the later stages (i.e., peripheral phase). An in vitro study using cultured glomerular endothelial cells showed that high-pI Angptl4 increases, and neutral-pI Angptl4 reduces, endothelial injury in the setting of oxidative stress.
A mediator of reduction in proteinuria is the Angptl4–αvβ5 integrin interaction in glomerular endothelium, as absence of β5 integrin or Angptl4 in knockout mice or blockage of this interaction using antibodies directed against the extracellular part of β5 integrin reduces the rate of decline of proteinuria. Another effect of the peripheral phase of Angptl4 production in PAN is the persistence of mild hypertriglyceridemia (day 21) even after proteinuria has subsided. Similar residual hypertriglyceridemia has been documented in children with MCD after they go into remission26. It is possible that circulating Angptl4 may interact with other glomerular cell–surface molecules as well to exert its protective effects. We had two reasons for pursuing the binding of Angptl4 to glomerular endothelial αvβ5 integrin. First, confocal imaging and immunogold electron microscopy showed that Angptl4-V5 secreted from adipose tissue in aP2-Angptl4 transgenic rats and recombinant V5-tagged human ANGPTL4 injected into wild-type mice binds to endothelial cells in the glomerulus. Second, αvβ5 is the only integrin expressed on glomerular endothelial cells in vivo that has been shown to interact with Angptl4. The other major glomerular endothelial integrin, αvβ3, does not interact with Angptl4 (ref. 24). The precise mechanism by which Angptl4 binding to endothelial αvβ5 integrin reduces proteinuria will need to be explored in the future. It is possible that putative endothelial-podocyte feedback loops are affected. The presence of faint staining for the V5 tag in Itgb5−/− mice injected with Angptl4-V5 suggests the presence of additional binding partners in the glomerulus.
Another interesting observation is that entry of Angptl4 into the circulation after monogenic overexpression is organ dependent. Similar to a heart-specific Angptl4-overexpressing transgenic mouse developed previously27, monogenic overexpression of Angptl4 in podocytes in NPHS2-Angptl4 transgenic rats does not automatically allow entry into the circulation, and consequently, these rats do not develop hypertriglyceridemia at 3 months of age. By contrast, over-expression in adipose tissue (aP2-Angptl4 transgenic rats7 and aP2-Angptl4 transgenic mice25) reliably raises circulating Angptl4 levels and produces hypertriglyceridemia. The entry of podocyte-secreted Angptl4 into the circulation and the development of hypertriglyceridemia, as noted in the Sprague Dawley rat PAN glomerular phase and in NPHS2-Angptl4 transgenic rats with PAN, likely requires the activity of other unidentified proteins that are produced in the glomerulus. This idea also fits in well with human glomerular disease, in which the expression of multiple genes and proteins is affected simultaneously. Therefore, the systemic availability and effects of circulating Angptl4 are likely to be affected by other genes or proteins that are altered in multiple organs as part of the disease process, as well as by urinary loss of Angptl4 and LPL in the nephrotic state.
Administration of mutant human ANGPTL4 protein is able to bypass the local feedback loop, and a single injection reduces proteinuria significantly (mean peak reduction around 60%) for at least 2 weeks without affecting plasma triglyceride levels. A part of the prolonged anti-proteinuric effect of recombinant ANGPTL4 may be related to its ability to bind high-density lipoprotein particles in circulation25 and form very–high order oligomers7, both of which could reduce urinary losses, especially when compared with a monomeric protein of similar size such as albumin. These recombinant proteins hold promise for further development as therapeutic agents for human glomerular disease. Similar principles could be used to identify disease mechanisms from animal models that use human disease sera28 and identify soluble proteins that could become therapeutic agents or targets. The molecular relationship between proteinuria and hypercholesterolemia, the other major part of hyperlipidemia in nephrotic syndrome, remains unresolved and should be explored in the future.
In summary, circulating Angptl4 is an important biological mediator of nephrotic syndrome and is a critical link between proteinuria and hypertriglyceridemia.
ONLINE METHODS
ELISA for human and rat ANGPTL4
We purchased a sandwich ELISA (R&D Systems) to measure native ANGPTL4 in human subjects and control plasma samples, quantify human recombinant ANGPTL4 harvested from stable cell lines and assess plasma levels of recombinant human ANGPTL4 injected into rats. The standard curve was calibrated between 1.25 and 40 ng ml−1 and had an R2 value of 0.98 (Supplementary Fig. 6a). There was no crossreactivity of this ELISA with native rat Angptl4.
To measure native rat Angptl4 in plasma, we developed a new ELISA assay. We raised a sheep anti-rat Angptl4 antibody (5006B) against amino acids 22–101 and characterized its specificity by western blot analysis using a previously published rabbit anti-rat Angptl4 antibody7 as a positive control. Activity was also absorbed out using recombinant Angptl4, and loss of reactivity as determined by western blot analysis and immunofluorescence was documented. We standardized this assay using concentrated supernatant from a previously published HEK293-based stable cell line that secretes recombinant rat Angptl4 (ref. 7). We coated wells with 0.1–0.5 mg of concentrated stable cell line supernatant. After blocking and washes, we added 10 µg of the sheep anti-rat Angptl4 antibody, which was followed by washes, the addition of 16 ng per well of donkey anti-sheep immunoglobulin horseradish peroxidase (HRP) (Jackson Laboratories, 713-035-147, 1:5,000), additional washes and the addition of tetramethylbenzidine (TMB) peroxidase substrate and solution (KPL, Inc.), and we then measured OD450 on a Labsystems Multiskan MCC/340 ELISA plate reader (Thermo Fisher Scientific). We obtained a standard curve with a linear relationship and R2 of 0.998 (Supplementary Fig. 6b). To measure rat or mouse Angptl4 levels in plasma, we coated wells with 50 µl of plasma and then followed the steps described above. We subtracted readings of the control wells, which contained all reagents except for the study sample, from the study sample readings. We measured a minimum of four samples for each time point. The assay was tenfold more sensitive for rat Angptl4 compared to mouse Angptl4.
We obtained human plasma samples after receiving informed consent through Institutional Review Board (IRB)-approved studies conducted at the University of Alabama at Birmingham (principal investigator S.S.C.), Instituto Nacional De Cardiologia, Mexico City (principal investigator C.A.-C.), the FSGS Clinical Trial29 and from previously published studies7.
Animal studies
The generation and characterization of NPHS2-Angptl4 and aP2-Angptl4 transgenic rats has been published7. We obtained Buffalo Mna rats through a Material Transfer Agreement (MTA) from Kyoto University, Kyoto, Japan. Wherever appropriate, we made all comparisons of Buffalo Mna rats with age- and sex-matched Sprague Dawley and Wistar rats. Because the results were similar, only data from comparisons with Sprague Dawley rats are presented. We purchased Itgb5−/− and control 129S1/SvImJ mice from Jackson Laboratories (Bar Harbor). Angptl4−/− mice were provided to S.K. by Eli Lilly Corporation (Indianapolis). All studies with Angptl4−/− mice were approved by the Animal Ethics Committee at Wageningen University. We obtained tissues and plasma from 13-week-old male Nagase analbuminemic rats, a strain that was originally derived from Sprague Dawley rats19, from a colony housed at the University Medical Center Utrecht in accordance with Animal Ethics Committee guidelines. All other animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham. We chose the number of animals for each study on the basis of the minimum number that would be approved by IACUC or ethics committees to obtain adequate biological samples. We performed animal studies at least twice. All animals were randomly assigned to study or control groups. We did not exclude animals from analysis. None of these studies was blinded. We used only male rats or mice (age 10–12 weeks, unless otherwise indicated).
Induction of single intravenous–dose PAN (n = 4 rats per group), PHN (n = 4 rats per group) and anti-Thy1.1 nephritis (n = 4 rats per group) are previously described13,14. For full-dose PAN, we used 15 mg per 100 g puromycin aminonucleoside (Sigma Chemical Company). For low-dose and intermediate-dose PAN, we used 7.5 mg per 100 g and 10 mg per 100 g, respectively. We induced complement- and leukocyte-independent nephrotic syndrome using the γ2 fraction of sheep anti-rat NTS as previously described30 in Itgb5−/− mice (n = 5 mice per group) and Angptl4−/− mice (n = 7 mice per group). For animal studies in which rabbit anti-rat Angptl4 antibody7 was injected into rats with PAN (n = 3 rats per group), we used 500 µl of antibody or preimmune serum per dose intravenously daily for 4 d starting on day 5.
For multiorgan gene expression studies, we snap froze organ samples in liquid nitrogen immediately after euthanasia (three rats or mice per organ sample pooled). We obtained WAT from the abdomen, BAT from the interscapular area, skeletal muscle from the thigh, intact liver or liver samples from both the left and right lobes, intact heart and rat kidneys, which we froze and used subsequently for glomerular isolation. In mouse experiments, we perfused kidneys with Dynabeads31 through the heart immediately after euthanasia and used them for glomerular isolation. We generated 12 cDNA templates from each pooled organ group and assessed gene expression by TaqMan real-time PCR.
We also used the following techniques, which have been previously described7,13,14,32: 18-h urine collection in metabolic cages, measurement of proteinuria, mouse urine albumin ELISA, mouse urine creatinine by mass spectrometry, two-dimensional gel electrophoresis and western blotting, confocal imaging, immunogold electron microscopy, extraction of total RNA, generation of cDNA templates (2 µg total RNA per template) and real-time PCR. In the real-time PCR studies, the threshold used for significance was a threefold change7,13,14,32. We purchased the following antibodies: mouse anti-V5 (Life Technologies, R96025, 1:2,500 (western blotting) or 1:50 (confocal and immunogold imaging)), mouse anti-PECAM1 (BD Pharmingen, 550300, 1:10) and secondary antibodies (Jackson ImmunoResearch laboratories). We assessed densitometry of the two-dimensional gel western blots using Image Quant TL 7.0 software (GE Healthcare).
We measured plasma triglyceride levels using an Alfa Wassermann Vetace autoanalyzer (Schiaparelli Biosystems) or a kit (Cayman Chemical Company). We measured plasma and urine FFA levels using a kit (Biovision), and we measured plasma or urine albumin from human and rat samples using an Alfa Wassermann Vetace autoanalyzer. We calculated the ratio of FFA to albumin by dividing the level of FFA (in µM) by the level of albumin (in g l−1). We used the albumin depletion kit (Pierce) to affinity absorb albumin from human sera.
Post-heparin plasma LPL activity
We injected rats with 10 units per 100 g body weight of porcine heparin intravenously 15 min before euthanasia and harvested the plasma after euthanasia. We measured post-heparin plasma LPL activity using the 3H-labeled triolein method33. We conducted the assay in the presence or absence of heat-inactivated serum from fasting rats (a source of the LPL activator ApoCII) and subtracted the readings without serum from the readings with serum. In addition, we conducted plasma LPL assays in the presence or absence of 1 M NaCl. In the absence of 1 M NaCl, the assay measured LPL and hepatic lipase activity. In the presence of 1 M NaCl, LPL was completely inhibited, and the assay measured hepatic lipase activity only. We calculated LPL activity by subtracting the activity in the presence of NaCl from the activity without NaCl.
We conducted western blot analyses for LPL using a goat LPL-specific antibody (Santa Cruz Biotechnology, sc-32382, 1:50) and a 5D2 monoclonal antibody that specifically identifies active dimeric LPL18.
Intralipid and oleic acid study
After collecting baseline 18-h urine and tail-vein blood samples in the fasting state, we treated 10-month-old Buffalo Mna rats (n = 4 rats per group) with 100 mg oleic acid (Sigma Chemical Company) by gavage, 200 mg Intralipid (Sigma Chemical Company) intraperitoneally or an equal volume of control normal saline intraperitoneally daily for 3 d. We obtained a tail-vein blood sample 5 h after the first dose in the fasting state. At the end of the study, we repeated an 18-h urine collection and collected cardiac blood and multiple organs in a fasting state immediately after euthanasia.
Injection of recombinant rat Angptl4
Harvesting of sialylated rat Angptl4 at neutral pI or hyposialylated rat Angptl4 from a HEK293 cell–based stable cell line has been described previously7. We injected concentrated supernatant from a stable HEK293 Angptl4 cell line containing rat Angptl4 or empty pcDNA 3.1 V5 His vector (1.8 mg per dose derived from approximately 200 ml of medium) intravenously into Buffalo Mna rats (n = 4 rats per group) and anti-Thy1.1 nephritis rats (n = 4 rats per group). To study glomerular binding of recombinant Angptl4 by confocal imaging, we injected 300 µg of concentrated supernatant containing rat Angptl4-V5 intravenously into wild-type and Itgb5 −/− mice and euthanized them after 2 h.
Development of human ANGPTL4 mutant constructs and stable cell lines
We mutated a human ANGPTL4 cDNA clone using PCR-based mutagenesis. We used pcDNA 3.1 V5 His B vector-based constructs of wild-type and mutant human ANGPTL4 clones to develop HEK293 (MP Biomedical)-based stable cell lines using methods previously described7. Proteins were harvested in serum-free conditions, and supernatant was concentrated as described previously7. When used for harvesting protein, we supplemented serum-free DMEM with 25 mM ManNAc, which is a precursor of sialic acid, to ensure adequate sialylation and the neutral pI of recombinant proteins. Recombinant human ANGPTL4 was quantified by ELISA.
For studies in which recombinant human wild-type ANGPTL4, mutant ANGPTL4 and control protein were injected into Buffalo Mna rats (n = 3 rats per group), we conducted both ELISA and conventional protein assays to quantify protein and used 55 µg (by ELISA) of recombinant ANGPTL4 per dose or equal amounts of control stable cell line supernatant (equalized by protein assay). We quantified plasma levels of the recombinant human protein in rats using reagents from the human ANGPTL4 ELISA kit. For the ZDF rat studies (n = 4 rats per group), we used 15 µg (by ELISA) of recombinant human wild-type or mutant ANGPTL4.
Endothelial cell study
We grew cultured rat glomerular endothelial cells (GEnCs; gift from F. Danesh, MD Anderson Cancer Center) in serum-free conditions for 24 h, subjected them to H2O2 (200 µM)-induced stress and co-incubated different groups with equal amounts of sialylated rat Angptl4, hyposialylated rat Angptl4 or supernatant protein from control HEK293 cell lines. We measured lactate dehydrogenase levels in the supernatant using a cytotoxicity kit (Roche Applied Science) after 24, 48 and 72 h of incubation.
αvβ5 integrin plate assay
We coated 96-well plates with 5 ng purified human αvβ5 integrin (Millipore) per well and blocked them with Tris-buffered saline plus Tween 20 (TBST) containing 1% BSA, which was followed by incubation with increasing amounts of sialylated rat Angptl4-V5, hyposialylated rat Angptl4-V5 or control protein. After washes, the V5 tag was detected using a V5-specific HRP antibody (Life Technologies, R96125, 1:2,500), and the reaction was developed using the TMB peroxidase substrate and solution and read on a Labsystems multiskan MCC/340 at 450 nm.
Generation and characterization of β5 integrin–specific antibodies
Fusion proteins were generated against parts of the extracellular segment of human β5 integrin to generate two polyclonal antibodies in rabbits (Proteintech, Inc.; antibody 8472A, amino acids 35–460, includes the integrin β domain; and antibody 8472B, amino acids 461–719, includes the integrin β tail). We tested both antibodies for specificity by western blot analysis before and after absorbing out reactivity to recombinant human β5 integrin. We conducted pilot studies by inducing PAN in Sprague Dawley rats (n = 3 rats per group) and injecting two doses of each antibody (250 µl per dose) intravenously during the recovery phase to assess for in vivo blockage of β5 integrin (i.e., slower recovery of proteinuria). Only 8472A (Supplementary Fig. 6c) was effective in slowing recovery of proteinuria. To test binding to glomerular endothelium, we injected 500 µl of each antibody intravenously into Sprague Dawley rats, which was followed by euthanasia after 3 h and confocal imaging using donkey anti-rabbit IgG (Supplementary Fig. 6d). To inhibit the Angptl4–β5 integrin interaction in vivo, we injected the 8472A β5 integrin–specific antibody or preimmune serum into Sprague Daley rats with PAN (250 µl per dose, with four doses total administered on alternate days starting on day 11) and in aP2-Angptl4 transgenic rats with PAN (500 µl per dose, with seven doses total administered on alternate days starting on day 11).
Statistical analyses
Analyses of differences between two groups were conducted using standard unpaired Student’s t test. If the variance was unequal, Welch’s t test was used.
Supplementary Material
ACKNOWLEDGEMENTS
We thank investigators of the FSGS clinical trial for providing baseline plasma and urine samples from patients with FSGS; M. del Nogal-Avila (University of Alabama at Birmingham (UAB)) for selected real-time PCR studies; H. Donoro (UAB) for assistance with animal colony management; E. Soria (Instituto Nacional de Cardiologia) for immunogold electron microscopy studies; H. Chung (UAB) for advice on LPL assays; V. Kumar (UAB) for help in collecting Institutional Review Board–approved patient sera from transplant patients; D. Salant (Boston University) for γ2-NTS; A. Köster (Eli Lilly) for Angptl4−/− mice; M. Mitsuyama (Kyoto University) for Buffalo Mna rats; J. Brunzell (University of Washington) for 5D2 monoclonal antibody; F. Danesh (MD Anderson Cancer Center) for cultured rat glomerular endothelial cells; UAB–University of California-San Diego George O’Brien Center Core C for measuring urine and serum creatinine by mass spectrometry; UAB Nephrology Research and Training Center for equipment use; and UAB Research Foundation for filing patent PCT/US2011/039255 for the use of ANGPTL4 mutants as therapeutic agents for nephrotic syndrome. This work is supported by the US National Institutes of Health (R01DK077073 and R01DK090035 to S.S.C., K01DK096127 to L.C.C. and T32DK007545 to C.M.).
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
L.C.C. maintained transgenic rat colonies and conducted rat experiments, developed stable cell lines and conducted imaging studies and selected gene expression studies. C.M. maintained the Itgb5−/− mouse colony and conducted mouse studies, did assays for Angptl4, V5-tagged proteins, triglycerides and free fatty acids and performed two-dimensional gel studies, western blotting, selected gene expression studies, protein interaction studies and albumin depletion studies. C.A.-C. interpreted and analyzed light microscopy, electron microscopy and immunogold electron microscopy studies. J.A.J. obtained blood and tissue from Nagase rats and provided useful advice on Nagase rat biology. S.K. conducted experiments with Angptl4−/− mice and made substantial contributions to the preparation and revision of the manuscript. S.S.C. acted as senior investigator, planned and supervised the study, generated mutant ANGPTL4 constructs to develop stable cell lines, conducted selected gene expression and animal studies and wrote and revised the manuscript with input from other authors.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63:1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 2.Marsh JB, Drabkin DL. Experimental reconstruction of metabolic pattern of lipid nephrosis: key role of hepatic protein synthesis in hyperlipemia. Metabolism. 1960;9:946–955. [PubMed] [Google Scholar]
- 3.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock PH, et al. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J. Clin. Invest. 1995;96:2555–2568. doi: 10.1172/JCI118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shearer GC, Kaysen GA. Endothelial bound lipoprotein lipase (LpL) depletion in hypoalbuminemia results from decreased endothelial binding, not decreased secretion. Kidney Int. 2006;70:647–653. doi: 10.1038/sj.ki.5000318. [DOI] [PubMed] [Google Scholar]
- 6.Ghiggeri GM, et al. Characterization of cationic albumin in minimal change nephropathy. Kidney Int. 1987;32:547–553. doi: 10.1038/ki.1987.243. [DOI] [PubMed] [Google Scholar]
- 7.Clement LC, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Clement LC, Macé C. New insights into human minimal change disease: lessons from animal models. Am. J. Kidney Dis. 2012;59:284–292. doi: 10.1053/j.ajkd.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 2002;43:1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Romeo S, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin W, et al. Genetic variation in ANGPTL4 provides insights into protein processing and function. J. Biol. Chem. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement LC, et al. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 2007;72:337–347. doi: 10.1038/sj.ki.5002302. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Clement L, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J. Biol. Chem. 2006;281:39681–39692. doi: 10.1074/jbc.M606664200. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, et al. Sclerotic lesions in the glomeruli of Buffalo/Mna rats. Nephron. 1986;43:50–55. doi: 10.1159/000183718. [DOI] [PubMed] [Google Scholar]
- 16.Le Berre L, et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J. Clin. Invest. 2002;109:491–498. doi: 10.1172/JCI12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuger L, et al. Effects of heparin on the uptake of lipoprotein lipase in rat liver. BMC Physiol. 2004;4:13. doi: 10.1186/1472-6793-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SF, Reich B, Brunzell JD, Will H. Detailed characterization of the binding site of the lipoprotein lipase-specific monoclonal antibody 5D2. J. Lipid Res. 1998;39:2350–2359. [PubMed] [Google Scholar]
- 19.Nagase S, Shimamune K, Shumiya S. Albumin-deficient rat mutant. Science. 1979;205:590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi H, Tamura S, Nagase S, Tsuiki S. Hypertriacylglycerolemia and adipose tissue lipoprotein lipase activity in the Nagase analbuminemic rat. Biochim. Biophys. Acta. 1983;744:165–170. doi: 10.1016/0167-4838(83)90086-9. [DOI] [PubMed] [Google Scholar]
- 21.Kersten S, et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 22.Staiger H, et al. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-δ and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgiadi A, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor β/δ and protects against fatty acid-induced oxidative stress. Circ. Res. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 24.Zhu P, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2 −:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19:401–415. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Mandard S, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 26.Zilleruelo G, Hsia SL, Freundlich M, Gorman HM, Strauss J. Persistence of serum lipid abnormalities in children with idiopathic nephrotic syndrome. J. Pediatr. 1984;104:61–64. doi: 10.1016/s0022-3476(84)80590-9. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, et al. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc. Natl. Acad. Sci. USA. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avila-Casado MC, et al. Proteinuria in rats induced by serum from patients with collapsing glomerulopathy. Kidney Int. 2004;66:133–143. doi: 10.1111/j.1523-1755.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferris M, et al. Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT) Clin. Transl. Sci. 2013;6:13–20. doi: 10.1111/cts.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chugh S, et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 2001;59:601–613. doi: 10.1046/j.1523-1755.2001.059002601.x. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto M, et al. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J. Clin. Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bengtsson-Olivecrona G, Olivecrona T. Lipoprotein analysis—a practical approach. In: Converse CA, Skinner ER, editors. Practical Approach Series. New York: Oxford University Press; 1992. pp. 169–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.