Abstract
This study analyzes the clinical impact of NPM1 and FLT3 mutations in AML patients 65 years of age or older treated with cytotoxic chemotherapy at our institution. A total of 557 patients were retrospectively reviewed. No difference in survivalwasnoted for patients with isolated FLT3 orNPM1mutations. However, elderlyAMLpatients who were NPM1 mutated and FLT3 wild type had significantly improved survival with cytotoxic chemotherapy.
Background
The effect of prognostically important gene mutations (MUTs), nucleophosmin (nucleolar phosphoprotein B23, numatrin) (NPM1) and fms-related tyrosine kinase 3 (FLT3), in elderly patients with acute myeloid leukemia (AML) is not well defined.
Patients and Methods
We analyzed 557 patients, 65 years of age or older with newly diagnosed AML, treated at our institution between 2000 and 2010 with cytotoxic chemotherapy. NPM1 and FLT3 analysis were available in 146 patients (26%) and 388 patients (70%), respectively.
Results
NPM1 and FLT3 MUTs occurred in 16% and 12% of patients, respectively. No difference in median overall survival was observed between FLT3-MUT and NPM1-MUT patients who received cytotoxic chemotherapy. Outcome was significantly better among patients with NPM1-MUT/FLT3-wild type (WT) genotype (n = 14) compared with patients carrying FLT3/NPM1 genotypes other than NPM1-MUT/FLT3-WT (n = 125). The complete remission rates were 71% and 49%, respectively (P = .11). The median survival was 21.5 months vs. 9.0 months and estimated 2-year survival rates were 51% vs. 38%, respectively (P = .003). NPM1 and FLT3 MUTs appear to occur less frequently in elderly AML patients. The prognostic effect of isolated NPM1- or isolated FLT3-MUT is minimal.
Conclusion
Elderly AML patients with NPM1-MUT/FLT3-WT genotype have significantly improved outcomes compared with patients with other NPM1/FLT3 genotypes when treated with cytotoxic chemotherapy.
Keywords: Acute myeloid leukemia, Cytotoxic chemotherapy, Elderly, FLT3, NPM1
Introduction
Acute myeloid leukemia (AML) usually afflicts older people. The median age of patients with AML at presentation is 67 years.1 The prognosis of patients with AML is determined by a number of factors. Survival is worse in patients with older age, high risk cytogenetic characteristics (ie, -5, -7, monosomal or complex karyotypes), poor performance status, and comorbidities.2,3 Advanced age (typically defined as older than 65 years) is also associated with a higher probability of resistant disease, because older patients with de novo AML are more likely to have cytogenetic abnormalities similar to those seen in therapy-related AML.4 Induction chemotherapy with standard regimens (ie, cytosine arabinoside in combination with an anthracycline) in elderly patients with AML is associated with complete remission (CR) rates of 35% to 50%, treatment-related mortality of up to 30%, and a median overall survival (OS) of 5 to 6 months.5-9
Frequently recurring molecular mutations (MUTs) add valuable prognostic and predictive information for patients with newly diagnosed AML.10 This is most helpful for risk stratification of patients whose AML cells have a normal karyotype. In an analysis of 872 adults younger than 60 years of age with cytogenetically normal AML (CN-AML), nucleophosmin (nucleolar phosphoprotein B23, numatrin) (NPM1), fms-related tyrosine kinase 3 (FLT3) internal tandem duplication (FLT3-ITD), and neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS) MUTs were identified in 53%, 31%, and 13% of patients, respectively.11 Patients with mutated NPM1 in the absence of FLT3-ITD MUTs had higher CR rates, approaching those seen in core-binding factor AML. Other favorable risk molecular markers included the presence of biallelic CCAAT/enhancer binding protein (C/EBP), alpha (CEBPA) MUTs. Conversely, several reports have shown that patients harboring FLT3-ITD MUTs had a significantly higher induction death rate, increased relapse risk, inferior event-free survival, and shorter OS.12-16
The objective of this study was to analyze the incidence of FLT3 and NPM1 MUTs and better define the prognostic and predictive effect of these gene alterations on outcomes and response to therapy in elderly patients (age 65 years or older) with AML and treated with standard cytotoxic chemotherapy.
Patients and Methods
Patient Population
The medical records of patients older than 65 years of age with newly diagnosed AML treated at M.D. Anderson Cancer Center (MDACC) between January, 2000 and December, 2010 with frontline cytotoxic chemotherapy (including standard dose or high dose cytotoxic chemotherapy) were reviewed. Patients who received epigenetic therapy (ie, azacitidine or decitabine-based therapy) were excluded from the analysis. Patients with core-binding factor AML or acute promyelocytic leukemia (APL) were excluded because such AML subtypes are well established to be associated with significantly better prognosis than other AML subtypes. All clinical trials were approved by the Institutional Review Board, and were conducted in accordance with the Declaration of Helsinki.
Mutational Analysis
Genomic DNA from bone marrow aspirates was isolated using the Autopure extractor (QIAGEN/Gentra). FLT3-ITD and codon 835/836 tyrosine-kinase domain mutational status were determined using DNA from unsorted bone marrow aspirate samples obtained at initial presentation using a polymerase chain reaction (PCR)-based method with an analytical sensitivity of 1% to 2% MUT-bearing cells. FLT3- ITD and codon 835/836 point MUT levels were determined using semiquantitative DNA-based PCR-capillary electrophoresis assay, as described previously.17 PCR amplifications to detect NPM1 exon 12 MUTs, and exons 1 and 2 MUTs in NRAS and v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog genes were carried out as previously described.18,19 Conventional cytogenetic analysis (ie, G-banding technique) was also performed on bone marrow aspirate samples and results were reported according to the International System for Human Cytogenetics Nomenclature.20
Therapeutic Regimens
Standard AML induction chemotherapy for most patients at our institution is a combination of cytarabine at a dose of 1.5 g/m2 given continuously (intravenous) daily for 3 days and idarubicin 12 mg/m2/d for 3 days (referred to as the “AI regimen” herein). Other cytarabine- and noncytarabine-based regimens were also included. All patients who received frontline cytotoxic chemotherapy regimens (cytarabine-based and otherwise) were included. Patients who received frontline epigenetic therapy with hypomethylating agents (decitabine or azacitidine) were excluded.
Definitions of Outcomes
Acute myeloid leukemia was defined by the presence of ≥ 20% blasts in bone marrow aspirate material or peripheral blood, as previously published in the 2008 revision of the World Health Organization classification of myeloid neoplasms.21 Response criteria were based on the revised recommendations of the International Working Group.22 CR was defined as normal bone marrow morphology with ≤ 5% blasts, with recovery of neutrophils to ≥ 1 × 109/L and platelets to ≥ 100 × 109/L. CR with incomplete platelet recovery (CRp) was defined as CR with platelet count <100 × 109/L. Overall response rate (ORR) included all patients who achieved CR or CRp. Early mortality was defined as death occurring within 8 weeks of presentation. Cytogenetic risk groups were as follows: poor cytogenetic risk included abnormalities in chromosome 5 or 7, complex karyotype (ie, 3 or more abnormalities), and trisomy 8; good cytogenetic risk included the core-binding factor abnormalities, inv(16) or t(8;21) and APL [t(15;17)]; intermediate cytogenetic risk included diploid karyotype, and abnormalities that did not fall into the poor or good risk categories.
Statistical Considerations
Differences among variables were evaluated using χ2 for categorical variables and Kruskal-Wallis and Mann-Whitney U tests for continuous variables. A Cox proportional hazard regression module was used to determine the independent predictors of survival. Time-to-event analyses were performed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. A 2-sided P value < .05 was considered statistically significant.
Results
Patient Characteristics
A total of 671 patients with newly diagnosed AML were older than 65 years of age at the time of presentation to our institution between January, 2000 and December, 2010. Five hundred fifty-seven patients (83%) received frontline cytotoxic chemotherapy and were included for analysis. One hundred fourteen (17%) patients received epigenetic therapy using either decitabine or azacytdine and were excluded from our study. Table 1 shows baseline characteristics for the 557 patients treated with cytotoxic chemotherapy. NPM1- and FLT3-MUT analysis was available in 146 patients (26%) and 388 patients (70%), respectively. The incidence of NPM1- and FLT3-MUT was 16% and 12%, respectively. The discrepancy in the number of patients who had NPM1 and FLT3 mutational analysis (26% vs. 70%) is because of the fact that FLT3 mutational analysis has been available at MDACC since 2003 whereas NPM1 analysis became available in 2005. Also, as part of their research activities, our pathology department has retrospectively performed FLT3 analysis on stored bone marrow samples from 2000 to 2003.
Table 1.
Baseline Characteristics of Elderly Patients With AML Treated With Standard Cytotoxic Chemotherapy (n = 557)
| Patient Characteristics | n (%) or Median [Range] |
|---|---|
| Male Sex | 358 (64) |
| Age, Years | 71 [65-89] |
| ECOG Performance Status 0 to 2 | 527 (95) |
| White Blood Cells × 109/L | 5.8 [0.4-433.0] |
| Hemoglobin, g/dL | 8.3 [3.8-14.2] |
| Platelets × 109/L | 51 [3-787] |
| Bone Marrow Blast, % | 43 [0-98] |
| Peripheral Blast, % | 15 [0-99] |
| Cytogenetic Characteristics | |
| −5 or −7 alone | 15 (3) |
| −5 or −7 with other abnormalities | 127 (23) |
| Complex (≥ 3 abnormalities) | 42 (8) |
| Noncomplex (≤ 2 abnormalities) | 14 (3) |
| 11q23 | 98 (18) |
| Diploid | 238 (43) |
| Not done/insufficient metaphases | 23 (4) |
| MDACC Risk Model for 8-wk Mortality | |
| Low | 192 (34) |
| Intermediate | 334 (60) |
| High | 31 (6) |
| MDACC Risk Model for Survival | |
| Low | 90 (16) |
| Intermediate | 352 (63) |
| High | 115 (21) |
| NPM1 Status | |
| Mutated | 23 (16) |
| Wild Type | 123 (84) |
| FLT3 Status | |
| Mutated | 47 (12) |
| Wild Type | 341 (88) |
Abbreviations: AML = acute myeloid leukemia; ECOG = Eastern Cooperative Oncology Group; MDACC = M.D. Anderson Cancer Center.
Five hundred fifty-seven patients received standard chemotherapy. Of these, 178 patients (32%) received the AI regimen,23 93 (17%) received other high-dose cytarabine-based induction chemotherapy regimens,24-26 and 286 (51%) received other chemotherapy combinations, with or without conventional or low-dose cytarabine.
Effect of FLT3-MUT
Table 2 and Figure 1 show outcomes of cytotoxic chemotherapy according to FLT3-MUT status. Among the 388 patients who had FLT3-MUT analysis, 47 patients (12%) had the FLT3-MUT. Among the 47 FLT3-MUT patients receiving cytotoxic chemotherapy, 24 (51%) achieved CR and 2 (4%) achieved CRp, for an ORR of 55%. Median OS, 2-year, and 5-year survival rates were 5.8 months, 4%, and 4%, respectively.
Table 2.
Outcomes According to FLT3 Mutational Status in Elderly AML Patients Treated With Cytotoxic Chemotherapy (n = 388)
| Variable | FLT3-MUT | FLT3-WT |
|---|---|---|
| Patients, n | 47 | 341 |
| Response Rates, n (%) | ||
| CR | 24 (51) | 154 (45) |
| CRp | 2 (4) | 18 (5) |
| Mortality (< 8 wk) | 6 (13) | 53 (16) |
| Mortality (> 8 wk) | 0 | 2 (1) |
| No response | 15 (32) | 114 (33) |
| OS | ||
| Median, mo | 5.8 | 8.8 |
| 2-y | 4% | 23% |
| 5-y | 4% | 8% |
| P | .06 |
Abbreviations: AML = acute myeloid leukemia; CRp = CR with incomplete platelet recovery; MUT = mutation; OS = overall survival; WT = wild type.
Figure 1.
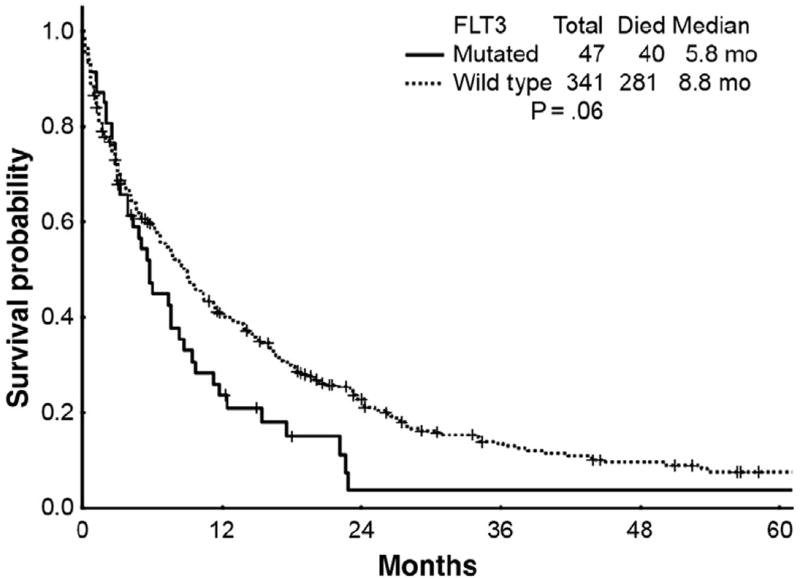
Survival Curves for Patients With Mutated FLT3 (n = 47) Alleles vs. Patients With Wild Type FLT3 (n = 341) Alleles Who Received Cytotoxic Chemotherapy (P = .06)
There were a total of 341 elderly AML patients who carried wild type (WT) FLT3 alleles (FLT3-WT) and received cytotoxic chemotherapy. One hundred fifty-four (45%) achieved a CR and 18 (5%) achieved a CRp for an ORR of 50%. Median OS, 2-year, and 5-year survival rates were 8.8 months, 23%, and 8%, respectively. Thus, FLT3-WT patients have a trend toward improved survival with cytotoxic chemotherapy compared with FLT3-MUT patients (P = .06).
Effect of NPM1-MUT
Next, we analyzed the response to cytotoxic chemotherapy according to NPM1-MUT status (Table 3 and Figure 2). Overall, 23 (16%) of the 146 patients that underwent NPM1-MUT analysis harbored an NPM1-MUT. The CR, CRp, and ORR rate for NPM1-MUT patients who received cytotoxic chemotherapy were 70%, 9%, and 79%, respectively. The median OS for NPM1-MUT patients who received cytotoxic chemotherapy was 11.8 months with 2-year and 5-year survival rates of 37% and 28%, respectively.
Table 3.
Outcomes According to NPM1 Mutational Status in Elderly AML Patients Treated With Cytotoxic Chemotherapy (n = 146)
| Variable | NPM1-MUT | NPM1-WT |
|---|---|---|
| Patients, n | 23 | 123 |
| Response Rates, n (%) | ||
| CR | 16 (70) | 58 (47) |
| CRp | 2 (9) | 8 (7) |
| Mortality (< 8 wk) | 2 (9) | 17 (14) |
| Mortality (> 8 wk) | 0 | 1 (1) |
| No response | 3 (12) | 39 (31) |
| OS | ||
| Median, mo | 11.8 | 9.7 |
| 2-y | 37% | 22% |
| 5-y | 28% | 8% |
| P | .13 |
Abbreviations: AML = acute myeloid leukemia; CRp = CR with incomplete platelet recovery; MUT = mutation; OS = overall survival; WT = wild type.
Figure 2.
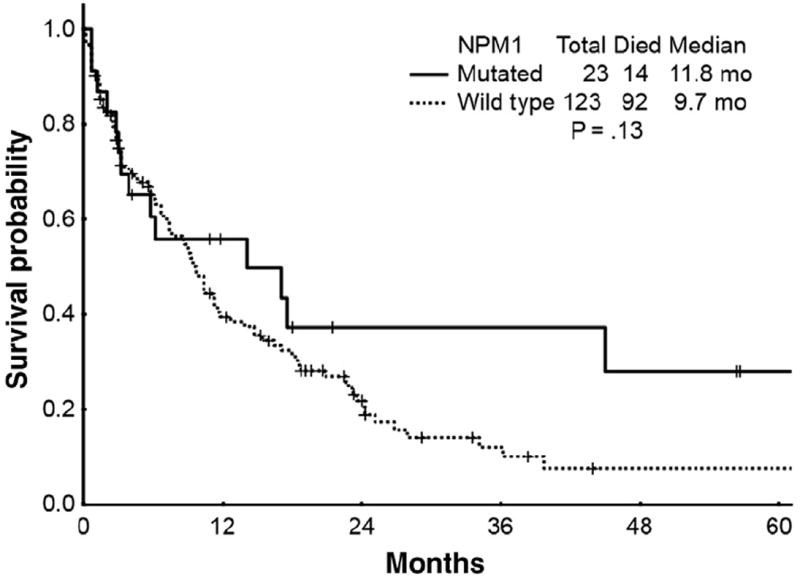
Survival Curves for Patients With Mutated NPM1 (n = 23) Alleles vs. Patients With Wild Type NPM1 (n = 123) Alleles Who Received Cytotoxic Chemotherapy (P = .13)
A total of 123 patients carried WT NPM1 alleles (NPM1-WT). CR, CRp, and ORR rates for these patients were 47%, 7%, and 54%, respectively. Median OS, 2-year, and 5-year survival rates were 9.7 months, 22%, and 8%, respectively. Thus, there was no significant difference in survival in patients given cytotoxic chemotherapy for NPM1-MUT vs. NPM1-WT (P = .13).
Effect of Combined NPM1- and FLT3-MUT
Although NPM1- and FLT3-MUT status are prognostic when considered independently, combined analysis of both molecular markers further refines risk stratification, particularly among patients with CN-AML. A total of 139 patients had available mutational analysis results for FLT3 and NPM1 (Table 4 and Figure 3). Fourteen patients (10%) had FLT3-WT and NPM1-MUT (FLT3-WT/NPM1-MUT). Chemotherapy resulted in CR in 71%, and CRp in 7% for an ORR rate of 78% with median OS, 2-year, and 5-year survival rates of 21.5 months, 51%, and 20%, respectively.
Table 4.
Outcomes According to Combined FLT3 and NPM1 Mutational Status in Elderly AML Patients Treated With Cytotoxic Chemotherapy (n = 139)
| Variable | NPM1-MUT/FLT3-WT | Other Genotypes |
|---|---|---|
| Patients, n | 14 | 125 |
| Response Rates, n (%) | ||
| CR | 10 (71) | 61 (49) |
| CRp | 1 (7) | 9 (7) |
| Mortality (< 8 wk) | 1 (7) | 18 (14) |
| Mortality (> 8 wk) | 0 | 1 (1) |
| No response | 2 (15) | 36 (29) |
| OS | ||
| Median, mo | 21.5 | 9.0 |
| 2-y | 51% | 38% |
| 5-y | 20% | 6% |
| P | .003 |
Abbreviations: AML = acute myeloid leukemia; CRp = CR with incomplete platelet recovery; MUT = mutation; OS = overall survival; WT = wild type.
Figure 3.
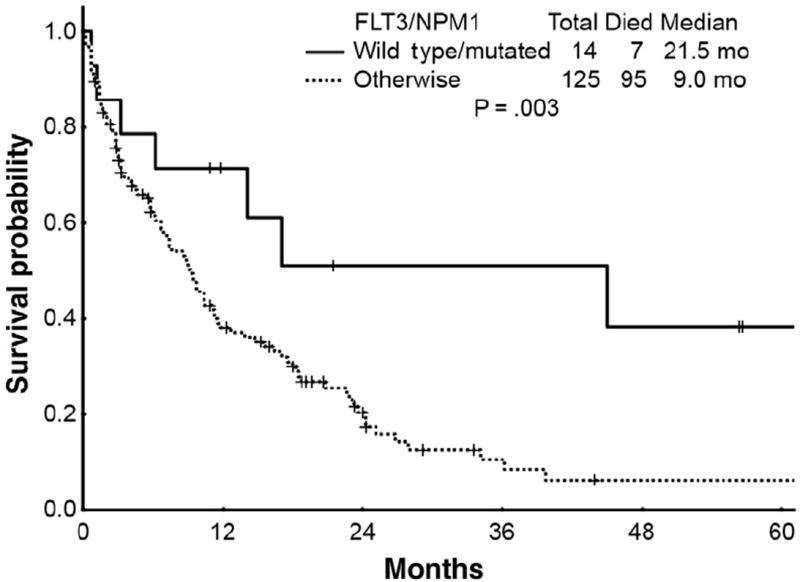
Survival Curves for Patients With Wild Type FLT3 and Mutated NPM1 (n = 14) Genotype vs. Patients With Other FLT3/NPM1 (n = 125) Genotypes (P = .003)
The remaining 125 patients (90%) who had combined FLT3/NPM1 mutational genotypes that were not FLT3-WT/NPM1-MUT received cytotoxic chemotherapy with a CR rate of 49% and a CRp rate of 7% (ORR rate of 56%). Median OS, 2-year, and 5-year survival rates were 9.0 months, 38%, and 6 %, respectively, which were inferior to those achieved by patients with NPM1-MUT/FLT3-WT (P = .003). Thus, patients with the NPM1-MUT/FLT3-WT genotype have significantly improved outcomes compared with patients with other NPM1/FLT3 genotypes when treated with cytotoxic chemotherapy.
Discussion
The traditional predictors of poor outcome in AML include age,2 high-risk cytogenetic characteristics,27 the presence of an antecedent hematological disorder, and AML arising after chemotherapy or radiation therapy for other malignancies or diseases.28 Advanced age (typically defined as 65 years or older) independently predicts a higher incidence of induction (8-week) mortality, lower CR rates, and shorter OS.29-34 Advanced age is more frequently associated with unfavorable cytogenetic characteristics (involving chromosome 5, 7, and 17),5,35 antecedent hematological disorders, inferior performance status, and high expression of multidrug resistance transport protein 1.32,33 In younger patients, the presence of MUTs in the alleles encoding the NPM1 or CEBPA have been linked to favorable outcomes, particularly among patients with normal karyotypes. The presence of NPM1-MUT represents a very favorable prognostic marker for CR during standard induction chemotherapy. 11,36 FLT3-ITD MUTs, in contrast, have been associated with lower CR rates and shorter OS.37 The effect of these gene MUTs on the outcomes of elderly patients (age 65 years or older) with AML has not been well established.
In the cohort presented herein, NPM1- and FLT3-MUT status was available in 146 (26%) and 388 (70%) of the patients reviewed, respectively. The incidence of NPM1-MUT and FLT3-MUT were 16% and 12%, respectively. These are markedly lower than the incidence of NPM1-MUT and FLT3-MUT reported in younger AML counterparts (approximately 50% and approximately 35%, respectively).11,13 Teleologically, this could be explained by the fact that high-risk cytogenetic characteristics are more prevalent among patients of advanced age, perhaps ameliorating the selection pressure to acquire more virulent genotypes via MUT of genes such as FLT3. Stirewalt et al, however, have reported that FLT3-ITD MUTs occur in 34% of evaluable elderly patients with AML,38 in stark contrast with our patient population. The reason for the difference in incidence rates across our studies remains poorly understood. However, in accord with Stirewalt et al and in contrast with studies in younger patients with AML, we found that FLT3-MUT was not associated with significantly inferior clinical outcomes in our patient cohort, although there was a trend to improved OS in FLT3-WT patients who received cytotoxic chemotherapy (P = .06). A possible explanation for the lack of clear prognostic value of FLT3-MUT in elderly AML patients as opposed to their younger counterparts might relate to the fact that elderly AML patients have very poor outcomes because of a much higher frequency of other powerful poor prognostic factors (eg, advanced age, comorbidities, p53 MUTs). The presence of the latter might diminish the negative effect of FLT3-ITD MUTs on survival.
We could not detect significant differences in outcomes among patients treated with cytotoxic chemotherapy according to isolated FLT3 or NPM1 mutational analyses. However, as expected, elderly patients with a FLT3-WT/NPM1-MUT genotype fared significantly better than patients with other FLT3/NPM1 genotypes when treated with cytotoxic chemotherapy. These results are in keeping with series of patients with AML including younger counterparts. The FLT3-WT/NPM1-MUT combination seems to confer a highly favorable prognostic effect with excellent CR rates and OS. In our patient cohort, the median OS for patients with the NPM1-MUT/FLT3-WT genotype was nearly 22 months, which was significantly longer than the OS noted in other genotypic combinations including NPM1-MUT/FLT3-MUT, NPM1-WT/FLT3-WT, or NPM1-WT/FLT3-MUT. Although less of a concern in patients of advanced age compared with younger counterparts, it has been suggested that the NPM1-MUT/FLT3-WT genotype might serve as a predictive marker for prolonged relapse-free survival, and that patients harboring such a genotype might not benefit from an allogeneic stem cell transplant in first CR, especially in elderly patients who have a tendency to do poorly with allogeneic transplantation.36 Thus, elderly AML patients with the NPM1-MUT/FLT3-WT genotype might benefit from a frontline cytotoxic chemotherapeutic approach.
Conclusion
In contrast to available data in younger patients with AML, NPM1-MUT and FLT3-MUT appear to occur at lower frequencies. The prognostic effect of each individual MUT is minimal for elderly AML patients treated with cytotoxic chemotherapy. Patients with the NPM1-MUT/FLT3-WT genotype have significantly improved outcomes compared with patients with other NPM1/FLT3 genotypes when treated with cytotoxic chemotherapy.
Clinical Practice Points.
NPM1 and FLT3 mutations appear to occur less frequently in elderly AML patients when compared to their younger counterparts.
The prognostic effect of isolated FLT3 and NPM1 mutations is minimal for elderly AML patients treated with cytotoxic chemotherapy.
Elderly AML patients who are NPM1 mutated/FLT3 wild-type have improved outcomes compared with other NPM1/FLT3 genotypes, when treated with cytotoxic chemotherapy.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: [April 2013]. [Web site]. Available at: http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 2.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–15. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 3.Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:89–99. doi: 10.1002/ajh.22246. [DOI] [PubMed] [Google Scholar]
- 4.Estey EH. Older adults: should the paradigm shift from standard therapy? Best Pract Res Clin Haematol. 2008;21:61–6. doi: 10.1016/j.beha.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Frohling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–8. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 7.Farag SS, Archer KJ, Mrozek K, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–9. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29:475–86. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 11.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 12.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 13.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 14.Schnittger S, Schoch C, Kern W, et al. FLT3 length mutations in AML: correlation to cytogenetics, FAB-subtype, and prognosis in 652 patients. Blood. 2000;96 doi: 10.1182/blood.v100.1.59. abstract 826a. [DOI] [PubMed] [Google Scholar]
- 15.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80. [PubMed] [Google Scholar]
- 17.Lin P, Jones D, Medeiros LJ, et al. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am J Clin Pathol. 2006;126:530–3. doi: 10.1309/JT5BE2L1FGG8P8Y6. [DOI] [PubMed] [Google Scholar]
- 18.Jones D, Yao H, Romans A, et al. Modeling interactions between leukemia-specific chromosomal changes, somatic mutations, and gene expression patterns during progression of core-binding factor leukemias. Genes Chromosomes Cancer. 2010;49:182–91. doi: 10.1002/gcc.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konoplev S, Huang X, Drabkin HA, et al. Cytoplasmic localization of nucleophosmin in bone marrow blasts of acute myeloid leukemia patients is not completely concordant with NPM1 mutation and is not predictive of prognosis. Cancer. 2009;115:4737–44. doi: 10.1002/cncr.24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaffer LG, Tommerup N, editors. ISCN 2005: An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S Karger; 2005. [Google Scholar]
- 21.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Estey EH, Kantarjian H, Keating M. Idarubicin plus continuous-infusion highdose cytarabine as treatment for patients with acute myelogenous leukemia or myelodysplastic syndrome. Semin Oncol. 1993;20:1–5. [PubMed] [Google Scholar]
- 24.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 25.Jabbour E, Kantarjian H, Ravandi F, et al. A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer. 2011;117:1236–44. doi: 10.1002/cncr.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estey EH, Thall PF, Pierce S, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/- all-trans retinoic acid +/- granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–84. [PubMed] [Google Scholar]
- 27.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33. [PubMed] [Google Scholar]
- 28.Goldstone AH, Burnett AK, Avivi I, et al. Secondary acute myeloid leukaemia has a worse outcome than de novo AML, even taking into account cytogenetics and age. AML 10,11,12 MRC trials. Blood. 2002;100 abstract 88a. [Google Scholar]
- 29.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 30.Schoch C, Kern W, Schnittger S, et al. The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica. 2004;89:1082–90. [PubMed] [Google Scholar]
- 31.Buchner T, Berdel WE, Wormann B, et al. Treatment of older patients with AML. Crit Rev Oncol Hematol. 2005;56:247–59. doi: 10.1016/j.critrevonc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estey E, Smith TL, Keating MJ, et al. Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia. 1989;3:257–63. [PubMed] [Google Scholar]
- 34.Estey EH, Thall PF, Cortes JE, et al. Comparison of idarubicin + ara-C-, fludarabine + ara-C, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98:3575–83. doi: 10.1182/blood.v98.13.3575. [DOI] [PubMed] [Google Scholar]
- 35.Moorman AV, Roman E, Willett EV, et al. Karyotype and age in acute myeloid leukemia. Are they linked? Cancer Genet Cytogenet. 2001;126:155–61. doi: 10.1016/s0165-4608(00)00414-3. [DOI] [PubMed] [Google Scholar]
- 36.Schlenk RF, Dohner K. Impact of new prognostic markers in treatment decisions in acute myeloid leukemia. Curr Opin Hematol. 2009;16:98–104. doi: 10.1097/MOH.0b013e3283257adb. [DOI] [PubMed] [Google Scholar]
- 37.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 38.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–95. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]


