Abstract
Background
Ingestion of sweet food is driven by central reward circuits and restrained by endocrine and neurocrine satiety signals. The specific influence of sucrose intake on central affective and reward circuitry and alterations of these mechanisms in the obese are incompletely understood. For this, we hypothesized that (i) similar brain regions are engaged by the stimulation of sweet taste receptors by sucrose and by non-nutrient sweeteners and (ii) during visual food-related cues, obese subjects show greater brain responses to sucrose compared with lean controls.
Methods
In a double-blind, crossover design, 10 obese and 10 lean healthy females received a sucrose or a non-nutrient sweetened beverage prior to viewing food or neutral images. BOLD signal was measured using a 1.5 Tesla MRI scanner.
Key Results
Viewing food images after ingestion of either drink was associated with engagement of similar brain regions (amygdala, hippocampus, thalamus, anterior insula). Obese differed from lean subjects in behavioral and brain responses rating both beverages as less tasteful and satisfying, yet demonstrating greater brain responses. Obese subjects also showed engagement of an additional brain network (including anterior insula, anterior cingulate, hippocampus, and amygdala) only after sucrose ingestion.
Conclusions & Inferences
Obese subjects had a reduced behavioral hedonic response, yet a greater engagement of affective brain networks, particularly after sucrose ingestion, suggesting that in obese subjects, lingual and gut-derived signaling generate less central hedonic effects than food-related memories in response to visual cues, analogous to response patterns implicated in food addiction.
Keywords: artificial sweeteners, fMRI, obesity
INTRODUCTION
In many parts of the industrialized world, obesity has reached epidemic proportions. In the United States, 34% of the population is obese and an additional 34% is considered overweight.1 Obesity-attributable medical expenditures related to the metabolic syndrome and other associated diseases reached $75 billion in the United States in 2003.2 Even with the billion-dollar diet industry, long-term weight loss results with dieting have been disappointing. Despite extensive research into possible targets for drug development aimed at the obesity epidemic, only surgical interventions including gastric bypass surgeries have shown persistent benefits.3
Although tremendous progress has been made in the characterization of peripheral, vagal, and hypothalamic mechanisms related to food intake and satiety in rodents,4–9 affective, reward, and cognitive processes related to ingestive behavior and obesity have only been studied recently in human subjects.10,11 Based on these studies, it has been suggested that overeating may be the result of an imbalance between vagally and endocrine-mediated satiety mechanisms from the intestine with central reward circuits and prefrontal circuits involved in executive control of ingestive behavior. A similar shift among interoceptive, reward, and executive control mechanisms is seen in drug addiction where an altered interaction of these networks results in an enhanced reinforcing value of the drug and a distorted reward threshold.10,12
A number of investigators have looked into the role of food-related memories in obesity, by studying the impact of food images on brain activation.13–15 Studies comparing lean and obese subjects have established differences in brain response to images of high-calorie vs low-calorie foods.13,14 It has also been demonstrated that brain activity changes in response to these food images after a subject consumes glucose vs water, and that this interaction between visual food cues and ingested nutrients differs in obese compared with lean groups.15 A limitation to these studies is the fact that subjects can taste the difference between high- and low-calorie foods and between glucose and water ingestion; thereby confounding the results by the subject’s preconceived expectations based on what they are seeing, smelling, and tasting. Non-caloric sweeteners stimulate the same sweet taste receptor as glucose and sucrose, thereby theoretically providing the same hedonic value without the calories.16,17 For this reason, they are an ideal means to study the specific effect of sucrose absorption on brain activation in obese and lean subjects.
In this study, we aimed to test the following main hypotheses: (i) Brain responses to the ingestion of a non-nutrient sweetened and a sucrose-sweetened drink will be similar, presumably involving similar engagement of sweet taste receptors; and (ii) during visual food-related cues, obese subjects will show greater affective and/or hedonic brain and behavioral responses to sucrose compared with lean controls. To test these hypotheses, we assessed brain responses using functional magnetic resonance imaging (fMRI) in lean and obese healthy female subjects during two drink conditions (a 300-calorie sucrose drink and an under 10-calorie non-nutrient sweetened beverage) that both stimulate sweet taste receptors.
MATERIALS AND METHODS
Participant selection
Twenty healthy lean and obese female participants were recruited from the clinical research unit of the UCLA Center for Neurobiology of Stress, from the UCLA Center for Human Nutrition, and from community advertisements. Subjects were between the ages of 18 and 40 years and were age-matched. The 10 lean subjects had a body mass index (BMI) between 19 and 25 kg m−2 and the 10 obese subjects had a BMI between 30 and 37 kg m−2. All subjects were right-handed, were regularly menstruating, and were studied during the follicular phase (4–12 days after the first day of last menstrual period) given brain reward mechanisms vary with reproductive phase.18
Exclusion criteria were as follows: (i) a history of any gastrointestinal surgery, psychiatric and neurologic disorders, or head trauma with loss of consciousness; (ii) a past or current history of an eating disorder; (iii) a current history of chronic pain; (iv) being pregnant or breast-feeding; (v) tobacco use of more than five cigarettes per month; (vi) a history of excessive exercise; (vii) postmenopausal status; (viii) use of any medications/drugs that affect the central nervous system, gastrointestinal motility, autonomic activity, or pain sensation within 4 weeks of enrollment; and (ix) a history of serious psychiatric, neurologic, cardiovascular, respiratory, or renal illnesses.
The UCLA Office of Protection of Research Subjects (OPRS) approved the study protocol. All subjects provided written informed consent before participation.
Psychosocial evaluations
Because psychological processes may influence gastrointestinal sensory and motor function as well as brain responses,19 all subjects were evaluated using the Mini-International Neuropsychiatric Interview (MINI)+5.0,20 the Hospital Anxiety and Depression scale (HAD),21 and the Spielberger State and Trait Anxiety Inventory (STAI).22 The MINI+5.0 is a brief structured interview for the major Axis I psychiatric disorders in DSM-IV and ICD-10. The HAD scale is a measure of current anxiety and depression symptoms validated for non-psychiatric samples. The STAI is a 40-item self-report assessment that differentiates between state anxiety and trait anxiety.
Appetite assessments
Taste, hunger, satiation, and satisfaction were assessed with two visual analog scales (VAS). The 10-point VAS for appetite examines taste and desire for specific food and has been shown to be reproducible and not influenced by prior diet standardization.23 The 10-point Fullness Questionnaire (FQ) is used to measure hunger and satisfaction and has been shown to correlate positively with the weight and caloric composition of foods, and negatively with palatability.24
Functional MRI paradigm
This study was part of a larger study that used both resting state and task functional MRI to evaluate differences in obese and lean women after a sucrose drink vs a non-nutrient sweetened beverage. In this article, we are reporting only on the second part of the study (study 2), which focuses on the evoked brain responses to visual food cues after nutrient ingestion in obese and lean subjects.
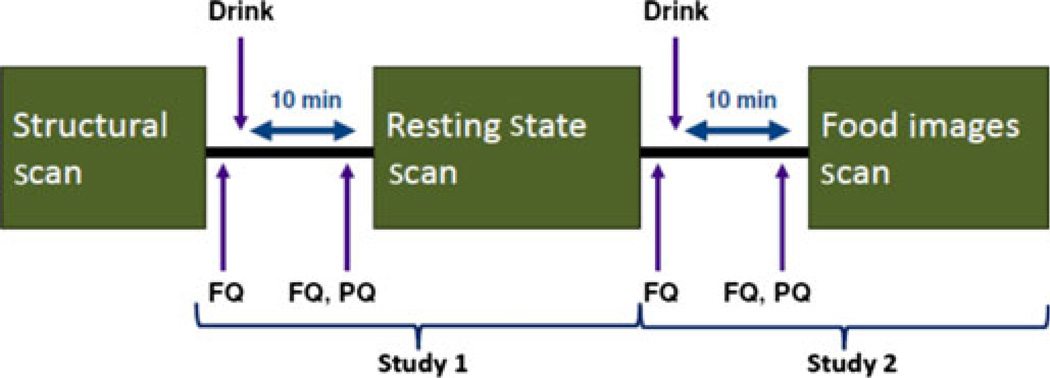
A double-blind randomized crossover design was utilized over two separate days of fMRI scanning, no less than 2 days apart. The details of the study protocol are shown in Fig. 1. Subjects fasted for 6 h prior to scan appointments, which occurred between 9:00 am and 11:00 am. The two scanning days were identical except the drink order was counterbalanced. At the start of each scan day, the subjects were given a synopsis of the study tests and placed in the scanner for a brief structural scan followed by two additional scans: a resting and a task-evoked functional food images scan.
Figure 1.
Study protocol. On the scanning day, subjects underwent three types of scans: a brief structural scan, a resting state scan, and a food images scan. Ten minutes before both the resting state and food images scans, subjects received a fullness questionnaire (FQ) and either a non-nutrient sweetened drink or a sucrose beverage. Immediately prior to scanning, subjects were given another FQ as well as a palatability questionnaire (PQ). This scanning day was repeated with the order of the drinks counterbalanced. The resting state scan along with the drink and questionnaires given prior to the scan were analyzed together as study 1 (results presented in a different paper). The food images scan and accompanying drink and questionnaires (study 2) are analyzed in this article.
The 15-min functional food images scan had three 5-min functional runs (runs 1–3). During each run, 36 images (18 food, 18 neutral images of brick walls) were presented in random order. Subjects were instructed to focus their attention on the stimuli. Three images were shown for 4 s each followed by a 12 s dark screen with a central fixation cross. A total of 108 images were displayed (54 food, 54 scenery). In contrast to pictures from the International Affective Picture System (IAPS), no standardized food images are currently available. As done by other investigators who have used food images,13–15,25 the images were selected from an internet repository. The wide array of appetizing food images were chosen to represent a balance of food preferences. After the scans, subjects rated the images to ensure their palatability.
Ten minutes before both the resting scan and the functional food images scan, subjects consumed a 10 oz beverage consisting of either a non-nutrient sweetened beverage (Diet Ocean Spray Cranberry Juice with 10 tsp of Truvia; <10 calories) or a sucrose beverage (Ocean Spray Cranberry Juice with 10 tsp of sugar; 300 calories). The beverages were designed to be similar in taste and sweetness. Pilot testing in five healthy individuals confirmed that the drinks could not be differentiated on the basis of taste. Subjects consumed both beverages on each scan day, but the drink order was counterbalanced. The two beverages were given 25 min apart. Randomization was performed using Excel random number generator function. Subjects and investigators were blinded to the drink order. The beverage was presented in a non-descriptive container. Nose clips were applied to minimize olfactory influences and subjects were instructed to drink through a straw. As the study was not aimed at identifying the endocrine mediator(s) of the brain response to the test drinks, no measurements of plasma levels of glucose, insulin, or other incretins were performed.
Subjects completed four FQs: a baseline FQ, a second FQ 10 min after the first beverage, a third FQ immediately prior to the second beverage, and a fourth FQ 10 min after the second beverage. A VAS for appetite questionnaires was completed 10 min after each beverage.
fMRI acquisition and preprocessing
MRI scanning was performed using a 1.5T MRI scanner (Siemens Sonata; Siemens, Erlangen, Germany). A high resolution structural image was acquired from each subject with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2200 ms, echo time (TE) = 4.38 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 1 mm3 voxel size.
Food-related image stimuli were presented using E-Prime 2.0 Professional through MR compatible goggles. Functional blood oxygen-level dependent (BOLD) images were acquired with an echo-planar T2*-weighted imaging sequence, TR = 2000 ms, TE = 45 ms, flip angle = 77°, slice thickness = 5 mm, 220 × 220 voxel matrices, and 3.4 × 3.4 × 5 mm voxel size.
Using SPM5 software (Welcome Department of Cognitive Neurology, London, UK), data were slice-time and motion corrected, spatially normalized to the Montreal Neurological Institute (MNI) template using the structural images, and spatially smoothed at both 3 mm3 and 8 mm3 Gaussian kernel. The first two volumes were discarded to allow for stabilization of the magnetic field.
Data analysis
Behavioral analyses
Behavioral analyses were performed in PASW v17.0 (IBM, Chicago, IL, USA). Group differences in STAI and HAD anxiety and depression ratings were evaluated by independent samples t-tests. Taste ratings were evaluated in a Group (lean; obese) × Condition (sucrose; non-nutrient sweetener) analyses of variance (ANOVA). Appetite ratings for satisfaction and hunger were evaluated in Group (lean; obese) × Condition (sucrose; non-nutrient sweetener) × Time (before ingestion; after ingestion) repeated measures ANOVAS. All data are given as mean ± SE.
fMRI analyses
To examine similarities between group brain responses to beverage consumption, we performed a region of interest (ROI)-based conjunction analysis on the within-group activation maps for the food–neutral image contrast obtained from applying a general linear model (GLM) in SPM5. Using the flexible factorial model option, subjects, groups (lean vs obese), and conditions (sucrose food images, sucrose neutral images, non-nutrient sweetener food images, and non-nutrient sweetener neutral images) were included as factors and the main effects of group and condition as well as their interaction were estimated. As no order effect was detected, order was not included in the final model. Region of interest analyses were conducted by applying a small volume correction for the ROIs and significance was defined at a probability value less than 0.05 corrected using the family-wise error algorithm. Anatomically based ROIs were selected a priori based on areas known to be involved in both hedonic and homeostatic networks (amygdala, hippocampus, hypothalamus, thalamus, and anterior and posterior insula). Region of interests were created using the Wake Forest University PickAtlas toolbox in SPM5. To control for type I error inherent in testing multiple ROIs (n = 6), the significance of each region of interest was only considered after applying a modified Bonferroni procedure.26
Multiple regression and the ROIs described above were used to determine group (lean non-nutrient sweetener, lean sucrose, obese non-nutrient sweetener, obese sucrose) differences in association between hunger scores and brain activity during the viewing of food images and viewing neutral images. Hunger scores were collected after the consumption of the second drink.
A partial least squares (PLS) analysis was employed to identify distributed patterns of regions associated with viewing pleasant images of food during non-nutrient and sucrose beverage consumption in lean and obese women. PLS is a multivariate statistical technique considered to be more sensitive than standard univariate analyses of neuroimaging data such as SPM.27,28 PLS is analogous to principal components analysis, but the solutions can be restricted to the part of the covariance structure that is attributable to conditions or groups in an experimental design. Task PLS identifies experimental contrasts accounting for the maximum amount of variance in the data and the brain regions whose activity relates, as a whole, to these contrasts. In addition, a non-rotated PLS analysis was employed to examine Group × Condition interactions. The difference between a non-rotated and a task PLS analysis is that a priori contrasts of interest are used in the non-rotated, but not in the task PLS. Contrasts representing group differences in response to food images in the high- and low-calorie conditions were entered into the analysis. PLS was implemented with freely available code (http://www.rotman-baycrest.on.ca) and performed on data spatially smoothed at 3 mm3 voxel reliability that was determined using bootstrap estimation (500 samples). The ratio of the observed weight to the bootstrap standard error was calculated and voxels were considered reliable if the absolute value of the bootstrap ratio (BSR) exceeded 1.96. Clusters with a peak >3.30 and >60 voxels are reported.
RESULTS
Patient population
Clinical variables
A total of 22 subjects were enrolled in the study (Table 1). The first two subjects were excluded from the GLM and PLS analyses due to suboptimal quality of our initial food and neutral images. Therefore, 20 subjects were used for the GLM and PLS analyses. The mean BMI of the lean group was 22.4 kg m−2 (SE 0.5), and 32.9 kg m−2 (SE 0.7) for the obese group (P < 0.05). All obese and lean subjects had similar STAI and HAD depression and anxiety ratings that were within the normal range. No subject had current depression or anxiety based on the MINI+ interview.
Table 1.
Demographics and baseline characteristics
| Lean mean (SE), n = 10 | Obese mean (SE), n = 10 | |
|---|---|---|
| Age (year) | 24.60 (1.33) | 26.50 (1.64) |
| BMI (kg m−2) | 22.40 (0.50) | 32.91 (0.74) |
| HAD depression | 1.20 (0.39) | 2.20 (0.44) |
| HAD anxiety | 5.70 (1.25) | 4.93 (1.06) |
| STAI | 49.20 (3.56) | 46.67 (2.82) |
HAD, hospital anxiety and depression; STAI, Spielberger State and Trait Anxiety Inventory.
Appetite measures
There was no difference between the sucrose and the non-nutrient sweetened drinks, in how their taste was rated by the two groups (Fig. S1). Beverage consumption significantly reduced hunger ratings (P = 0.005), increased satisfaction ratings (P < 0.001), and reduced desire for sweetness (P < 0.001) across all subjects, without any differences between the two drinks. However, obese subjects rated the taste of both beverages significantly lower than the lean subjects (P = 0.005), and obese women reported less satisfaction compared with lean subjects after both drinks, consistent with reduced subjective hedonic responses in the obese (P = 0.016) (Table S1).
Correlation of subjective sensations with brain responses
In both obese and lean subjects, a greater subjective hunger score on the FQ correlated with a greater engagement of the left posterior insula using ROI analyses (z = 3.70; P = 0.045) when viewing food images after beverage consumption. Compared with lean subjects, obese subjects had a trend for greater correlation of the right anterior insula with the subjective feeling of hunger (z = 3.29; P = 0.074). No significant associations were detected for the other ROIs.
Brain responses related to test drinks in both lean and obese
Conjunction analyses of within-group activation maps for food-neutral image contrast demonstrated that in both groups, similar brain regions were engaged after ingestion of the two test drinks when viewing food-neutral images. Specifically, there was significant engagement of bilateral amygdala, bilateral hippocampus, and bilateral thalamus, and right anterior insula, consistent with engagement of a network of brain regions related to affect, memory, and interoception (Table 2).
Table 2.
Similar brain regions were engaged when looking at images of food after ingestion of both sucrose and the non-nutrient sweetener beverages in lean and obese subjects, using ROI conjunction analysis, as described in the Methods section. The results for the following ROIs were not significant: posterior insula, hypothalamus
| ROI | Coordinate (x, y, z) |
Cluster value (Ke) |
P value (FWE) |
Z score |
|---|---|---|---|---|
| R Amygdala | (−24, −6, −14) | 117 | 0.020 | 3.24 |
| L Amygdala | (24, −6, −16) | 136 | 0.007 | 3.60 |
| R Hippocampus | (−28, −36, 2) | 32 | 0.009 | 3.52 |
| L Hippocampus | (26, −30, −6) | 56 | 0.002 | 4.00 |
| R Thalamus | (22, −26, −2) | 364 | 0.007 | 3.98 |
| L Thalamus | (−20, −30, 0) | 281 | 0.004 | 4.14 |
| R Anterior insula | (−24, −6, −14) | 117 | 0.020 | 3.24 |
| L Anterior insula | (−26, 30, −8) | 18 | 0.321 | 2.68 |
ROI, region of interest; R, right; L, left; FWE, family-wise error.
Brain responses to food cues and differences between obese and lean subjects
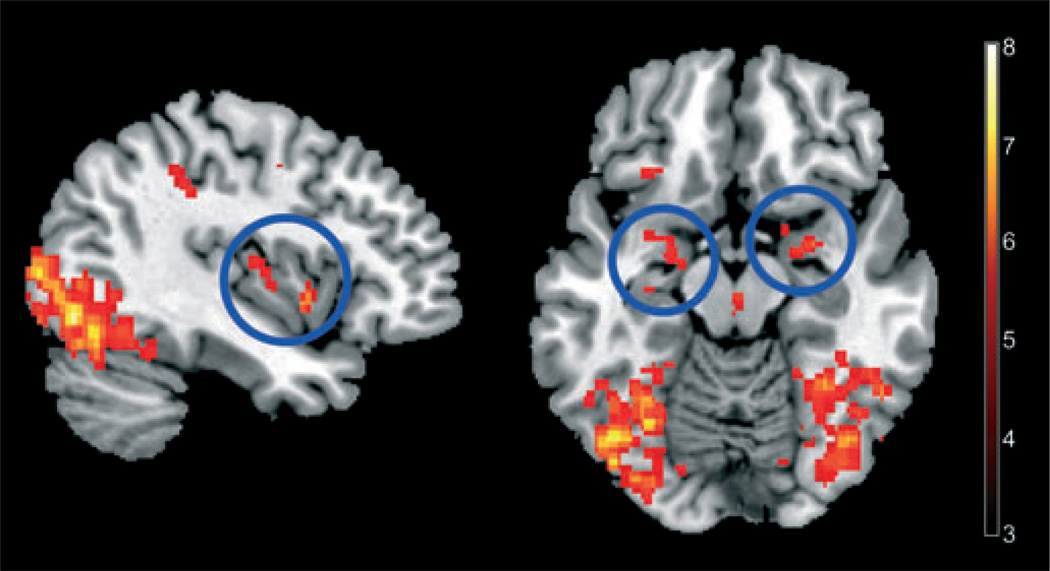
A multivariate analysis (task PLS) was used to identify a network of interactive regions that were engaged differently between groups during the four conditions. In both subject groups, the viewing of food images was associated with activation of a network that included the left insula, bilateral pregenual cingulate, bilateral amygdala, and left hippocampus (Table 3). This network accounted for 41% of the crossblock variance (P < 0.001), and was engaged to a greater extent in the obese compared with lean subjects (Fig. 2).
Table 3.
Network analyses revealed a network of regions associated with viewing of food images in both lean and obese groups that accounted for 41% of the crossblock variance (P < .001) and was more engaged in the obese subjects (task PLS) and an additional network more engaged by obese subjects only after consumption of the high-calorie sucrose beverage that accounted for 27% of the crossblock variance (P = 0.028) (non-rotated PLS). Analyses detailed in the Methods section
| Analysis | Region | Coordinate (x, y, z) | Cluster value (voxels) | Approx. P value | Bootstrap ratio |
|---|---|---|---|---|---|
| Task PLS food > neutral | L BA17/18/19 | (−44, −84, 2) | 5281 | 0 | 9.2475 |
| R BA18/19/39 | (42, −78, 8) | 5986 | 0 | 8.2686 | |
| L Cerebellum | (−18, −34, −46) | 71 | 0 | 5.5753 | |
| R Cerebellum | (24, −34, −40) | 100 | 0 | 5.5712 | |
| L BA24 | (−4, 4, 28) | 66 | 0 | 5.5022 | |
| L BA47 | (−30, 30, −12) | 270 | 0 | 5.3495 | |
| R amygdala/PHG/basal ganglia | (28, −2, −14) | 393 | 0 | 5.0527 | |
| L Insula | (−38, −10, 10) | 192 | 0 | 5.0482 | |
| L BA6 | (32, −8, 56) | 89 | 0 | 5.0031 | |
| L amygdala/PHG/hippocampus | (−18, −8, −18) | 324 | 0 | 4.9989 | |
| R Caudate | (34, −36, −2) | 64 | 0 | 4.9537 | |
| R BA9/46 | (52, 4, 36) | 493 | 0 | 4.544 | |
| L BA9/46 | (−58, 6, 30) | 252 | 0 | 4.4831 | |
| R Insula | (40, −6, 4) | 83 | 0 | 4.2976 | |
| L/R BA6/32 | (−4, 12, 52) | 208 | 0 | 4.181 | |
| R Thalamus | (4, −40, −12) | 166 | 0 | 4.1239 | |
| L BA40 | (−42, −42, 42) | 219 | 0 | 4.0752 | |
| R BA6 | (0, 14, 66) | 125 | 0.0001 | 4.0478 | |
| L/R Midbrain | (2, −20, −14) | 92 | 0.0001 | 4.0306 | |
| R Cerebellum | (16, −54, −46) | 124 | 0.0001 | 4.0259 | |
| L BA17/18 | (−12, −94, 2) | 97 | 0.0001 | 4.0206 | |
| L Insula | (−28, −30, 20) | 72 | 0.0001 | 3.9012 | |
| L/R BA8/32 | (−10, 32, 42) | 173 | 0.0001 | 3.8883 | |
| L BA24/32 | (−6, 32, 20) | 137 | 0.0003 | 3.6452 | |
| R BA45/46 | (46, 32, 4) | 92 | 0.0003 | 3.6238 | |
| L Cerebellum | (−28, −72, −48) | 97 | 0.0003 | 3.6037 | |
| R BA32 | (14, 20, 30) | 95 | 0.0003 | 3.5874 | |
| L Cerebellum | (−6, −80, −28) | 72 | 0.0009 | 3.3164 | |
| Non-rotated task PLS food > neutral | R BA 18/19 | (30, −80, 24) | 3200 | 0 | 10.5634 |
| L BA 18/19 | (−44, −84, 2) | 2314 | 0 | 8.4669 | |
| L BA 40 | (−48, −36, 48) | 139 | 0 | 7.0144 | |
| R BA 7 | (30, −62, 62) | 79 | 0 | 6.8714 | |
| R Cerebellum | (18, −42, −44) | 103 | 0 | 6.6146 | |
| R Insula | (52, 14, 0) | 209 | 0 | 6.5611 | |
| L BA 7 | (−22, −64, 42) | 364 | 0 | 6.5473 | |
| R BA 32 | (12, 12, 40) | 211 | 0 | 6.336 | |
| R BA 9 | (50, 4, 42) | 259 | 0 | 6.0134 | |
| R BA 20 | (42, −18, −26) | 119 | 0 | 5.906 | |
| L BA 6 | (−4, 12, 54) | 279 | 0 | 5.8971 | |
| L PHG/Hippocampus | (−20, −30, −8) | 181 | 0 | 5.8168 | |
| L Insula | (−40, 8, 2) | 131 | 0 | 5.6259 | |
| R BA 6 | (32, −8, 54) | 94 | 0 | 5.32 | |
| L Amygdala | (−32, 0, −24) | 136 | 0 | 4.843 | |
| R BA 2/3 | (54, −20, 30) | 73 | 0 | 4.351 | |
| R Thalamus | (4, −24, 0) | 71 | 0 | 4.3483 | |
| R Amygdala | (18, −2, −8) | 96 | 0 | 4.2853 | |
| L BA 6 | (−44, 0, 34) | 134 | 0.0001 | 3.9377 | |
| L BA 47 | (−40, 24, −10) | 103 | 0.0002 | 3.7485 | |
| L BA 4 | (−36, −22, 36) | 71 | 0.0005 | 3.4908 |
PLS, partial least squares.
Figure 2.
Obese subjects show greater engagement of brain network including posterior INS (left panel) and bilateral amygdala (right panel) to viewing food images, using a multivariate functional magnetic resonance imaging analysis approach, as described in the Methods section. A blue circle marks respective brain regions.
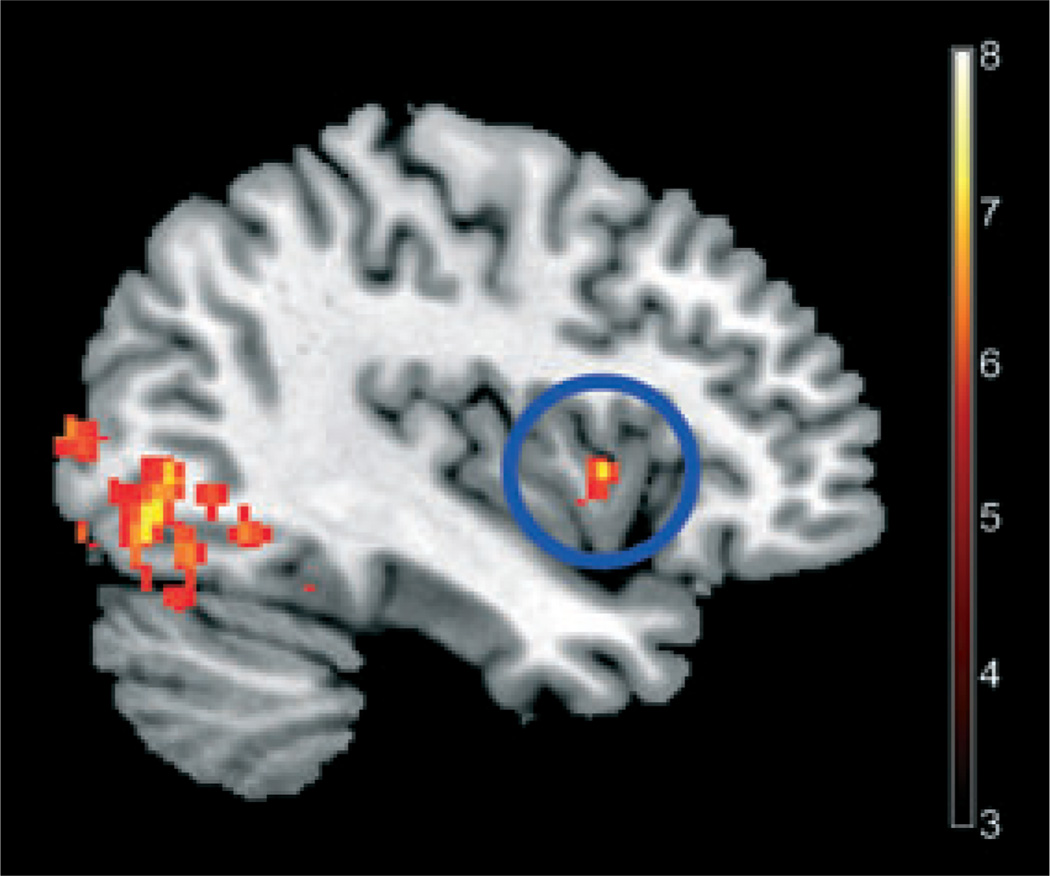
Using a non-rotated PLS, we found an additional network that was more engaged by obese subjects only after the high-calorie sucrose beverage compared with all other group/conditions. This network included bilateral anterior insula, right anterior cingulate cortex, bilateral amygdala, the left hippocampus, and the visual cortex (Table 3). This network accounted for 27% of the crossblock variance (P = 0.028; Fig. 3).
Figure 3.
Obese subjects had greater engagement of a network including the anterior INS (blue circle) following the high-calorie sucrose beverage, using multivariate functional magnetic resonance imaging analysis approach, as described in the Methods section (P < .001).
DISCUSSION
The main findings of the study are as follows: (i) Similar brain regions were engaged after ingestion of either sucrose or a non-nutrient sweetener while viewing hedonic food images; (ii) In both groups, regardless of drink type, the viewing of pleasant food images was associated with brain networks involving interoceptive, affective, and cognitive brain regions; (iii) While all subjects rated the taste and the satisfaction of the two drinks similarly, obese women rated both drinks as less tasteful and satisfying than lean women, consistent with a reduced hedonic response to the drinks; and (iv) This reduced behavioral hedonic response in the obese was associated with greater responses in affective and memory-related brain regions to both viewing of food images, and to the ingestion of the sucrose drink. Even though other interpretations are possible, these results suggest that in obese subjects, gut-derived sucrose-related signaling, presumably transmitted to the brain via vagal afferent and/or endocrine pathways, generates less hedonic effects than recalling memories of pleasant food in response to visual cues. As the latter response appears to be reinforced by sucrose ingestion, it may be a mechanism whereby sucrose ingestion perpetuates the craving for more sweets.
Similarities in brain responses to sucrose and non-nutrient sweetener
In the combined sample, similar brain regions were engaged after ingestion of the two test drinks while viewing hedonic food images. The regions included the bilateral amygdala, bilateral hippocampus, bilateral thalamus, and right anterior insula. These findings are consistent with the multidimensional encoding of interoceptive, affective, memory related, and cognitive aspects of the experience of a sweet test meal, as previously reported.23 Non-nutrient sweeteners bind to lingual taste receptors with equal, if not greater, affinity as sucrose.16,17 The similarity of the brain response between the two drinks suggests that the interoceptive, presumably vagally mediated input to the brain results primarily from lingual and possibly intestinal sweet taste receptor activation, and does not require other encoding mechanisms, which require glucose absorption and interaction with glucose-sensing mechanisms in the portal vein, the pancreas, and the brain (hypothalamus, nucleus tract solitarus).29,30 However, as blood glucose or insulin levels were not assessed in this study, the possible role of such endocrine signals in the observed brain responses cannot be answered directly.
Differences in subjective ratings of the test meal by obese and lean subjects
Despite the difference in caloric content between the two drinks, we found that subjects rated the taste of the sucrose and the non-nutrient sweetened beverage similarly, suggesting similar activation of lingual sweet taste receptors by the two drinks. Sweet taste perception of both non-nutrient sweeteners and sucrose is peripherally mediated by tongue heteromic T1R2/T1R3 sweet taste receptors. The sensory information is then transmitted by cranial nerves VII, IX, and X to the nucleus tract solitarus, and to the human gustatory cortex within the anterior insula.31 Previous studies have shown that artificial sweeteners bind to these taste receptors with equal, if not greater, affinity compared with sucrose.16,17,32 For example, it has been reported that both sucrose and sucralose applied lingually active functionally connected primary taste pathways and related brain regions.31
Obese subjects rated the taste of both beverages lower than lean subjects, and reported less satisfaction after consuming the beverages compared with lean subjects, consistent with a reduction in the hedonic aspect associated with ingestion of a sweet drink. The subjective perception of sweet taste is a multidimensional experience and reflects the modulation of anterior insula activity by inputs from interoceptive, affective, reward, and prefrontal/orbitofrontal inputs.21 Taste perception includes the assessment of taste quality, hedonic ‘liking’, and the incentive motivational component ‘wanting’.31,33 Reduced satisfaction to actual food ingestion (‘liking’), despite greater engagement of hedonic circuits during expectation of food intake (‘wanting’), has been proposed as a mechanism underlying food addiction.34 This pattern is similar to drug addiction where craving for the drug is amplified, whereas actual satisfaction after drug use is reduced. 34 In our study, the subjects were shown palatable images of food after beverage consumption and in-between behavioral measure assessment. Altogether, these findings imply that in obese subjects, gut-derived signaling generates less hedonic effects than recalling memories of food in response to visual cues.
Recall of food-related memories by visual stimuli and interaction with interoceptive stimuli
It has long been known that amnesic patients readily eat a second meal offered immediately after a full meal11, suggesting that memory recall of food-related experiences are as equally significant as caloric need in the decision to eat. Representations of food-related experiences are generated in networks involving the prefrontal and orbitofrontal cortices, anterior insula, amygdala, hippocampus, and reward pathways.8,33 Consistent with previous reports, we found that in both lean and obese subjects, regardless of drink type (and possibly differences in endocrine mediators), the viewing of pleasant food images was associated with activation of interoceptive, affective, and memory-related brain regions.35,36 The fact that we did not see a difference in BOLD response to the two drinks in the hypothalamus in either group or condition comparison may be related to the small sample size.
Obese subjects have an exaggerated response to food images after a sweetened drink
Although similar brain regions were engaged with both the sucrose and the non-nutrient sweetened beverage in the combined lean and obese groups, there were differences in brain responses when the obese women were compared with the lean group. When obese subjects viewed food images after the sucrose beverage (but not the non-nutrient sweetened drink), a network including the anterior insula, anterior cingulate cortex, right lateral amygdala, right hippocampus, and the visual cortex was more engaged compared with lean subjects. Similarly, Rothemund et al.37 found that high-calorie food images yielded BMI-dependent activations in regions associated with taste information processing (anterior insula and lateral orbitofrontal cortex), motivation (orbitofrontal cortex), and emotion and memory functions (posterior cingulate). These findings are consistent with the concept that viewing food images produces a brain response involving recall of previous food experiences, and that this response is exaggerated in obese subjects.
Although both obese and lean subjects showed the expected positive correlation between the subjective feeling of hunger with the engagement of insular cortex when viewing food images postprandially,38,39 obese subjects had a greater correlation of the right anterior insula with the subjective feeling of hunger. This suggests a greater modulation in the obese subjects of this brain region by emotional and reward pathways when craving for food. Anterior insula activation has also been demonstrated in association with the conscious feeling of urge in drug addiction.12
Our findings suggest that in obesity, additional interoceptive inputs other than that generated by lingual/intestinal sweet taste receptor activation may play a role in the associated brain response to a sweetened beverage in the context of viewing food cues. In addition to sweet taste receptor activation on intestinal enteroendocrine cells, glucose-sensing mechanisms have been described in the pancreas, portal vein, hypothalamus, and the nucleus of the solitary tract.29,30,40–42 Although group differences between lean and obese subjects in peripheral glucose, insulin, or incretin levels after sucrose intake may play a role, this study was not designed to identify such differences.
As an alternative to peripheral differences in glucose sensing, central differences between obese and lean populations might also explain our findings. In the drug addiction model, as addiction increases, stimuli within the environment that are associated with drug use (cigarettes, bottles of alcohol, drug paraphernalia) become powerful reinforcing incentives to drive ongoing drug use. This suggests that the interoceptive cortex has a central role in conscious cue-induced urges by encoding a representation of the salient effects of drug use that become activated when an addicted person is exposed to drug cues. It is believed that the amygdala and hippocampus are also involved in conditioning to addictive substances and relative cues in addiction.43 Similarly, in obesity, an addiction to sucrose might prompt increased engagement of this salience network when viewing images of palatable food after ingesting sucrose.
Limitations
A potential limitation to our study is the fact that the volumes of the food were the same for obese and lean, possibly contributing to the finding that obese subjects were less satiated – obese women might require a larger volume to feel full. However, the absence of statistical differences in hunger ratings pre and post beverage consumption suggests that volume did not play a significant role in the satiety measures. Similarly, group differences in gastric emptying may have contributed to the observed differences. The findings in rodent studies44 that no difference in gastric emptying after infusion of sucrose or an artificial sweetener is observed argue against a group difference. Another potential limitation is the fact that no direct measurements of glucose, insulin, and incretin levels at baseline and following the test meal were performed. While being an interesting target for future mechanistic studies, such measurements were not essential to address the main hypotheses of this study related to group differences in behavioral and brain responses between lean and obese subjects.
Summary and possible clinical implications
In summary, we found several obesity-related differences in behavioral and brain responses to sucrose vs a non-nutrient sweetened beverage. Obese women verbally reported a reduced hedonic behavioral response to either sweetened beverage, yet they demonstrated a greater hedonic brain response particularly after sucrose ingestion. This increased brain response is driven more by recalling memories of food experiences in response to visual cues than by lingual and gut-derived signaling. Despite the extensive literature on obesity-related changes in peripheral satiety mechanisms,6,7 the findings of this study are most consistent with a difference in central modulation of ingestive behavior in the obese, and are suggestive of food addiction.
Supplementary Material
Acknowledgments
FUNDING
This report was supported by a NIH/NIDDK grant (P30DK041301) and grants P50 DK064539, R01 DK048351, R24 AT002681, K01 DK071626.
Footnotes
DISCLOSURE
No competing interests declared.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Gostin LO. Law as a tool to facilitate healthier lifestyles and prevent obesity. JAMA. 2007;297:87–90. doi: 10.1001/jama.297.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 4.Covasa M. Deficits in gastrointestinal responses controlling food intake and body weight. AmJPhysiol Regul Integr Comp Physiol. 2010;299:R1423–R1439. doi: 10.1152/ajpregu.00126.2010. [DOI] [PubMed] [Google Scholar]
- 5.de Lartigue G, de La Serre CB, Ray-bould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M. Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul Pept. 2009;156:24–27. doi: 10.1016/j.regpep.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav. 2009;97:572–580. doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 12.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallner-Liebmann S, Koschutnig K, Reishofer G, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010;18:1552–1557. doi: 10.1038/oby.2010.26. [DOI] [PubMed] [Google Scholar]
- 14.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls:an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBois GE. Unraveling the biochemistry of sweet and umami tastes. Proc Natl Acad Sci USA. 2004;101:13972–13973. doi: 10.1073/pnas.0405991101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oudenhove L, Coen SJ, Aziz Q. Functional brain imaging of gastrointestinal sensation in health and disease. World J Gastroenterol. 2007;13:3438–3445. doi: 10.3748/wjg.v13.i25.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 23.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 24.Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49:675–690. [PubMed] [Google Scholar]
- 25.Baicy K, London ED, Monterosso J, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 27.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Matveyenko AV, Donovan CM. Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes. 2006;55:1276–1282. doi: 10.2337/db05-1665. [DOI] [PubMed] [Google Scholar]
- 30.Heijboer AC, Pijl H, van den Hoek AM, Havekes LM, Romijn JA, Cors-smit EP. Gutbrain axis: regulation of glucose metabolism. J Neuroendocrinol. 2006;18:883–894. doi: 10.1111/j.1365-2826.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 31.Frank GK, Oberndorfer TA, Simmons AN, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 32.Grimm ER, Steinle NI. Genetics of eating behavior: established and emerging concepts. Nutr Rev. 2011;69:52–60. doi: 10.1111/j.1753-4887.2010.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 36.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Wang GJ, Volkow ND, Telang F, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Thorens B. Glucose sensing and the pathogenesis of obesity and type 2 diabetes. Int J Obes (Lond) 2008;32(Suppl 6):S62–S71. doi: 10.1038/ijo.2008.208. [DOI] [PubMed] [Google Scholar]
- 41.Langhans W, Grossmann F, Geary N. Intrameal hepaticportal infusion of glucose reduces spontaneous meal size in rats. Physiol Behav. 2001;73:499–507. doi: 10.1016/s0031-9384(01)00479-6. [DOI] [PubMed] [Google Scholar]
- 42.Grabauskas G, Song I, Zhou S, Owyang C. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J Physiol. 2010;588:617–632. doi: 10.1113/jphysiol.2009.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, O’Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry. 2007;164:708–710. doi: 10.1176/ajp.2007.164.5.708. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–G739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.