Abstract
Purpose
Depressive symptoms are common in cancer patients and their family caregivers (FCs). While these symptoms are characterized by substantial interindividual variability, the factors that predict this variability remain largely unknown. This study sought to confirm latent classes of oncology patients and FCs with distinct depressive symptom trajectories and to examine differences in phenotypic and genotypic characteristics among these classes.
Method
Among 167 oncology outpatients with breast, prostate, lung, or brain cancer and 85 of their FCs, growth mixture modeling (GMM) was used to identify latent classes of individuals based on Center for Epidemiological Studies-Depression (CES-D) scores obtained prior to, during, and for four months following completion of radiation therapy. One hundred four single nucleotide polymorphisms (SNPs) and haplotypes in 15 candidate cytokine genes were interrogated for differences between the two largest latent classes. Multivariate logistic regression analyses assessed effects of phenotypic and genotypic characteristics on class membership.
Results
Four latent classes were confirmed: Resilient (56.3%), Subsyndromal (32.5%), Delayed (5.2%), and Peak (6.0%). Participants who were younger, female, non-white, and who reported higher baseline trait and state anxiety were more likely to be in the Subsyndromal, Delayed, or Peak groups. Variation in three cytokine genes (i.e., interleukin 1 receptor 2 [IL1R2], IL10, tumor necrosis factor alpha [TNFA]), age,and performance status predicted membership in the Resilient versus Subsyndromal classes.
Conclusions
Findings confirm the four latent classes of depressive symptom trajectories previously identified in a sample of breast cancer patients. Variations in cytokine genes may influence variability in depressive symptom trajectories.
Keywords: depression, cancer, cytokines, genetics, growth mixture modelling, family caregivers, radiation therapy
Introduction
Depressive symptoms are experienced by 10%-40% of oncology patients (Massie, 2004; Pirl, 2004), have deleterious effects on quality of life (QOL) (Given et al., 2008; Rabin et al., 2008), and are associated with increased mortality (Satin et al., 2009). Family caregivers (FCs), who provide substantial physical and emotional support to patients (Yabroff and Kim, 2009), are at heightened risk for depressive symptoms (Institute of Medicine, 2007; Kim et al., 2005; Rhee et al., 2008), with similar negative consequences (Couper et al., 2006a; Couper et al., 2006b).
Despite the prevalence and impact of depressive symptoms in patients and FCs, most studies have examined these groups separately, based on assumptions that the stressors experienced by patients and FCs differ. However, recent evidence suggests that demographic, dispositional, and personality-related characteristics explain substantial variability in distress and depression in cancer patients (Deimling et al., 2006; Schou et al., 2004) and FCs (Carter and Acton, 2006), while disease characteristics explain relatively little (Bardwell et al., 2006; Deshields et al., 2006). Moreover, as underscored by the Stress Process model (Pearlin et al., 1990) and supported by both cancer and non-cancer literature (Gottlieb and Rooney, 2004; Hooker et al., 1998; Kim et al., 2008; Kurtz et al., 1997), FC stress is influenced by a FC's individual characteristics.
Cancer treatment and follow-up typically occur over long time periods (Deshields et al., 2006; Helgeson et al., 2004; Kurtz et al., 2004). While longitudinal studies have evaluated for changes in mean symptom scores, such approaches obscure underlying patterns (i.e., subgroups of individuals with similar symptom trajectories). Growth mixture modelling (GMM) is an approach that identifies latent classes with similar patterns of change (Muthen and Muthen, 2000). Few studies have employed GMM to identify latent classes of oncology patients with distinct symptom trajectories (Donovan et al., 2007; Helgeson et al., 2004; Henselmans et al., 2010; Hou et al., 2010; Lam et al., 2010; Lam et al., 2012; Legler et al., 2004; Rose et al., 2009).
In our previous study of patients with breast cancer (n=398), four latent classes were identified using Center for Epidemiological Studies-Depression (CES-D) scores assessed prior to and for six months after surgery (Dunn et al., 2011). The classes were named Resilient (38.9%), Subsyndromal (45.2%), Delayed (11.3%), and Peak (4.5%), based on the shape of the trajectories. Compared to the Resilient class, patients in the Subsyndromal class were younger and had higher anxiety scores prior to breast cancer surgery. However, differences in genotypic predictors between the two classes were not evaluated.
Genetic variation accounts for substantial heterogeneity in risk for depression (Levinson, 2006), primarily through mediation of neuroendocrine and immune pathways (Feder et al., 2009; Maes et al., 2009; Miller et al., 2009). Specifically, the cytokine signalling pathway is associated with inflammation, stress, and depression (Haroon et al., 2012; Miller et al., 2009). The “cytokine hypothesis of depression” is supported by findings of increased levels of cytokines in adults with major depression and in those with treatment-resistant depression (Maes et al., 1997; Zorrilla et al., 2001), as well as reductions in cytokine levels in individuals who respond to antidepressants (Miller et al., 2009). Pro-inflammatory cytokines may influence vulnerability to depression by reducing synthesis and increasing reuptake of key neurotransmitters associated with depression (Miller et al., 2009). For instance, pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα) and interleukin-1-beta (IL1β), upregulate the serotonin transporter (5-HTT) (Zhu et al., 2006). Sickness behavior, which includes symptoms of depression, is hypothesized to be associated with cytokine pathway dysregulation (Dantzer et al., 2008a; Dantzer and Kelley, 2007) and can be induced by administration of pro-inflammatory cytokines (Capuron et al., 2002; Dantzer et al., 2008b; Haroon et al., 2012; Raison et al., 2006; Reichenberg et al., 2001). For instance, administration of interferon-alpha to patients with melanoma or hepatitis C can induce depressive symptoms (Raison et al., 2006).
Preliminary evidence suggests that an underlying genetic predisposition exists for different levels of symptom severity in response to inflammation or other stressors. For instance, a common functional promoter polymorphism in the TNFA gene (c.G-308A) is associated with inflammatory diseases (Lee et al., 2007) and increased sleep disturbance (Aouizerat et al., 2009). However, relationships between cytokine gene variation and depressive symptoms in cancer patients and their FCs have not been evaluated.
Therefore, the purposes of this study, in a sample of oncology patients and their FCs, were to: confirm previously identified (Dunn et al., 2011) latent classes with distinct depressive symptom trajectories from the beginning to four months after the completion of RT; evaluate for differences in demographic, clinical, and underlying trait characteristics among these latent classes; and examine whether latent classes differed with respect to variation in a number of pro- and anti-inflammatory cytokine genes. Based on our prior work (Dunn et al., 2012), we predicted that latent classes would not be dependent on patient or FC status. Given substantial evidence for cytokine-induced sickness behaviour (Aouizerat et al., 2009; Capuron et al., 2002; Cerri et al., 2010; Collado-Hidalgo et al., 2008; Dantzer et al., 2008b; Haroon et al., 2012; Miaskowski et al., 2010; Raison et al., 2006; Reichenberg et al., 2001), we also hypothesized that variation in candidate cytokine genes would be associated with latent class membership.
Methods and Materials
Participants and Settings
Patients and their FCs were recruited from two radiation therapy (RT) departments located in a Comprehensive Cancer Center and a community-based oncology program at the time of the patient's simulation visit. Patients were eligible to participate if they were ≥18 years of age; were scheduled to receive primary or adjuvant RT for one of four cancer diagnoses (i.e., breast, prostate, lung, brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky Performance Status (KPS) score of ≥60. Patients were excluded if they had metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder. FCs were eligible to participate if they were adult (≥18 years of age); were able to read, write, and understand English; gave written informed consent; had a Karnofsky Performance Status (KPS) score of ≥ 60; were living with the patient; and did not have a diagnosed sleep disorder.
Self-Report Instruments
A demographic questionnaire obtained information on age, gender, marital status, education, ethnicity, employment status, and the presence of co-morbidities. Depressive symptoms were assessed using the CES-D scale (Radloff, 1977). A score of ≥16 is considered indicative of the need for clinical evaluation for major depression. State and trait anxiety were measured using the Spielberger State-Trait Anxiety Inventories (STAI-T and STAI-S) (Spielberger, 1983). Functional status was assessed using the Karnofsky Performance Status (KPS) scale (Karnofsky et al., 1948). Additional information on each of the measures is included in the Supplement.
Study Procedures
The study was approved by the institutional review boards at each of the research sites. At the time of simulation (i.e., approximately one week prior to the initiation of RT), patients and FCs were invited to participate. A research nurse explained the study protocol, determined eligibility, and obtained written informed consent.
At the time of the simulation visit (enrollment), participants completed self-report questionnaires. Subsequently, participants completed the CES-D at 4 weeks after the initiation of RT, at the end of RT (approximately 6-9 weeks later), and at 4, 8, 12, and 16 weeks after the completion of RT (i.e., 7 assessments over 6 months). In addition, patients' medical records were reviewed for disease and treatment information.
Analysis of the Phenotypic Data
Additional details on the phenotypic analyses are provided in the Supplement. Data were analyzed using SPSS version 19 (SPSS, 2010) and Mplus version 6.11 (Muthen and Muthen, 1998-2010). Descriptive statistics and frequency distributions were generated for the sample characteristics and symptom data. Independent samples t-tests, analyses of variance, and chi- square analyses evaluated for differences in demographic, clinical, and genotypic characteristics between patients and FCs and among the latent classes. A p-value of <.05 was considered statistically significant. Post hoc contrasts were done using the Bonferroni procedure.
GMM with robust maximum likelihood estimation was used to identify latent classes with distinct depressive symptom trajectories over the six months of the study (Muthen and Kaplan, 2004). Because 65% of participants were in patient-caregiver dyads, models were estimated with dyad as a clustering variable to ensure that any dependency between CES-D scores for patients and FCs in the same dyad was controlled for in the GMM analysis. After taking dependency within dyads into account, no significant differences were found between patients and FCs in the parameter estimates for GMM trajectories identified in the initial analysis.
Analysis of the Genomic Data
Additional details on the genomic analyses are provided in the Supplement.
Blood collection and genotyping
Genomic deoxyribonucleic acid (DNA) was extracted from archived buffy coats using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). Standard genotyping procedures were performed.
Gene and SNP selection
Fifteen genes for major pro- and anti-inflammatory cytokines and cytokine receptors were selected for analysis (Supplemental Table 1). The pro-inflammatory cytokines included interferon gamma (IFNG), IFNG receptor 1 (IFNGR1), interleukin 1 (IL1), IL1 receptor 1 (IL1R1), IL2, IL8, IL17A, nuclear factor-kappa B1 (NFKB1), NFKB2, and TNFA. Anti-inflammatory cytokines included IL1R2, IL4, IL10, and IL13. Cytokines with both pro- and anti-inflammatory functions (Seruga et al., 2008) included IFNG1, IL1B, and IL6. In total, 92 SNPs among the 15 candidate genes passed all quality control filters and were included in the genetic association analyses (see Supplementary Table 1).
Statistical Analyses for the Genetic Data
Hardy-Weinberg equilibrium was assessed by the Chi-square or Fisher Exact tests. Measures of linkage disequilibrium ((LD) i.e., D′ and r2) were computed using Haploview 4.2. LD-based haplotype block definition was based on D′ confidence interval (Gabriel et al., 2002). For SNPs in the same haploblock, haplotype analyses were done to detemine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1 (Stephens et al., 2001).
Ancestry informative markers (AIMS) were used to minimize confounding due to population stratification (Halder et al., 2008; Hoggart et al., 2003; Tian et al., 2008). Homogeneity in ancestry among participants was verified by principal component analysis (Price et al., 2006), using Helix Tree (Golden Helix, Bozeman, MT). One hundred and six AIMs were included in the analysis.
Three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value, was selected for each SNP. Haplotypes were evaluated assuming a dosage model.
A backwards stepwise approach was used to create the most parsimonious model. Except for self-reported race/ethnicity and AIMS, only predictors with a p-value of <.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using STATA version 9.
Results
Identification of Latent Classes Using GMM
The majority of participants were female, Caucasian, and well educated. Patients and FCs differed only by gender and marital status. Compared to patients, a greater proportion of FCs was female (p<0.0001) and married/partnered (p<0.0001).
Four distinct classes of depressive symptom trajectories were identified using GMM (Figure 1). A four-class solution provided the best model fit (Supplementary Table 2), as it had a reduced Bayesian information criterion, high entropy, and showed significant improvement over the three-class model (Jung and Wickrama, 2008).
Figure 1.
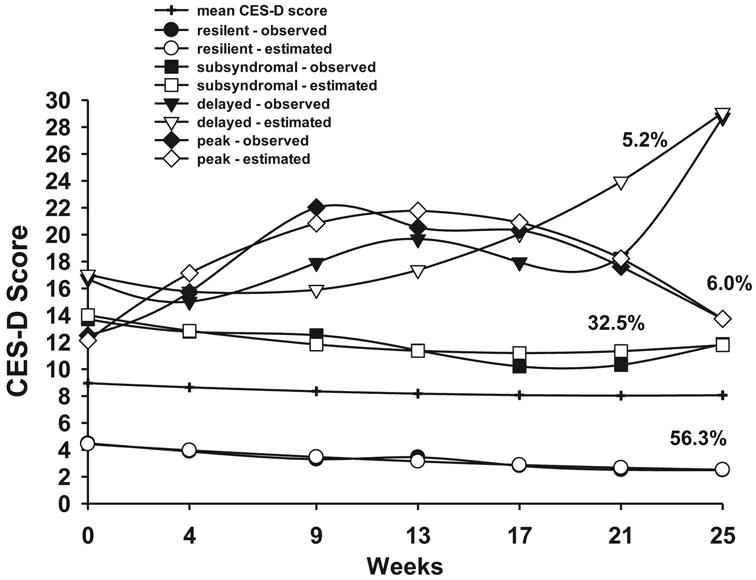
Observed and estimated CES-D trajectories for participants in each of the latent classes, as well as the mean CES-D scores for the total sample.
The parameter estimates for the four latent classes are listed in Supplementary Table 3. The largest percentage of participants was grouped in the Resilient class (56.3%). These participants had low CES-D scores at baseline (mean 4.6), which gradually declined over time. The second largest class, Subsyndromal (32.5%), had a mean baseline CES-D score of 14.7, which decreased slightly over time. The Delayed class (5.2%) had a slightly elevated mean baseline CES-D score (mean 16.4), with an initial decrease followed by an increase after the completion of RT. The Peak class (6.0%) had a lower mean CES-D score at baseline (mean 11.7) compared to the Subsyndromal group. This mean CES-D score increased during treatment, peaked after treatment, and decreased to baseline levels at six months.
Differences in Demographic and Clinical Characteristics Among the Latent Classes
Significant differences were found among the latent classes in age, gender, ethnicity, having children living at home, and KPS scores (Table 1). Specifically, participants in the Subsyndromal, Delayed, and Peak classes were younger than those in the Resilient class. Compared to the Resilient class, the Peak class had a higher proportion of women. Compared to the Subsyndromal class, the Resilient class had a higher proportion of White participants. Compared to the Resilient and Subsyndromal classes, a higher proportion of participants in the Peak class had children at home. Finally, no differences in the proportion of patients and FCs were found among the four latent classes.
Table 1. Differences in Baseline Demographic Characteristics Among the Four Latent Classes and Between Resilient and Subsyndromal Classes.
| Characteristic | Resilient (1) n=142 (56.3%) | Subsyndromal (2) n=82 (32.5%) | Delayed (3) n=13 (5.2%) | Peak (4) n=15 (6.0%) | Omnibus (4-group) Test Statistic and Post hoc Comparisons* | p-value for Resilient vs. Subsyndromal |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 64.7 (9.9) | 58.8 (10.9) | 53.0 (14.4) | 52.8 (12.3) | F(3,248)=12.13; p<0.0001; 1>2, 3, 4 | <0.001 |
| Education (years) | 16.1 (3.2) | 15.7 (2.9) | 15.6 (2.0) | 15.8 (2.9) | F(3,248)=0.33; p=0.801 | .39 |
| # Comorbid conditions | 4.3 (2.7) | 4.9 (2.9) | 4.8 (2.8) | 5.1 (2.2) | F(3,248)=1.10; p=0.35 | .11 |
| Weight (pounds) | 178.9 (37.6) | 171.7 (38.0) | 163.5 (44.9) | 171.8 (49.0) | F(3,245)=1.08; p=0.357 | .17 |
| KPS Score | 94.9 (9.2) | 87.5 (12.8) | 90.0 (16.0) | 89.3 (12.7) | F(3,242)=8.13; p<0.0001; 1>2 | <0.001 |
| n (%) | n (%) | n (%) | n (%) | |||
| Gender (% female) | 63 (44.4) | 50 (61.0) | 9 (69.2) | 13 (86.7) | χ2=14.53; p=0.002; 1<4 | .017 |
| Ethnicity (%) | ||||||
| White | 115 (81.0) | 52 (63.4) | 11 (84.6) | 10 (66.7) | χ2=9.66; p=0.028 | .011 |
| Asian/Pacific Islander | 4 (2.8) | 10 (12.2) | 0 (0.0) | 2 (13.3) | ||
| Black | 17 (12.1) | 14 (17.1) | 0 (0.0) | 3 (20.0) | ||
| Hispanic/Mixed/Other | 6 (4.3) | 6 (7.3) | 2 (15.4) | 0 (0.0) | ||
| Lives Alone (% yes) | 24 (25.8) | 23 (42.6) | 4 (40.0) | 3 (30.0) | χ2=4.70; p=0.195 | .035 |
| Married/partnered (% yes) | 108 (76.1) | 50 (62.5) | 8 (61.5) | 8 (53.3) | χ2=6.98; p=0.073 | .032 |
| Children at home (% yes) | 18 (14.9) | 9 (13.6) | 1 (9.1) | 7 (53.8) | χ2=14.16; p=0.003 1 and 2<4 | .82 |
| Older adult at home (% yes) | 2 (1.6) | 3 (4.5) | 1 (9.1) | 1 (7.7) | χ2=3.30; p=0.35 | .25 |
| Work for pay (% yes) | 67 (48.2) | 38 (46.9) | 3 (25.0) | 7 (46.7) | χ2=2.40; p=0.49 | .85 |
| Patient/FC (% Patient) | 93 (65.5) | 54 (65.9) | 10 (76.9) | 10 (66.7) | χ2=.71; p=0.872 | .96 |
Abbreviations: FC = family caregiver, KPS = Karnofsky Performance Status
Bonferroni post hoc pairwise comparisons with alpha = .05: Numbers refer to latent classes, e.g., for Age: 1>2,3,4 represents Resilient class had a higher mean age than the Subsyndromal, Delayed, or Peak classes. Only significant post hoc contrasts are shown.
Because of small sample sizes in the Delayed and Peak classes, genotype analyses focused on comparisons between the Resilient and Subsyndromal classes. In order to determine the appropriate covariates to include in the multivariate logistic regression analyses, these two groups were compared separately. As shown in Table 1, compared to the Resilient class, participants in the Subsyndromal class were significantly younger, had a lower KPS score, were more likely to be female, were less likely to be white, were more likely to live alone, and less likely to be married/partnered. In addition, participants in the Subsyndromal class had significantly higher baseline state (37.7 versus 26.0; p<.001) and trait (41.0 versus 29.2; p<.001) anxiety scores.
Differences in Cytokine Gene Variations Between the Resilient and Subsyndromal Classes
Of the 9 SNPs and four haplotypes that differed significantly between the Resilient and Subsyndromal classes in univariate analyses (Supplementary Table 1), four associations across three genes remained significant in multivariate regression analyses (i.e., IL1R2 haplotype A1, IL10 rs1518111, and TNFA rs2229094 and rs1800629).
Table 2 displays those genotypic predictors that remained significant after adjustment for age, functional status, and race/ethnicity. In the models fit for IL1R2, the model that included haplotype A1 as a genotypic predictor remained significant (p<.0001). Haplotype A1 was composed of alleles at three SNPs (i.e., rs4141134 [C rare allele], rs11674595 [T common allele], rs7570441 [G common allele]). The overall model explained 17.1% of the variance in GMM CES-D group membership. Each additional dose of IL1R2 haplotype A1 was associated with increased odds of belonging to the Subsyndromal class (OR: 2.10, 95% CI: 1.117, 3.959, p=.021). Figure 2 displays the IL1R2 linkage disequilibrium-based heatmap and haplotype analysis.
Table 2. Multiple Logistic Regression Analyses for IL1R2, IL10, and TNFA Candidate Gene Markers.
| GMM Class Comparison | Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|---|
| CES-D Resilient versus Subsyndromal (n=211) | IL1R2 Haplotype A1 | 2.10 | 0.679 | 1.117, 3.959 | 2.30 | 0.021 |
| Age | 0.74 | 0.064 | 0.626, 0.880 | -3.43 | 0.001 | |
| KPS | 0.53 | 0.084 | 0.393, 0.727 | -3.99 | <0.001 | |
| Overall model fit: χ2 = 47.5, p <.0001 R2 = 0.1709 | ||||||
| CES-D Resilient versus Subsyndromal (n=211) | IL10 Genotype | 3.91 | 2.544 | 1.091, 14.000 | 2.09 | 0.036 |
| Age | 0.74 | 0.065 | 0.620, 0.875 | -3.48 | 0.001 | |
| KPS | 0.54 | 0.084 | 0.401, 0.734 | -3.97 | <0.001 | |
| Overall model fit: χ2 = 46.6, p < .0001 R2 = 0.1677 | ||||||
| CES-D Resilient versus Subsyndromal (n=211) | TNFA Genotype 1 | 4.50 | 2.821 | 1.319, 15.373 | 2.40 | 0.016 |
| TNFA Genotype 2 | 0.43 | 0.180 | 0.192, 0.979 | -2.01 | 0.044 | |
| Age | 0.74 | 0.065 | 0.624, 0.881 | -3.40 | 0.001 | |
| KPS | 0.58 | 0.090 | 0.425, 0.783 | -3.53 | <0.001 | |
| Overall model fit: χ2 = 55.0, p < .0001 R2 = 0.1977 | ||||||
Multiple logistic regression analysis for the two GMM groups (i.e., Resilient versus Subsyndromal) based on Center for Epidemiological Studies - Depression Scale total score. For each model, the first three principal components identified from the analysis of ancestry informative markers as well as self-report race/ethnicity were retained in all models to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in each model included genotype (IL1R2 haplotype A1 composed of rs4141134-rs11674595-rs7570441: zero, one, or two doses of the C-T-G haplotype; IL10 rs1518111: GG + GA versus AA; TNFA rs2229094: TT + TC versus CC (genotype 1); TNFA rs1800629: GG versus GA + AA (genotype 2)), age at baseline (five-year increments), and functional status at baseline (KPS score, ten-point increments).
Abbreviations; CI =confidence interval; GMM = growth mixture model; CESD = Center for Epidemiological Studies -Depression scale; IL1R2 = interleukin 1 receptor 2; IL10 = interleukin 10; KPS = Karnofsky Performance Status; TNFA = tumor necrosis factor alpha.
Figure 2.
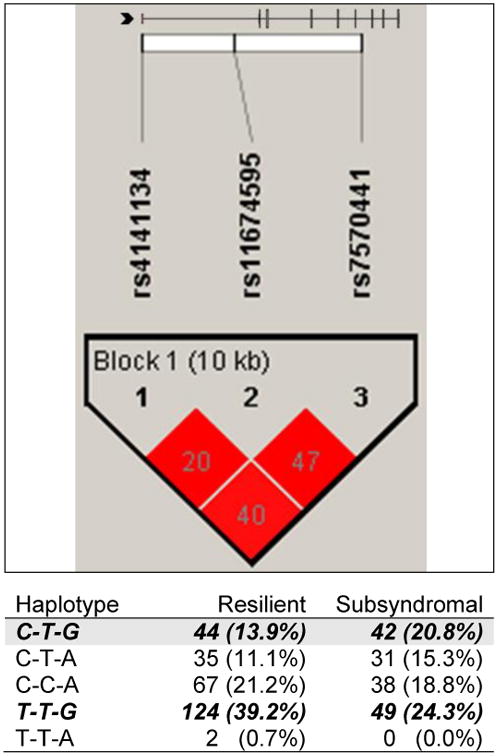
IL1R2 linkage disequilibrium-based heatmap and haplotype analysis.
In the figure embedded in the top row of the table, an ideogram of interleukin 1 receptor 2 (IL1R2) is presented above the white bar that represents the physical distance along human chromosome 2 (position 31, 96,370,336 to 96,380,807; genome build 36.3, contig NT_022171.14). Exons are represented as tick marks. Grey lines connecting the exons represent introns. The black chevron indicates the direction of gene transcription. Reference sequence identifiers (rsID) for each single nucleotide polymorphism (SNP) are plotted both in terms of their physical distance (i.e., the white bar at the top of the figure) and also equidistantly to render the pairwise linkage disequilibrium (LD) estimates that were calculated and visualized with Haploview 4.2. The gene structure for IL1R2 (i.e., reference sequence NM_004633) was rendered with FancyGene 1.4. The correlation statistics (r2 and D′) are provided in the heatmap. LD-based haplotype block definition was based on the D′ confidence interval method (Conde et al., 2006). The haploblock is indicated in a bolded triangle and its component SNPs are rendered in bold font. Pairwise D′ values (range: 0-1, inclusive) were rendered in color, with darker red diamonds representing D′ values approaching 1.0 and progressively lighter red- to pink-colored diamonds representing progressively smaller D′ values. Grey diamonds represent pairwise D′ values of 1.0, but with log of odds values of less than 2.0 (i.e., below the significance threshold). When the r2 values (range of 0-100, inclusive) are not equal to 0 or 100, they are provided in a given diamond. The haplotypes observed in the haploblock are listed in each row, starting with the nucleotide composition across the three SNPs that compose the haplotype (i.e., rs4141134, rs11674595, rs7570441) and both the count (n) and frequency (%) of each haplotype observed in the two GMM CESD groups. The two haplotypes (i.e. CTG, TTG) identified in the bivariate analyses (Supplemental Table 1) are italicized and rendered in bold, while the row featuring the haplotype (i.e., CTG) that remained significant after controlling for relevant confounders (Figure 2) is shaded grey.
For IL10, only the model fit for SNP rs1518111 (A rare allele) remained significant. The overall model explained 16.8% of the variance in GMM CES-D group membership. Individuals homozygous for the rare A allele had increased odds of belonging to the Subsyndromal class (OR: 3.91, 95% CI: 1.091, 14.000, p=.036). While the model fit for IL10 rs1518110 was significant (p<0.0001), it is not described because the SNP is collinear with rs1518111. In addition, though haplotype analysis of the IL10 gene region that included rs1518111 and rs1518110 detected an association with latent class membership, the haplotype was no longer significant after controlling for covariates.
In the model fit for TNFA, two SNPs (i.e., rs2229094 and rs1800629) remained significant. The overall model explained 19.8% of the variance in GMM CES-D group membership. Controlling for age, functional status, race/ethnicity, and rs1800629, individuals homozygous for the C rare allele in rs2229094 had an increased odds of belonging to the Subsyndromal class (OR: 4.50, 95% CI: 1.319, 15.373, p=.016). In contrast, controlling for age, functional status, race/ethnicity, and rs2229094, individuals who possessed the A rare allele (i.e., GA or AA genotypes) for rs1800629 had lower odds of belonging to the Subsyndromal class (OR: 0.43, 95% CI: 0.192, 0.979, p=.044).
Discussion
Using GMM, this study confirmed four latent classes of oncology patients and FCs with distinct depressive symptom trajectories during and following RT (Dunn et al., 2011). These distinct latent classes were not dependent on patient or FC status, which suggests that risk for depressive symptoms may be more related to an individual's phenotypic and genotypic characteristics than to treatment for cancer. Finally, several phenotypic and genotypic characteristics associated with the Resilient and Subsyndromal classes were identified.
While the mean CES-D score for the entire sample was relatively low (i.e., ∼9) and did not change substantially over six months (Figure 1), the GMM analysis identified subgroups of participants with distinct depressive symptom trajectories. This heterogeneity would have been obscured if only mean CES-D scores were evaluated. The subgroups identified in this study are nearly identical to those we previously identified in a sample of women pre- and post-breast cancer surgery (Dunn et al., 2011). In addition, findings from our two studies are consistent with previous work that evaluated psychological distress trajectories (Helgeson et al., 2004; Henselmans et al., 2010; Hou et al., 2010; Lam et al., 2010; Lam et al., 2012). Approximately 36%-66% of participants in these studies were in no-distress or low-distress groups (i.e., similar to our Resilient class, 56.3%). The findings that several demographic and clinical characteristics (i.e., younger age, female, non-white, higher baseline anxiety) were associated with membership in the Subsyndromal class are consistent with previous reports of predictors of depressive symptoms (Helgeson et al., 2004; Institute of Medicine, 2007; Kornblith et al., 2007; Massie, 2004; Perkins et al., 2007).
Of note, one-third of participants (32.5%, Subsyndromal class) had depressive symptom levels that might represent subthreshold, yet clinically meaningful, levels of depression. These participants' CES-D scores remained approximately 12 across the six months of this study. A number of studies have documented deleterious effects of subsyndromal depression in primary care patients (Lyness et al., 2007; Williams et al., 1995), FCs (Taylor et al., 2008) and the general population (Chachamovich et al., 2008; Chen et al., 2000). Additional research is warranted to understand the nature and consequences of subsyndromal depressive symptoms in both oncology patients and their FCs.
To our knowledge, this study is the first to report significant associations between genetic variations in cytokine genes and distinct depressive symptom trajectories in cancer patients and their FCs. Two of these associations were with genes with anti-inflammatory functions (i.e., IL1R2, IL10), and one was with a gene with pro-inflammatory functions (i.e., TNFA).
Variation in the IL1R2 haplotype A1 explained 1.9% of variance in latent class membership, such that each additional dose of the haplotype was associated with approximately twice the odds of belonging to the Subsyndromal class. While IL1R2 has not been studied in the context of depressive symptoms, IL1R2 inhibits inflammatory signalling by binding to pro-inflammatory IL1β that prevents its binding to IL1R1 (Colotta et al., 1993). It is plausible to hypothesize that SNPs that compose the haplotype, or an unmeasured SNP(s) in linkage disequilibrium (LD), result in decreased IL1R2 expression, which would decrease sequestration of pro-inflammatory IL1β. While the functionality of each SNP in the haplotype has not been studied, one SNP (rs4141134) is located in the promoter region of the IL1R2 gene and may impact IL1R2 expression. The other SNPs in the haplotype (rs11674595 and rs7570441) are located in non-coding regions. Evaluation of these three SNPs, through a number of databases (i.e., in silico analyses), failed to reveal a potential functional impact. While the impact of this haplotype on IL1R2 production is unknown, IL1R2 is an important regulator of the inflammatory response (Seruga et al., 2008). Therefore, its association with depressive symptoms warrants additional investigation.
Individuals homozygous for the rare allele for IL10 rs1518111 had approximately four times greater odds of being in the Subsyndromal class. This SNP explained 1.6% of the variance in GMM class membership. This SNP lies in a non-coding region of the IL10 gene and has no known function. However, a recent genome-wide association study identified this SNP as a marker of the autoimmune disorder Behçet's Disease (Remmers et al., 2010), which is associated with elevated depressive symptom scores on the Beck Depression Inventory (Gur et al., 2006). Moreover, interferon alpha-induced increases in IL10 production and depressive behavior in rodents are prevented by pre-treatment with the selective serotonin-reuptake inhibitor, paroxetine (Myint et al., 2007). Taken together, these findings suggest that IL10 may play a key role in the pathophysiology of depressive symptoms. Replication of this finding and functional analyses are needed.
Two SNPs (rs2229094 and rs1800629) in the pro-inflammatory TNFA gene explained 3.1% and 2.4%, respectively, of the variance in latent class membership. Jointly, these two SNPs explained 4.6% of variance. The joint estimate is lower than the individual estimates because these SNPs are partially correlated (see Figure 3). Of note, rs1800629 is a common functional promoter polymorphism (c.G-308A). For example, we found that, in this same sample, individuals homozygous for the common allele (GG) reported significantly higher levels of sleep disturbance and morning fatigue than individuals heterozygous or homozygous for the rare allele (GA + AA) (Aouizerat et al., 2009). Additionally, in one small study of older adults (n=50), an increased OR for major depression was found among those with the G (common) allele (Cerri et al., 2010). These findings suggest that TNFA may play a role in mood disturbance and other symptoms that are prevalent in cancer patients and FCs. However, additional studies are needed to replicate this finding and to examine the functional consequences.
Figure 3. TNFA gene region linkage disequilibrium-based heatmap.
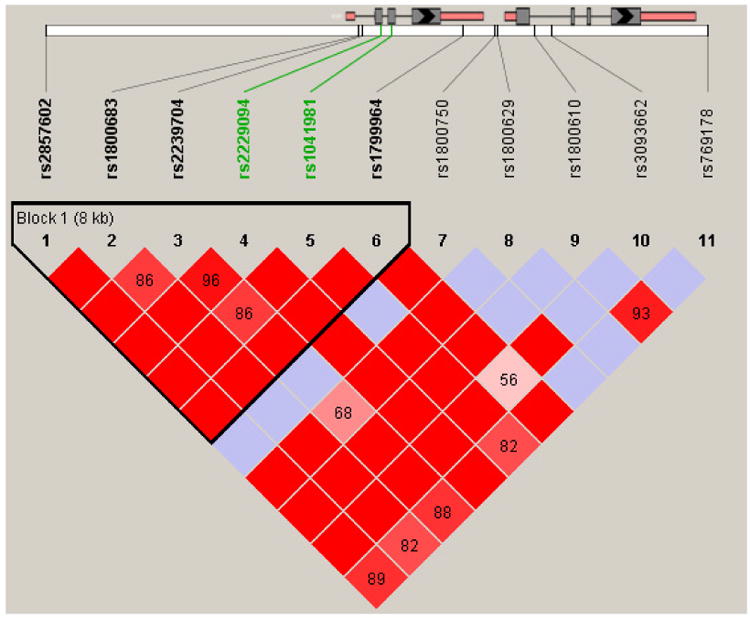
Ideograms of the two genes in the region (i.e., the gene on the left is lymphotoxin alpha [LTA], the gene on the right is tumor necrosis factor alpha [TNFA] are presented at the top of the figure above the white bar which represents the physical distance along human chromosome 6 [position 31,533,378 to 31,547,514; genome build 37.3, contig NT_007592.15]). Exons are represented as boxes with coding regions rendered in grey and untranslated regions rendered in pink. Grey lines connecting the exons represent introns. The black chevron indicates the direction of gene transcription. For both genes, transcription is initiated from the left. Reference sequence identifiers (rsID) for each single nucleotide polymorphism (SNP) are plotted both in terms of their physical distance (i.e., the white bar at the top of the figure) and equidistantly to render the pairwise linkage disequilibrium (LD) estimates, which were calculated and visualized with Haploview 4.2. Gene structures for TNFA (i.e., reference sequence NM_000594) and LTA (i.e., reference sequence NM_000592) were rendered with FancyGene 1.4. The correlation statistics (r2 and D′) are provided in the heatmap. LD-based haplotype block definition was based on D′ confidence interval (Conde et al., 2006). The haploblock is indicated in a bolded triangle and its component SNPs are rendered in bold font. SNPs that result in missense mutations are rendered in green font. Pairwise D′ values (range: 0-1, inclusive) were rendered in color, with darker red diamonds representing D′ values approaching 1.0 and progressively lighter red to pink-colored diamonds representing progressively smaller D′ values. Grey diamonds represent pairwise D′ values of 1.0, but with log of odds values of less than 2.0 (i.e., below the significance threshold). When the r2 values (range of 0-100, inclusive) are not equal to 0 or 100, they are provided in a given diamond.
The other TNFA SNP (rs2229094) is functional. In addition to being located in the promoter region of TNFA, it also resides in the coding region of an adjacent pro-inflammatory gene, lymphotoxin alpha (LTA). Whether LTA has a direct impact, or whether the promoter's influence on TNFA is responsible for the observed association cannot be disentangled with the current design. Nonetheless, this association highlights the importance of pro-inflammatory cytokines in relation to variation in depressive symptoms.
While information on depressive symptoms and anxiety was obtained through valid and reliable self-report measures, future studies should include a clinical evaluation of previous and concurrent psychiatric comorbidities. The major reasons for refusal were being too overwhelmed or too busy, which may have led to an underestimation of depressive symptoms in this sample. Finally, the sample size placed some constraints on these analyses. With a larger sample, more latent classes may be identified. However, a sample size of 252 is within the required limits to obtain meaningful and reliable estimates of latent classes (Nylund et al., 2007; Tofighi and Enders, 2008).
Additional research in larger, independent samples is needed to validate these genetic associations. Because not all SNPs in cytokine genes were interrogated, subtle associations may have been missed. Nonetheless, significant SNPs were identified using the current approach. In addition, measurement of plasma cytokine levels may allow for a more comprehensive evaluation of the association between cytokine genotype and severity of depressive symptoms.
Given the high prevalence of depressive symptoms in both oncology patients and FCs (Institute of Medicine, 2007; Kim et al., 2005; Massie, 2004; Pirl, 2004; Rhee et al., 2008), the deleterious impact of these symptoms on QOL (Couper et al., 2006a; Couper et al., 2006b; Kim and Given, 2008; Rabin et al., 2008), and robust evidence for associations among cytokines, depression, and a constellation of other symptoms, research combining newer longitudinal analytic methods with genetic approaches should contribute insights that could help identify those at greater risk for more severe depressive symptoms during and after cancer and its treatment.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift P, Dunn LB, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Bardwell WA, Natarajan L, Dimsdale JE, Rock CL, Mortimer JE, Hollenbach K, Pierce JP. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. Journal of Clinical Oncology. 2006;24:2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Carter PA, Acton GJ. Personality and coping: predictors of depression and sleep problems among caregivers of individuals who have cancer. Journal of Gerontological Nursing. 2006;32:45–53. doi: 10.3928/0098-9134-20060201-11. [DOI] [PubMed] [Google Scholar]
- Cerri AP, Arosio B, Viazzoli C, Confalonieri R, Vergani C, Annoni G. The -308 (G/A) single nucleotide polymorphism in the TNF-alpha gene and the risk of major depression in the elderly. International Journal of Geriatric Psychiatry. 2010;25:219–223. doi: 10.1002/gps.2323. [DOI] [PubMed] [Google Scholar]
- Chachamovich E, Fleck M, Laidlaw K, Power M. Impact of major depression and subsyndromal symptoms on quality of life and attitudes toward aging in an international sample of older adults. Gerontologist. 2008;48:593–602. doi: 10.1093/geront/48.5.593. [DOI] [PubMed] [Google Scholar]
- Chen L, Eaton WW, Gallo JJ, Nestadt G. Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study. Journal of Affective Disorders. 2000;59:1–11. doi: 10.1016/s0165-0327(99)00132-9. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behavior and Immunity. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Research. 2006;34:W621–625. doi: 10.1093/nar/gkl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology. 2006a;15:937–953. doi: 10.1002/pon.1031. [DOI] [PubMed] [Google Scholar]
- Couper JW, Bloch S, Love A, Duchesne G, Macvean M, Kissane DW. The psychosocial impact of prostate cancer on patients and their partners. Medical Journal of Australia. 2006b;185:428–432. doi: 10.5694/j.1326-5377.2006.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Rousey S, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008a;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behavior and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008b;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology. 2006;15:306–320. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- Deshields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychology. 2007;26:464–472. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, Paul SM, Wara W, Swift P, Miaskowski C. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. European Journal of Oncology Nursing. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Cooper BA, Neuhaus J, West C, Paul S, Aouizerat B, Abrams G, Edrington J, Hamolsky D, Miaskowski C. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychology. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Review Neuroscience. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Given B, Given CW, Sikorskii A, Jeon S, McCorkle R, Champion V, Decker D. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? Journal of Pain and Symptom Management. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb BH, Rooney JA. Coping effectiveness: determinants and relevance to the mental health and affect of family caregivers of persons with dementia. Aging and Mental Health. 2004;8:364–373. doi: 10.1080/13607860410001709719. [DOI] [PubMed] [Google Scholar]
- Gur A, Sarac AJ, Burkan YK, Nas K, Cevik R. Arthropathy, quality of life, depression, and anxiety in Behcet's disease: relationship between arthritis and these factors. Clinical Rheumatology. 2006;25:524–531. doi: 10.1007/s10067-005-0100-6. [DOI] [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Human Mutation. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychology. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychology. 2010;29:160–168. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM. Control of confounding of genetic associations in stratified populations. American Journal of Human Genetics. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker K, Monahan DJ, Bowman SR, Frazier LD, Shifren K. Personality counts for a lot: predictors of mental and physical health of spouse caregivers in two disease groups. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 1998;53:P73–85. doi: 10.1093/geronb/53b.2.p73. [DOI] [PubMed] [Google Scholar]
- Hou WK, Law CC, Yin J, Fu YT. Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: a growth mixture modeling approach. Health Psychology. 2010;29:484–495. doi: 10.1037/a0020809. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. The National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- Kim Y, Carver CS, Deci EL, Kasser T. Adult attachment and psychological well-being in cancer caregivers: the mediational role of spouses' motives for caregiving. Health Psychology. 2008;27:S144–154. doi: 10.1037/0278-6133.27.2(Suppl.).S144. [DOI] [PubMed] [Google Scholar]
- Kim Y, Duberstein PR, Sorensen S, Larson MR. Levels of depressive symptoms in spouses of people with lung cancer: effects of personality, social support, and caregiving burden. Psychosomatics. 2005;46:123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: across the trajectory of the illness. Cancer. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Powell M, Regan MM, Bennett S, Krasner C, Moy B, Younger J, Goodman A, Berkowitz R, Winer E. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16:895–903. doi: 10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Given CW, Given B. Predictors of postbereavement depressive symptomatology among family caregivers of cancer patients. Supportive Care in Cancer. 1997;5:53–60. doi: 10.1007/BF01681962. [DOI] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Given CW, Given BA. Depression and physical health among family caregivers of geriatric patients with cancer--a longitudinal view. Medical Science Monitoring. 2004;10:CR447–456. [PubMed] [Google Scholar]
- Lam WW, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, Or A, Fielding R. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology. 2010;19:1044–1051. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- Lam WW, Shing YT, Bonanno GA, Mancini AD, Fielding R. 2010b Distress trajectories at the first year diagnosis of breast cancer in relation to 6 years survivorship. Psychooncology. 2012;21:90–99. doi: 10.1002/pon.1876. [DOI] [PubMed] [Google Scholar]
- Lee YH, Ji JD, Song GG. Tumor necrosis factor-alpha promoter -308 A/G polymorphism and rheumatoid arthritis susceptibility: a metaanalysis. Journal of Rheumatology. 2007;34:43–49. [PubMed] [Google Scholar]
- Legler JM, Davis WW, Potosky AL, Hoffman RM. Latent variable modelling of recovery trajectories: sexual function following radical prostatectomy. Statistics in Medicine. 2004;23:2875–2893. doi: 10.1002/sim.1864. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biological Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Kim J, Tang W, Tu X, Conwell Y, King DA, Caine ED. The clinical significance of subsyndromal depression in older primary care patients. American Journal of Geriatric Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic Brain Disease. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. Journal of the National Cancer Institute Monographs. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift PS, Dunn LB, Aouizerat BE. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. Journal of Pain and Symptom Management. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcoholism, Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Muthen BO, Kaplan DW. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The Sage Handbook of Quantitative Methodology for the Social Sciences. Sage Publications; Newbury Park, CA: 2004. pp. 345–368. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. 6th. Muthen & Muthen; Los Angeles, CA: 1998-2010. [Google Scholar]
- Myint AM, O'Mahony S, Kubera M, Kim YK, Kenny C, Kaim-Basta A, Steinbusch HW, Leonard BE. Role of paroxetine in interferon-alpha-induced immune and behavioural changes in male Wistar rats. Journal of Psychopharmacology. 2007;21:843–850. doi: 10.1177/0269881107077165. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Perkins EA, Small BJ, Balducci L, Extermann M, Robb C, Haley WE. Individual differences in well-being in older breast cancer survivors. Critical Review of Oncology and Hematology. 2007;62:74–83. doi: 10.1016/j.critrevonc.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. Journal of the National Cancer Institute Monographs. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Rabin EG, Heldt E, Hirakata VN, Fleck MP. Quality of life predictors in breast cancer women. European Journal of Oncology Nursing. 2008;12:53–57. doi: 10.1016/j.ejon.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, Le JM, Yang B, Korman BD, Cakiris A, Aglar O, Emrence Z, Azakli H, Ustek D, Tugal-Tutkun I, Akman-Demir G, Chen W, Amos CI, Dizon MB, Kose AA, Azizlerli G, Erer B, Brand OJ, Kaklamani VG, Kaklamanis P, Ben-Chetrit E, Stanford M, Fortune F, Ghabra M, Ollier WE, Cho YH, Bang D, O'Shea J, Wallace GR, Gadina M, Kastner DL, Gul A. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet's disease. Nature Genetics. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee YS, Yun YH, Park S, Shin DO, Lee KM, Yoo HJ, Kim JH, Kim SO, Lee R, Lee YO, Kim NS. Depression in family caregivers of cancer patients: the feeling of burden as a predictor of depression. Journal of Clinical Oncology. 2008;26:5890–5895. doi: 10.1200/JCO.2007.15.3957. [DOI] [PubMed] [Google Scholar]
- Rose JH, Kypriotakis G, Bowman KF, Einstadter D, O'Toole EE, Mechekano R, Dawson NV. Patterns of adaptation in patients living long term with advanced cancer. Cancer. 2009;115:4298–4310. doi: 10.1002/cncr.24584. [DOI] [PubMed] [Google Scholar]
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- Schou I, Ekeberg O, Ruland CM, Sandvik L, Karesen R. Pessimism as a predictor of emotional morbidity one year following breast cancer surgery. Psychooncology. 2004;13:309–320. doi: 10.1002/pon.747. [DOI] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Review Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- SPSS. IBM SPSS for Windows (Version 19) SPSS, Inc.; Chicago, Illinois: 2010. [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DH, Jr, Ezell M, Kuchibhatla M, Ostbye T, Clipp EC. Identifying trajectories of depressive symptoms for women caring for their husbands with dementia. Journal of American Geriatrics Society. 2008;56:322–327. doi: 10.1111/j.1532-5415.2007.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Human Molecular Genetics. 2008;17:R143–150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. In: Hancock GR, Samuelsen SM, editors. Advances in Latent Variable Mixture Models. Information Age Publishing; Charlotte, NC: 2008. pp. 317–341. [Google Scholar]
- Williams JW, Jr, Kerber CA, Mulrow CD, Medina A, Aguilar C. Depressive disorders in primary care: prevalence, functional disability, and identification. Journal of General Internal Medicine. 1995;10:7–12. doi: 10.1007/BF02599568. [DOI] [PubMed] [Google Scholar]
- Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behavior and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


