Abstract
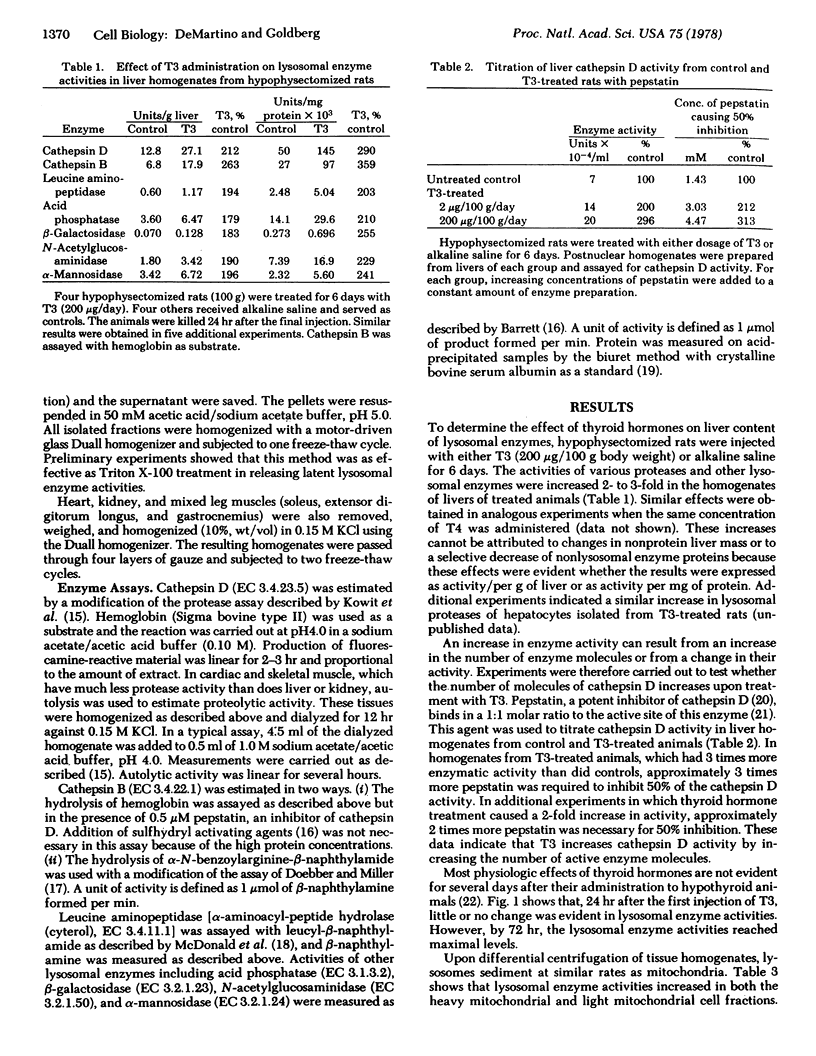
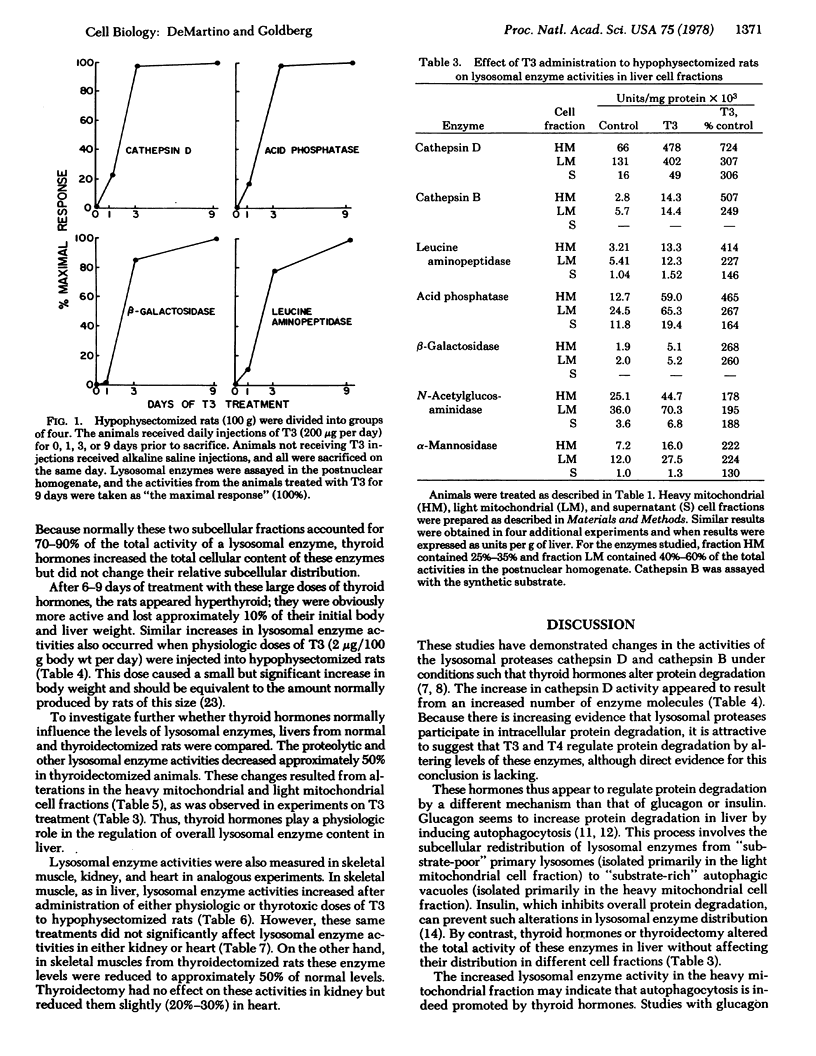
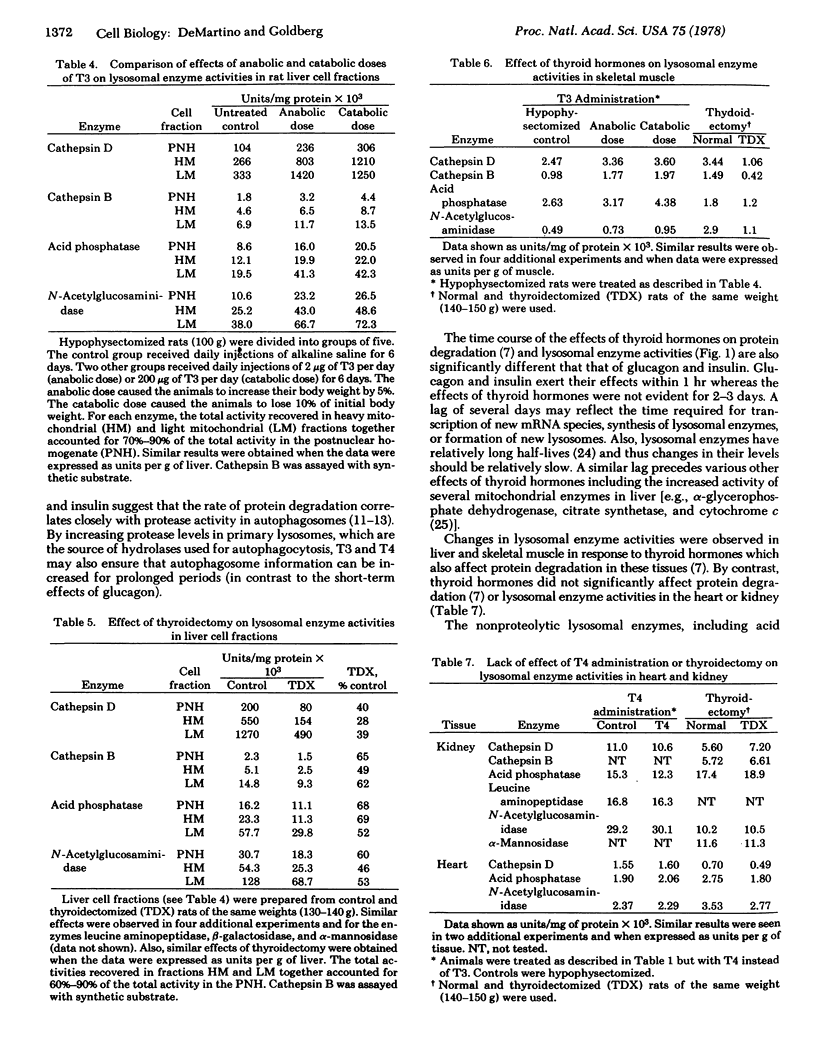
Because protein degradation in liver and skeletal muscle is increased by thyroid hormones and decreased by thyroidectomy; we investigated the influence of thyroid hormones on the level of lysosomal enzymes. Hypophysectomized rats received daily injections of L-thyroxine or L-triiodothyronine. After 3 days of this regimen, homogenates of liver and skeletal muscle showed a 2- to 3-fold increase in the activities of cathepsin D, cathepsin B, and other lysosomal enzymes including leucine aminopeptidase, acid phosphatase, β-galactosidase, N-acetylglucosaminidase, and α-mannosidase. In liver, this effect reflected increased enzyme activity in the two subcellular fractions that normally contain lysosomes. Titration of cathepsin D with pepstatin indicated that the increase in this activity resulted from an increase in the number of enzyme molecules. These effects occurred with both pharmacologic (thyrotoxic) and physiologic (growth-promoting) doses of thyroid hormones. Liver and skeletal muscle from thyroidectomized rats had approximately 50% of the normal levels of lysosomal enzyme activities. Under these various conditions, heart and kidney, tissues in which protein degradation does not appear to be influenced by thyroid hormones, showed no significant changes in lysosomal hydrolases. Thus, thyroid hormones regulate proteolytic and other lysosomal enzyme activities in those tissues in which these hormones influence protein degradation. Many characteristic features of hyperthyroidism and hypothyroidism may result from changes in levels of lysosomal enzymes.
Keywords: intracellular proteases, protein turnover, thyroxine, triiodothyronine, hyperthyroidism and hypothyroidism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Beaudet A. L., Lipson M. H., Ferry G. D., Nichols B. L., Jr Acid lipase in cultured fibroblasts: cholesterol ester storage disease. J Lab Clin Med. 1974 Jul;84(1):54–61. [PubMed] [Google Scholar]
- Burke J. A., Schubert W. K. Deficient activity of hepatic acid lipase in cholesterol ester storage disease. Science. 1972 Apr 21;176(4032):309–310. doi: 10.1126/science.176.4032.309. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla W. D. Role of the pituitary and thyroid glands in the decline of minimal O2 consumption with age. J Clin Invest. 1974 Feb;53(2):572–581. doi: 10.1172/JCI107592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L. Quantitative characterization of dense body, autophagic vacuole, and acid phosphatase-bearing particle populations during the early phases of glucagon-induced autophagy in rat liver. J Cell Biol. 1971 Mar;48(3):473–489. doi: 10.1083/jcb.48.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebber T. W., Miller L. L. Circumvention of interference by sulfhydryl compounds in azo dye determination of beta-naphthylamine from synthetic protease substrates. Anal Biochem. 1976 Jan;70(1):39–44. doi: 10.1016/s0003-2697(76)80045-0. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Howell E. M., Li J. B., Martel S. B., Prouty W. F. Physiological significance of protein degradation in animal and bacterial cells. Fed Proc. 1974 Apr;33(4):1112–1120. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Iodice A. A., Chin J., Perker S., Weinstock I. M. Cathepsins A, B, C, D and autolysis during development of breast muscle of normal and dystrophic chickens. Arch Biochem Biophys. 1972 Sep;152(1):166–174. doi: 10.1016/0003-9861(72)90204-4. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER L. L. DIRECT ACTIONS OF INSULIN, GLUCAGON, AND EPINEPHRINE ON THE ISOLATED PERFUSED RAT LIVER. Fed Proc. 1965 May-Jun;24:737–744. [PubMed] [Google Scholar]
- MILLER L. L. Glucagon: a protein catabolic hormone in the isolated perfused rat liver. Nature. 1960 Jan 23;185:248–248. doi: 10.1038/185248a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Zeitman B. B., Ellis S. Leucine naphthylamide: an inappropriate [corrected] substrate for the histochemical detection of cathepsins B and B'. Nature. 1970 Mar 14;225(5237):1048–1049. doi: 10.1038/2251048a0. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Peters J. P., Man E. B. THE INTERRELATIONS OF SERUM LIPIDS IN PATIENTS WITH THYROID DISEASE. J Clin Invest. 1943 Sep;22(5):715–720. doi: 10.1172/JCI101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPPEL A. L., ZALKIN H., CALDWELL K. A., DESAI I. D., SHIBKO S. Increased lysosomal enzymes in genetic muscular dystrophy. Arch Biochem Biophys. 1962 Feb;96:340–346. doi: 10.1016/0003-9861(62)90418-6. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Winder W. W., Baldwin K. M., Terjung R. L., Holloszy J. O. Effects of thyroid hormone administration on skeletal muscle mitochondria. Am J Physiol. 1975 May;228(5):1341–1345. doi: 10.1152/ajplegacy.1975.228.5.1341. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Pepstatin inhibits the digestion of hemoglobin and protein-polysaccharide complex by cathepsin D. Biochem Biophys Res Commun. 1972 May 26;47(4):965–970. doi: 10.1016/0006-291x(72)90587-6. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Ward W. F., Mortimore G. E. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974 Sep 10;249(17):5458–5463. [PubMed] [Google Scholar]


