Abstract
Amygdala dysfunction has been proposed as a critical contributor to social impairment in autism spectrum disorders (ASD). The current study investigated biochemical abnormalities in the amygdala in 20 high functioning adults with autistic disorder or Asperger’s disorder and 19 typically developing adults matched on age and IQ. Magnetic resonance spectroscopy was used to measure n-acetyl aspartate (NAA), creatine/phosphocreatine (Cre), choline/choline containing compounds (Cho), and Myoinositol (mI) in the right and left amygdala. There were no significant between-group differences in any of the metabolites. However, NAA and Cre levels were significantly correlated to clinical ratings on the Autism Diagnostic Interview-Revised. This suggests that altered metabolite levels in the amygdala may be associated with a more severe early developmental course in ASD.
Keywords: amygdala, autism, Asperger’s disorder, MRS
Social deficits are a core feature of individuals with autism that persist throughout the lifespan. The search for the neurobiological substrates of social dysfunction in autism has focused on the limbic system, with special attention paid to the amygdala. Amygdala abnormalities were initially hypothesized as a potential candidate involved in the pathophysiology of autism by Baron-Cohen and colleagues (Baron-Cohen et al., 2000). The amygdalae play a key role in social cognition and are essential for recognizing emotions, engaging in appropriate social interactions, and evaluating the emotional and/or social value of the stimulus being perceived (Adolphs, 2001; Adolphs et al., 1999; Bachevalier & Loveland, 2006; Baron-Cohen et al., 2000).
Although neural abnormalities in autism are widespread and heterogeneous, there is a growing body of evidence to suggest that the amygdala is impaired in autism and the degree of amygdala dysfunction may be critical to mediating the severity of social deficits in individuals with autism spectrum disorders (ASD). Social dysfunction may arise from structural and biochemical abnormalities in the amygdala. Cross-sectional studies of amygdala volume indicate an abnormal developmental trajectory. Amygdala growth is characterized by enlargement in very young children with ASD (Munson et al., 2006; Schumann et al., 2004; Sparks et al., 2002), followed by size normalization or even volume reduction relative to controls in adolescents and adults (Aylward et al., 1999; Nacewicz et al., 2006; Pierce, Muller, Ambrose, Allen, & Courchesne, 2001). The degree to which the pattern of amygdala growth deviates from the normal developmental trajectory may be related to clinical severity. In a study of young children with ASD, Munson et al. (Munson et al., 2006) found larger right amygdalar volume at age 3–4 years was predictive of a more severe clinical course from 3–6 years of age, as reflected in rate of acquisition of social and communicative skills. In contrast, adolescents and adults with the smallest amygdala volumes exhibited the most severe current deficits in processing facial emotions and the greatest severity on childhood nonverbal social behaviors estimated from the ADI-R (Nacewicz et al., 2006). This theoretical developmental model posits that the most severely affected individuals undergo excessive amygdalar growth early in childhood and subsequent atrophy in adolescence and adulthood. This pattern has been suggested to be the result of hyperactivity that leads to excitotoxic changes in the amygdala (Nacewicz et al., 2006).
Abnormal amygdala functional activation in ASD has been reported in response to emotional face processing (Ashwin, Baron-Cohen, Wheelwright, O’Riordan, & Bullmore, 2006; Critchley et al., 2000), discrimination (Dalton et al., 2005), and attribution (Wang, Dapretto, Hariri, Sigman, & Bookheimer, 2004) and consists of abnormally increased activation (Dalton et al., 2005), decreased activation (Ashwin et al., 2006; Baron-Cohen et al., 1999; Critchley et al., 2000), reduced task-related modulation of the amygdala (Ashwin et al., 2006; Wang et al., 2004; Williams et al., 2006), and reduced functional connectivity (Kleinhans et al., 2008).
A handful of studies have investigated the biochemical integrity of the amygdala in-vivo using magnetic resonance spectroscopy with mixed results. Reduced NAA, a marker of neuronal integrity, is the most consistent finding (Endo et al., 2007; Gabis et al., 2008; Otsuka, Harada, Mori, Hisaoka, & Nishitani, 1999) but one group found no difference from controls (Page et al., 2006). Other reports include increased levels of Cre and glutamate/glutamine (Glx) (Page et al., 2006), increased Cho/Cre (Gabis et al., 2008) and increased myo-inosotol (MI)/Cre (Gabis et al., 2008), in the amygdala-hippocampal region in the ASD group. Though ¾ studies found reduced NAA in the amygdala-hippocampal region, methodological differences may complicate interpretation of the results. Two of the three studies reporting NAA reduction used a ratio approach which is dependent on an assumption of normal levels of creatine in both patients and controls (Pfefferbaum, Adalsteinsson, Spielman, Sullivan, & Lim, 1999). Page et al (2006), who did not report reduced NAA in the amygdala, reported abnormally increased levels of Cre in their ASD group. This raises the question as to whether previously reported reductions in NAA/Cre may be driven by abnormally high levels of Cre.
The purpose of our study was to address two main questions: 1) do high-functioning individuals with ASD have biochemical alterations in the amygdala and 2) are biochemical alterations in the amygdala related to clinical severity or levels of anxiety/social avoidance? Few studies have addressed the questions of biochemical abnormalities in the amygdala in ASD. Our study makes an important contribution to the literature by studying rigorously diagnosed individuals with ASD and a control comparison group matched on age, gender, and IQ. Further, metabolite concentrations were studied using institutional units instead of ratios, removing the potential confound of incorporating abnormal levels of Cre into the measurement. We predicted that the ASD group would have reduced levels of NAA compared to the control group. We further tested whether there was a correlation between social dysfunction and neural integrity in the amygdala. While some theorize that the relationship between social dysfunction and amygdala abnormalities in autism are likely (see e.g., Adolphs, Sears, & Piven, 2001; Baron-Cohen et al., 2000) Amaral et al suggested that amygdala pathology most likely contributes to comorbid anxiety and abnormal fears in ASD (Amaral, Bauman, & Schumann, 2003). In order to test whether amygdala dysfunction is related to level of social anxiety, the Social Avoidance and Distress Scale (SADS, Watson & Friend, 1969) was correlated to metabolite measures in the amygdalae. The relationship between social dysfunction and amygdala abnormalities was tested by correlating metabolite levels and the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 2000) social score and the Autism Diagnostic Interview-Revised (ADI-R, Lord, Rutter, & Le Couteur, 1994) social score.
Methods
Participants
Twenty-four adults with ASD and 23 controls participated in the proton magnetic resonance spectroscopy (1H-MRS) protocol. Control participants were screened for current and past psychiatric disorders, history of a developmental learning disability, and contraindications to magnetic resonance imaging. Four individuals with ASD and four typically developing controls were excluded due to poor data quality. All included participants had valid data for every measured metabolite (i.e., n-acetyl aspartate, choline and choline containing compounds, creatine/phosphocreatine, and myoinositol). Participants had full-scale IQ and verbal IQ ≥ 80 as measured by the Wechsler Adult Intelligence Scale, Third Edition. The included control group was composed of 2 women and 17 men. The included ASD group (n=20, 2 women) consisted of 9 individuals with autistic disorder and 11 individuals with Asperger’s disorder. Diagnoses were confirmed with the Autism Diagnostic Interview-Revised (ADI-R, Lord, Rutter, & Le Couteur, 1994), the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 2000), and clinical judgment based on all available information and DSM-IV criteria. Current treatment status was unknown. In addition, the Social Avoidance and Distress Scale (SADS, Watson & Friend, 1969) was administered to all study participants. There were no significant differences between the ASD and control groups on age, gender, verbal IQ, performance IQ, or full-scale IQ. Clinical and demographic information is reported in table 1.
Table 1.
Participant charateristics
| ASD (n=20) | Control (n=19) | p value | |||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | ||
| Age | 23.57 | (6.60) | 23.32 | (5.15) | .90 |
| Full scale IQ | 113.3 | (14.22) | 112.05 | (15.17) | .79 |
| Verbal IQ | 114.2 | (14.89) | 111.94 | (14.74) | .64 |
| Performance IQ | 109.3 | (16.05) | 109.16 | (16.22) | .98 |
| SADS | 16.4 | (6.98) | 3.57 | (4.40) | <.001 |
| ADOS subscales | |||||
| Communication | 3.9 | (1.30) | |||
| Social | 8.55 | (2.76) | |||
| ADI-R subscales | |||||
| Communication | 14.25 | (5.23) | |||
| Social | 16.85 | (5.28) | |||
| Repetitive behavior | 4.85 | (2.48) | |||
This study was approved by the University of Washington (UW) Human Subjects Institutional Review Board. Informed written consent was obtained from all study participants.
1H-MRS data acquisition
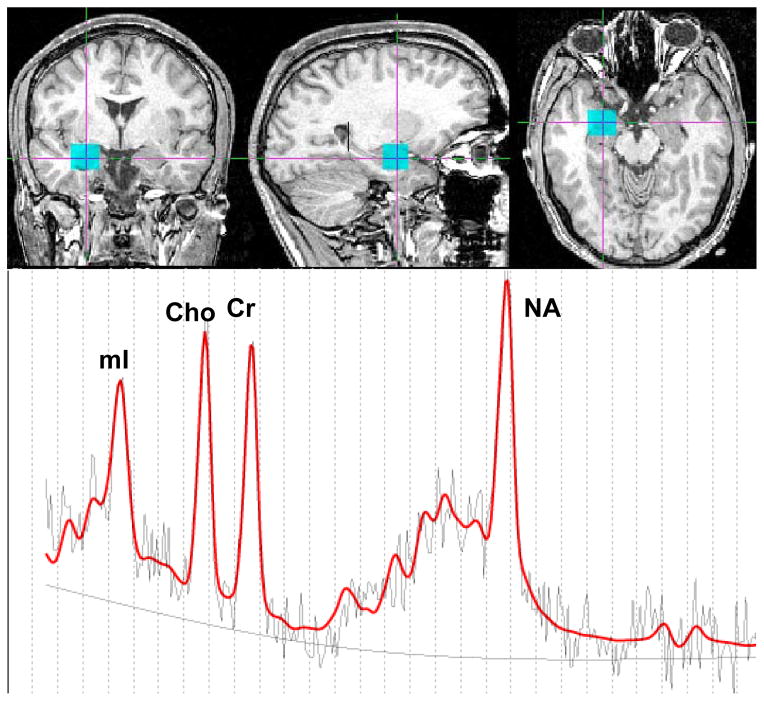
Scans were performed at the UW Diagnostic Imaging Sciences Research Center on a 1.5-T General Electric Signa Scanner (General Electric Medical Systems, Milwaukee, WI). Single-voxel proton magnetic resonance spectra were obtained from the left and right amygdala, often extending to the hippocampal region. See Figure 1 for an example of voxel placement and corresponding spectrum. 1H-MRS data were obtained with the automated GE pulse sequence PROBE-P, a PRESS sequence [TE = 30 ms, TR = 2000 ms, band width = 16k HZ, FOV = 24x24, 4 NEX, extended dynamic, voxel size = 15(A/P) x 15(S/I) x 15(R/L), 128 acquisitions, spectral width = 2500 Hz, and 2048 data points]. A high resolution anatomical SPGR was collected for anatomical localization and tissue segmentation of the 1H-MRS voxel. The following parameters were used: TR= 33 milliseconds, TE= minimum, flip angle =30°, field of view =24 cm, 256 x 256 matrix, scan thickness =1.5 mm, acquisition plane= coronal plane.
Figure 1.
Example right amygdala voxel location (blue box) and corresponding MRS spectrum. The red line in the MRS spectrum represents the LC-model fit for the underlying raw data, the black line is the least squares fit baseline, and the dotted line is the 0 line.
Example voxel placement and spectrum line fitting with LC Model.
1H-MRS data processing
Data were analyzed using software developed in our laboratory, written in MATLAB (5.3, Mathworks) and Fortran (Language Systems Fortran for the Macintosh and F77 Fortran for Silicon Graphics Unix operating system), which employed the LCModel package (Provencher, 1993) for spectral fitting. To optimize LCModel parameters, phantoms with known concentrations of brain metabolites were prepared and scanned at various acquisition settings. From these spectra, libraries of phantom data, or basis-sets, were created as detailed in the LCModel manual (Provencher SW. LcModel and LcMgui user’s manual. Available at: http://s-provencher.com/pages/lcm-manual.shtml). Metabolite concentrations were computed as detailed in the LCModel manual consistent with other techniques employing water-referencing (Barker et al., 1993; Brooks, Friedman, & Stidley, 1999). Following line-fitting, both metabolites and water amplitudes were adjusted for acquisition parameters and voxel size compared to the phantom data. Water amplitudes were adjusted using estimates of water molarity and attenuation (Barker et al., 1993) and multiplied by the tissue fraction within the voxel (see below for segmentation method). Dividing adjusted metabolite peak amplitudes by this corrected water term yields metabolite concentrations.
Segmentation of the 1H MRS voxels
The location and boundaries of the 1H MRS voxels were identified on each participant’s SPGR image via a MATLAB script. Gray matter, white matter, and cerebral spinal fluid (CSF) within the MRS voxels were segmented using MIPAV (Medical Image Processing, Analysis, and Visualization) and a plug-in, FANTASM (Fuzzy and Noise Tolerant Adaptive Segmentation Method), which is an extension of the fuzzy c-means algorithm (FCM) and the adaptive fuzzy c-means algorithm (AFCM) (http://iacl.ece.jhu.edu/projects/fantasm/). Each MRI voxel contained within the 1H MRS voxel was assigned to a tissue type (white matter, grey matter, CSF). Following this procedure, the percentage of CSF, gray matter, and white matter within the 1H MRS voxel was computed.
Results
The metabolite concentrations of the right and left amygdala-hippocampal regions were averaged together in order to reduce the data. Independent samples t-tests were run using SPSS for Windows 10. No significant between group differences were observed for any of the metabolites. No significant between-group differences were found between the proportion of grey matter or white matter within the MRS voxel (see table 2). Results of NAA, Cho, MI were also reported as ratios to Cre for informational purposes (see table 2). Semipartial correlational analyses were conducted to investigate the relationship between metabolite concentrations and clinical measures in the ASD group, controlling for age and FSIQ. Significant semipartial correlations were found between the ADI-R communication scale and NAA, ADI-R Restricted Interests scale and NAA and Cre, and the ADI-R Social scale and Cre in the ASD group (see table 3). These correlations indicate that individuals with the lowest current concentrations of NAA and Cre had greater clinical impairment as children. No significant semipartial correlations were found between the ADOS measures or the SADS and the metabolites. Pearson correlations between metabolite concentrations and IQ and the SADS were tested in the control group. Only NAA and FSIQ were significantly correlated (see table 3).
Table 2.
Concentrations of metabolites and tissue composition in the combined amygdala voxels.
| ASD (n=20) | Control (n=19) | % Diff | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Metabolite concentrations | ||||||
| N-acetyl aspartate | 8.586 | 0.531 | 8.362 | 0.534 | 2.68 | .20 |
| Choline | 2.119 | 0.197 | 2.143 | 0.315 | −1.16 | .77 |
| Creatine + phosphocreatine | 6.666 | 0.453 | 6.627 | 0.677 | 0.59 | .83 |
| myo-Inositol | 6.108 | 0.795 | 5.935 | 0.754 | 2.91 | .49 |
| Ratio to Cr + PCr | ||||||
| N-acetyl aspartate | 1.270 | 0.106 | 1.292 | 0.101 | −1.73 | .51 |
| Choline | 0.323 | 0.032 | 0.319 | 0.033 | 1.42 | .67 |
| myo-Inositol | 0.899 | 0.104 | 0.917 | 0.108 | −1.96 | .60 |
| Tissue composition | ||||||
| Gray matter volume (%) | 78.640 | 5.405 | 79.340 | 3.079 | −0.88 | .63 |
| White matter volume (%) | 16.810 | 5.523 | 16.790 | 2.857 | 0.12 | .99 |
| Average % SD | ||||||
| N-acetyl aspartate | 6.750 | 0.870 | 7.000 | 1.230 | −3.57 | .35 |
| Choline | 8.575 | 1.217 | 8.684 | 1.579 | −1.26 | .76 |
| Creatine + phosphocreatine | 8.650 | 1.122 | 8.921 | 1.124 | −3.04 | .36 |
| myo-Inositol | 10.325 | 2.200 | 10.868 | 1.948 | −5.00 | .26 |
Note. % Diff = ((ASD-Control)/Control)*100
Table 3.
Correlations between metabolite concentrations and clincial measures
| NAA | Cho | Cre | mI | ||
|---|---|---|---|---|---|
| ASD (n=20) | |||||
| ADI-R Communication | r | −.552* | .324 | −.321 | .046 |
| p value | .021 | .205 | .209 | .861 | |
| ADI-R Repetitive | r | −.612** | .003 | −.559* | −.114 |
| p value | .009 | .990 | .020 | .663 | |
| ADI-R Social | r | −.397 | −.027 | −.676** | −.047 |
| p value | .114 | .918 | .003 | .859 | |
| ADOS Communication | r | .281 | −.043 | .403 | .270 |
| p value | .274 | .870 | .109 | .295 | |
| ADOS Social | r | .080 | .025 | −.269 | .062 |
| p value | .761 | .924 | .296 | .812 | |
| SADS | r | .296 | .187 | .029 | −.136 |
| p value | .249 | .471 | .912 | .604 | |
| Control (n=19) | |||||
| SADS | r | .187 | −.057 | −.108 | .085 |
| p value | .444 | .816 | .660 | .731 | |
| FSIQ | r | 0.473* | .127 | .299 | .143 |
| p value | .041 | .605 | .214 | .558 | |
| VIQ | r | .359 | −.059 | .087 | −.019 |
| p value | .132 | .812 | .722 | .938 | |
| PIQ | r | .436 | .256 | .416 | .265 |
| p value | .062 | .290 | .077 | .273 | |
Note. Semipartial correlations, controlling for age and FSIQ, were conducted in the ASD group.
correlation is significant at p < .05
correlation is significant at p < .01
Discussion
This study used single-voxel MRS to investigate chemical metabolites within the amygdala in high-functioning individuals with ASD and typically developing controls matched on age, gender, and IQ. No significant between-group differences were found in overall level of NAA, Cre, Cho, or MI. However, several significant semipartial correlations were present between metabolite concentrations and measures of clinical severity even when controlling for the effects of age and FSIQ. Specifically, NAA and Cre were inversely correlated to the three ADI-R summary scores (see table 3). This finding is consistent with our previous fMRI study which found weaker functional connectivity between the fusiform face area and the amygdala was associated with greater clinical severity on the ADI-R but not the ADOS (Kleinhans et al., 2008). No correlation was found between any of the metabolites and measures of current social functioning or anxiety, suggesting that biochemical alterations within the amygdala may be specifically related to early developmental factors rather than current clinical features of the disorder. These results address the hypothesized link between the amygdala and social anxiety and avoidance in ASD. Amaral et al (2003) reported a series of elegant studies of maternally reared macaque monkeys who received bilateral amygdala lesions as infants. His group’s work found that the lesioned monkeys developed appropriate social behaviors including social interest, typical eye gaze, and emotional facial expressions. In contrast, abnormalities in fear processing were observed, such as difficulty evaluating dangerous objects or situations. This led to the proposal that the amygdala dysfunction may contribute to abnormal fear and anxiety levels in autism rather than causally contributing to the social dysfunction that typifies this disorder. Our study provides preliminary evidence that neuronal integrity and cellular metabolism in the amygdala in adults are related to early emerging clinical features of autism, but not current levels of social avoidance and anxiety. Thus, it is possible that the infant monkey lesion model may not fully reproduce the aberrant neurodevelopmental processes in autism spectrum disorders.
Correlations between IQ, anxiety and neurometabolites were also tested in the control group. Full-scale IQ and NAA were significantly correlated; a trend-level correlation was also found between Performance IQ and NAA. The relationship between metabolites in healthy young adults and intellectual level has not been widely studied. One research group has reported that higher IQ is associated with higher levels of NAA and lower levels of Cho in parietal-occipital white matter (Jung et al., 1999; Jung, Yeo, Chiulli, Sibbitt, & Brooks, 2000). A different study investigated the relationship between amygdala volume and intellectual ability and reported negative findings (Amat et al., 2008). Based on these reports, we suggest that the relationship between NAA and IQ in the controls most likely reflects general neural functioning rather than individual differences within cellular properties of the amygdala specifically.
The negative results of the between-group comparisons of the metabolites was unexpected given previous MRS reports of the biochemical alterations in the amygdala-hippocampal region (Endo et al., 2007; Gabis et al., 2008; Otsuka et al., 1999; Page et al., 2006) and a quantitative postmortem study that reported reduced numbers of neurons in the amygdala (Schumann & Amaral, 2006), and may be due to a lack of power. However, the reports in the literature differ from the current study on several important methodological factors. With the exception of Page et al., the other groups did not report ADI-R or ADOS scores precluding comparisons to the current study in terms of symptom severity. It is possible that our ASD group was higher functioning than others. Because ADI-R and metabolites levels were correlated, if our ASD group had been more severely affected, group differences in the metabolites may have been present. Secondly, half of the previous studies used ratios as opposed to institutional units to study brain metabolites. The use of ratios with Cre as the denominator may cause errors in interpretation if the metabolite is affected by the disease (Pfefferbaum et al., 1999). This is relevant to the study of autism, as growing evidence indicates that Cre levels are altered (Friedman et al., 2003; Page et al., 2006), and as reported in this study, possibly correlated to symptom severity. Lastly, the studies reporting the most striking abnormalities tested wide age ranges that included children through adolescents/young adults and failed to match on intellectual functioning. A series a cross-sectional studies suggest that individuals with autism appear to undergo a severe early neurodevelopmental course that may largely normalize during adolescence and adulthood (Aylward, Minshew, Field, Sparks, & Singh, 2002; Courchesne et al., 2001; Redcay & Courchesne, 2005; Schumann et al., 2004). Although this pattern was identified predominantly though volumetric and head circumference studies, it is possible that this developmental course also reflects pathological changes at the cellular level that could account for the lack of group differences in the metabolites assayed in the current study. Notably, the correlations between metabolite levels in adulthood and level of clinical impairment during early childhood suggests that subtle yet persistent residual neural effects remain.
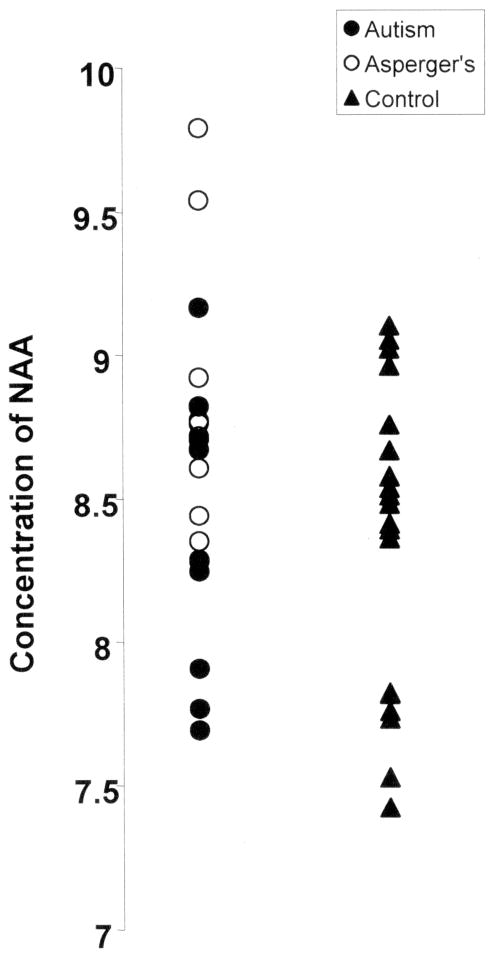
There are several limitations to the current study that warrant comment. We sought to investigate biochemical abnormalities in the amygdala in ASD and their relationship to clinical measures of social functioning and anxiety. However, since 1H MRS data was not collected from other brain regions, we are unable to determine whether these associations are specific to the amygdala or instead reflect a generalized brain process. Follow-up studies will be useful in resolving this question. This point is particularly important given that the MRS voxel likely included structures that extended beyond the amygdala. Second, this group was composed almost exclusively of very high functioning men with ASD. Thus, it is not certain whether the findings reported here are generalizable to the entire spectrum of clinical presentations, levels of functioning, females, and as noted earlier, ages. It is very possible that younger and lower functioning individuals with ASD may have more striking biochemical abnormalities than were observed in the current study. Third, we did not statistically correct for multiple comparisons and therefore our results may reflect type I error. Lastly, because of our relatively small sample, we had inadequate power to investigate differences between individuals with DSM-IV diagnoses of Autistic Disorder and Asperger’s Disorder within our sample. Visual inspection of the NAA data (see figure 2) suggests that the Asperger’s individuals may have increased NAA in the amygdala compared to those with autistic disorder. This would be consistent with a previous study that found increased NAA in ASD (with a sample comprised almost exclusively of individuals with Asperger’s Disorder)(Murphy et al., 2002) and our finding of an inverse correlation between the ADI-R and level of NAA. Obtaining a greater understanding of the pathophysiological mechanisms that underlie the varied clinical presentations in ASD may be important in pinpointing the etiology of these disorders.
Figure 2.
Scatter plot of average concentration of NAA in the right and left amygdala. NAA levels are reported in institutional units. No significant between group differences were found in NAA (p> .05). However, visual inspection suggests that studies with increased sample-sizes may identify significantly elevated NAA in Asperger’s compared to autistic disorder.
In conclusion, overall levels of brain metabolites that are measures of neuronal integrity, membrane turnover, and mitochondrial metabolism were intact in the amygdala in very high functioning individuals with ASD. We suggest that biochemical alterations previously reported in younger individuals with ASD may normalize during adolescence and adulthood. Further studies are needed to determine whether the same process of normalization occurs in lower-functioning adults. Lastly, within the ASD group, current metabolite levels were significantly associated with early clinical severity but not current social functioning or social anxiety. This suggests that putative cellular abnormalities linked to clinical symptomatology in early childhood have subtle, persistent effects that remain in very high functioning adults with ASD.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (U19 HD34565) and the National Institute of Mental Health (U54MH066399).
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2(5):295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Amat JA, Bansal R, Whiteman R, Haggerty R, Royal J, Peterson BS. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain and Cognition. 2008;66(2):105–114. doi: 10.1016/j.bandc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6(1):89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Friedman SD, Stidley CA. Reproducibility of 1H-MRS in vivo. Magn Reson Med. 1999;41(1):193–197. doi: 10.1002/(sici)1522-2594(199901)41:1<193::aid-mrm27>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Shioiri T, Kitamura H, Kimura T, Endo S, Masuzawa N, et al. Altered chemical metabolites in the amygdala-hippocampus region contribute to autistic symptoms of autism spectrum disorders. Biol Psychiatry. 2007;62(9):1030–1037. doi: 10.1016/j.biopsych.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, et al. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60(1):100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, et al. 1H-magnetic resonance spectroscopy markers of cognitive and language ability in clinical subtypes of autism spectrum disorders. J Child Neurol. 2008;23(7):766–774. doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL., Jr Biochemical markers of intelligence: a proton MR spectroscopy study of normal human brain. Proc Biol Sci. 1999;266(1426):1375–1379. doi: 10.1098/rspb.1999.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Brooks WM. Myths of neuropsychology: intelligence, neurometabolism, and cognitive ability. Clin Neuropsychol. 2000;14(4):535–545. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, et al. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, et al. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63(6):686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Critchley HD, Schmitz N, McAlonan G, Van Amelsvoort T, Robertson D, et al. Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59(10):885–891. doi: 10.1001/archpsyc.59.10.885. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63(12):1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41(7):517–519. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, et al. In Vivo 1H-Magnetic Resonance Spectroscopy Study of Amygdala-Hippocampal and Parietal Regions in Autism. 2006;163:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999;41(2):276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Watson D, Friend R. Measurement of social-evaluative anxiety. J Consult Clin Psychol. 1969;33(4):448–457. doi: 10.1037/h0027806. [DOI] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]