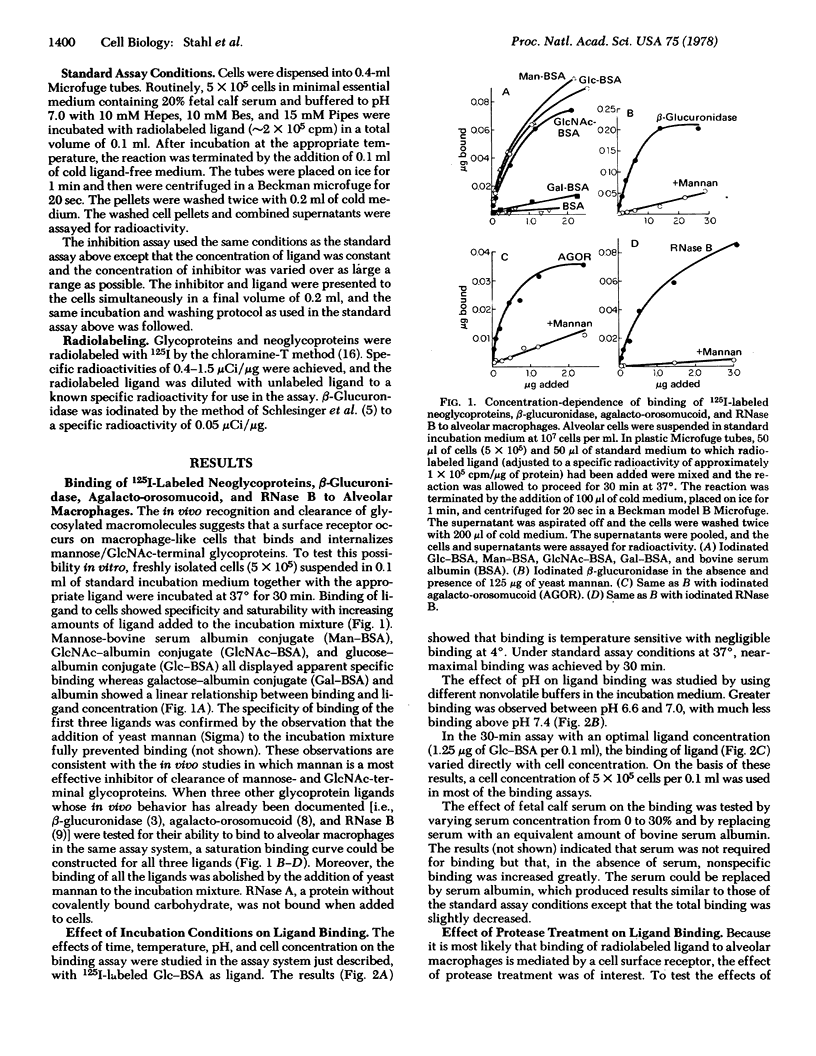
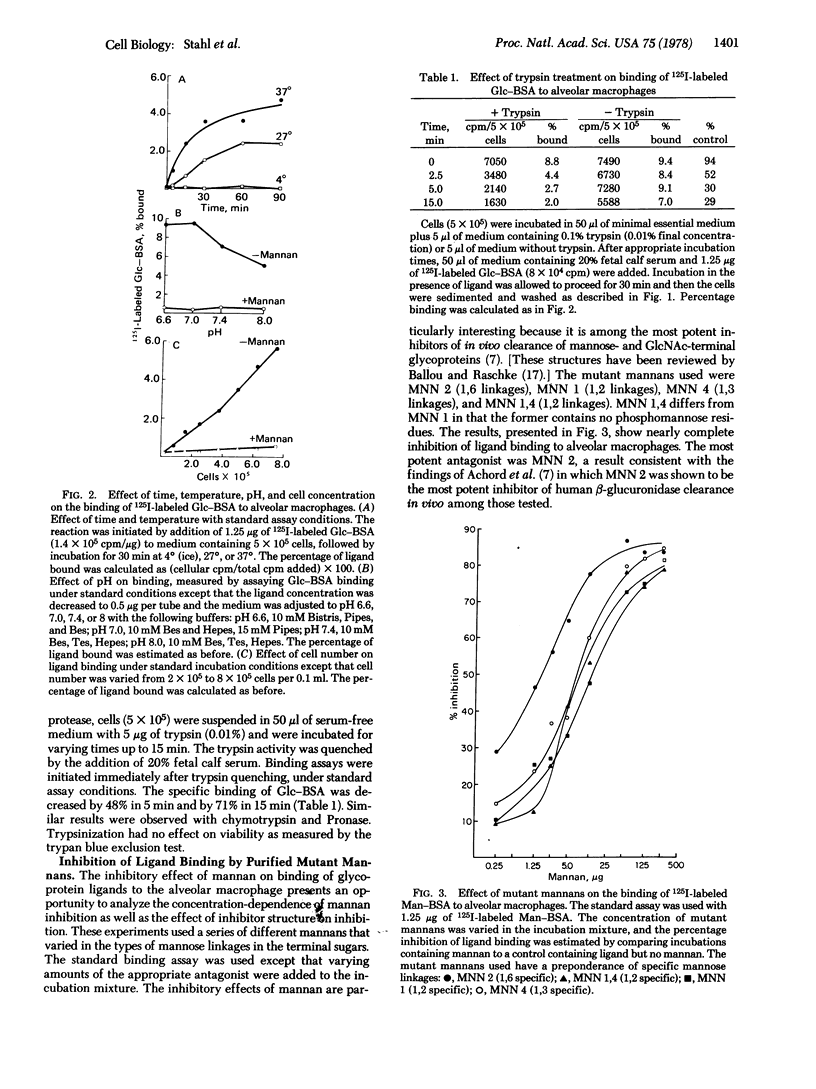
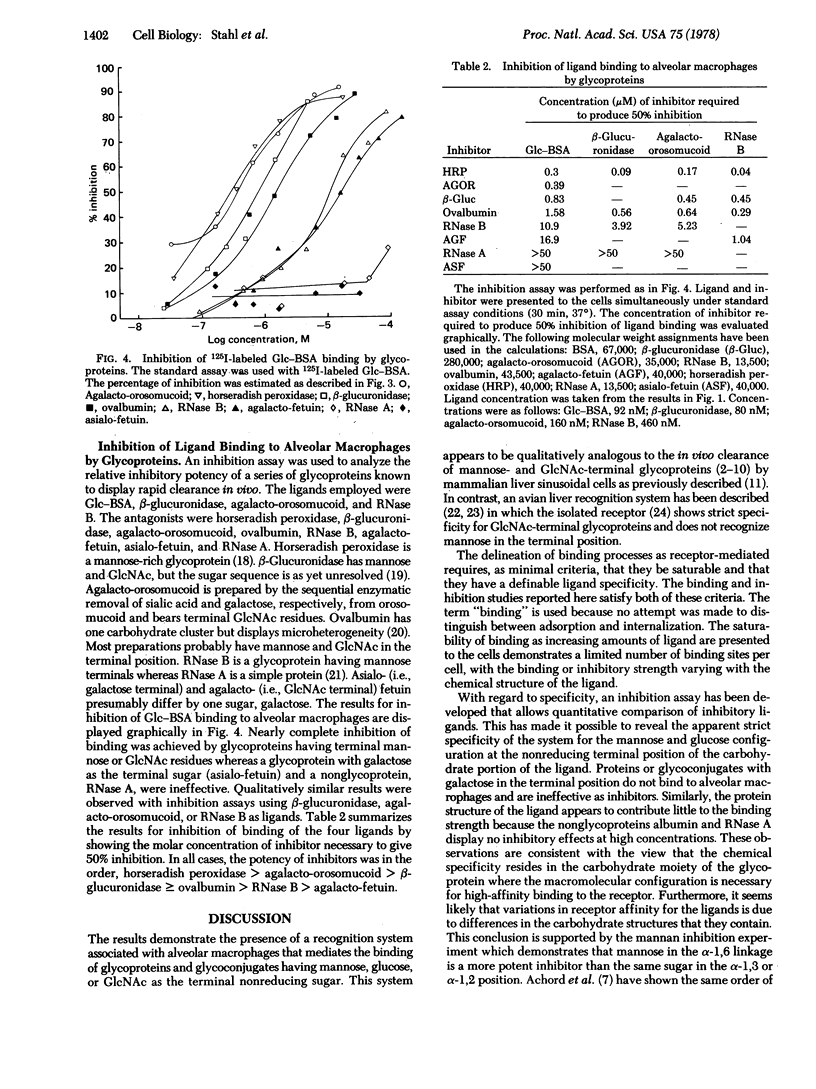
Abstract
Alveolar macrophages have been shown to bind glycoproteins and synthetic glycoconjugates (neoglycorpoteins) that have mannose, N-acetylglucosamine, or glucose in the exposed, nonreducing position. Galactose-terminal glycoproteins are not bound. Binding of radiolabeled ligands to cells is nearly completely impaired by the presence of an excess of yeast mannan. Binding is temperature sensitive and proceeds optimally at pH 7.0. Prior treatment of the cells with trypsin severely decreases their capacity to bind ligands. An inhibition assay has been developed, using radioiodinated glucose-albumin conjugate, agalacto-orosomucoid, beta-glucuronidase, and RNase B as ligands. Various glycoproteins have been shown to be effective inhibitors of ligand binding including horseradish peroxidase, agalacto-orosomucoid, beta-glucuronidase, ovalbumin, agalacto-fetuin, and RNase B. RNase A and asialo-fetuin are ineffective as antagonists. The results suggest the presence of a cell surface receptor on alveolar macrophages that binds glycoproteins having terminal sugars with the mannose or glucose configuration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Achord D., Brot F., Gonzalez-Noriega A., Sly W., Stahl P. Human beta-glucuronidase. II. Fate of infused human placental beta-glucuronidase in the rat. Pediatr Res. 1977 Jul;11(7):816–822. doi: 10.1203/00006450-197707000-00008. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Brain J. D., Frank R. Alveolar macrophage adhesion: wash electrolyte composition and free cell yield. J Appl Physiol. 1973 Jan;34(1):75–80. doi: 10.1152/jappl.1973.34.1.75. [DOI] [PubMed] [Google Scholar]
- Clarke J., Shannon L. M. The isolation and characterization of the glycopeptides from horseradish peroxidase isoenzyme C. Biochim Biophys Acta. 1976 Apr 14;427(2):428–442. doi: 10.1016/0005-2795(76)90186-0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Hirs C. H. The primary structure of porcine pancreatic ribonuclease. I. The distribution and sites of carbohydrate attachment. J Biol Chem. 1970 Feb 10;245(3):624–636. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Krantz M. J., Holtzman N. A., Stowell C. P., Lee Y. C. Attachment of thioglycosides to proteins: enhancement of liver membrane binding. Biochemistry. 1976 Sep 7;15(18):3963–3968. doi: 10.1021/bi00663a009. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Stowell C. P., Krantz M. J. 2-Imino-2-methoxyethyl 1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976 Sep 7;15(18):3956–3963. doi: 10.1021/bi00663a008. [DOI] [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. The purification and characterization of rat liver lysosomal alpha-L-fucosidase. J Biol Chem. 1977 Jan 25;252(2):739–743. [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W., Charlwood P. A. Carbohydrate-mediated elimination of avian plasma glycoprotein in mammals. Nature. 1975 Apr 24;254(5502):699–701. doi: 10.1038/254699a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger P., Rodman J. S., Frey M., Lang S., Stahl P. Clearance of lysosomal hydrolases following intravenous infusion. The role of liver in the clearance of beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):606–614. doi: 10.1016/0003-9861(76)90472-0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Touster O. Beta-glucuronidase of rat liver lysosomes. Purification, properties, subunits. J Biol Chem. 1971 Sep 10;246(17):5398–5406. [PubMed] [Google Scholar]
- Stahl P., Rodman J. S., Schlesinger P. Clearance of lysosomal hydrolases following intravenous infusion. Kinetic and competition experiments with beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):594–605. doi: 10.1016/0003-9861(76)90471-9. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976 Nov 4;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- Stahl P., Six H., Rodman J. S., Schlesinger P., Tulsiani D. R., Touster O. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4045–4049. doi: 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Keller R. K., Touster O. The preparation and chemical composition of the multiple forms of beta-glucuronidase from the female rat preputial gland. J Biol Chem. 1975 Jun 25;250(12):4770–4776. [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Aglycosylantibody. Effects of exoglycosidase treatments on autochthonous antibody survival time in the circulation. J Biol Chem. 1976 Feb 25;251(4):1074–1080. [PubMed] [Google Scholar]


