Abstract
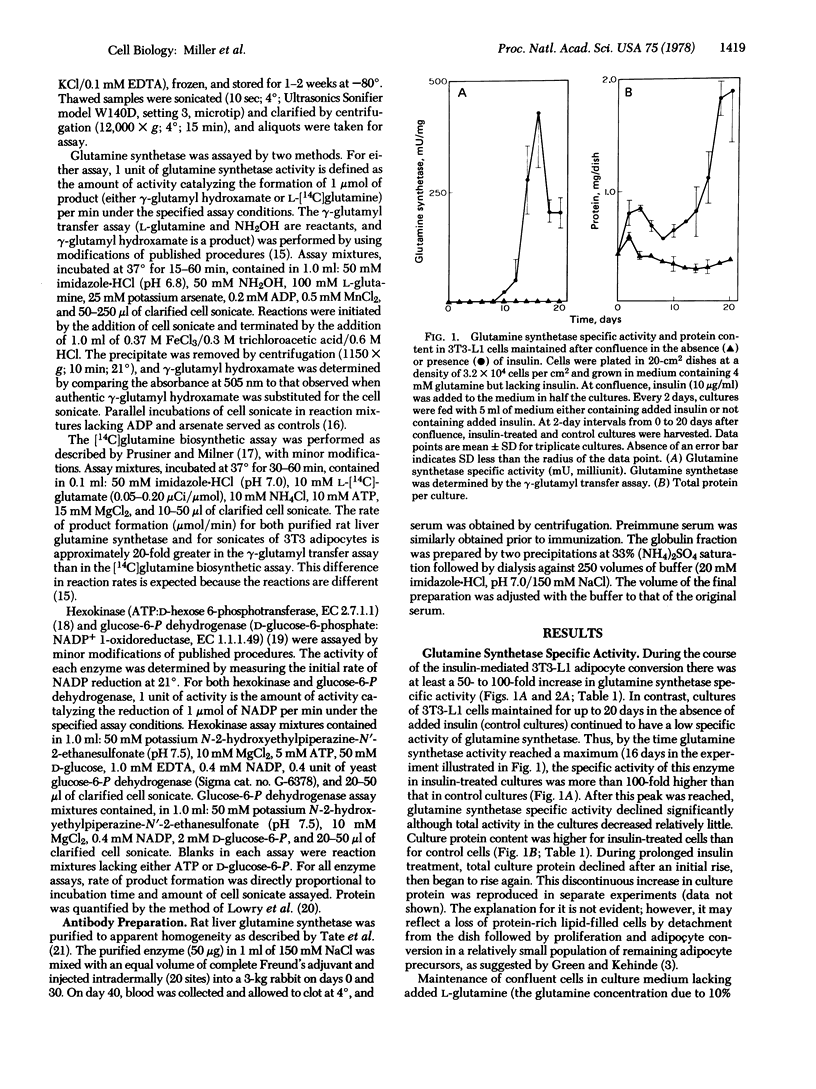
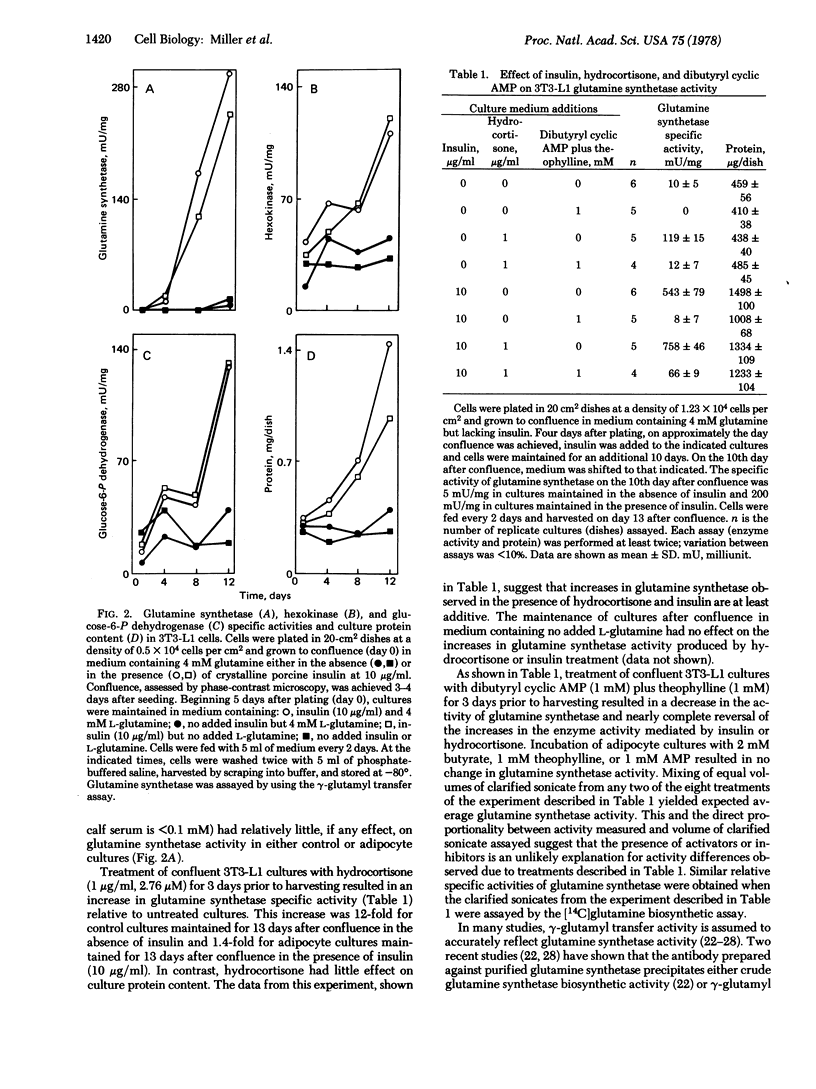
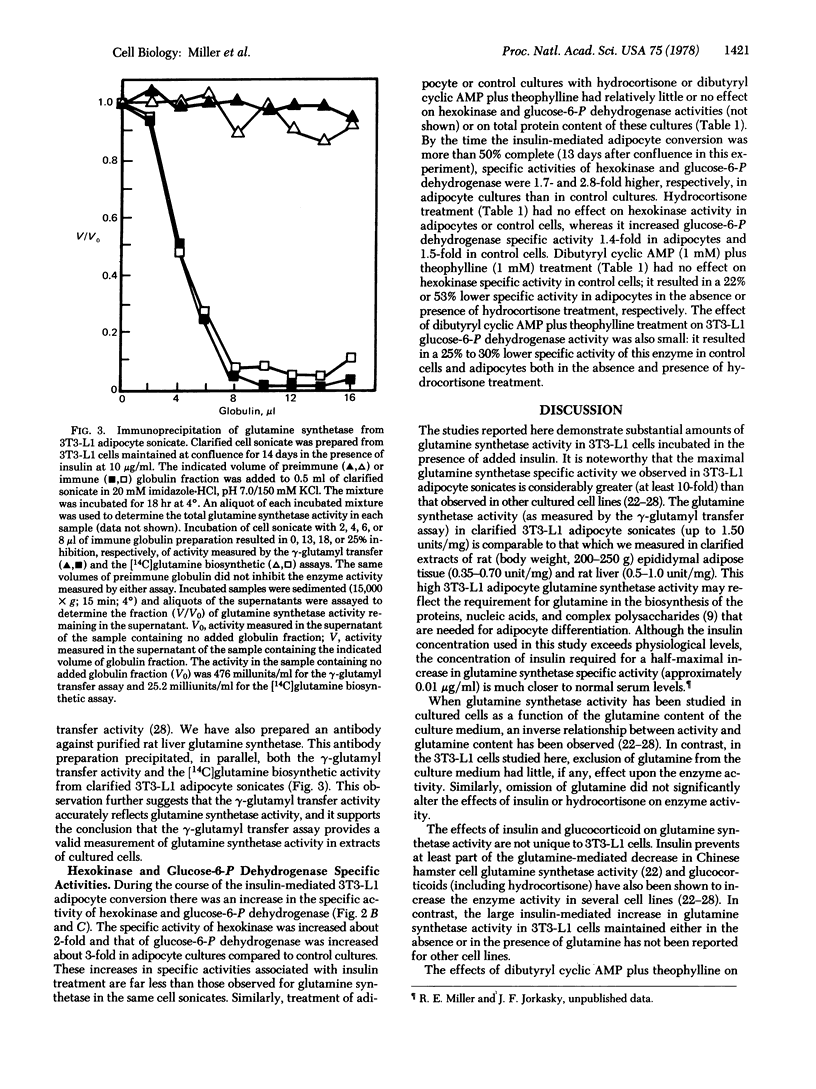
The 3T3-L1 mouse fibroblast cell line develops morphological and biochemical characteristics of adipocytes when maintained at confluence. This conversion to adipocytes is accelerated by addition of insulin to the culture medium [Green, H. & Kehinde, O. (1975) Cell 5, 19-27]. During the course of the insulin-mediated adipocyte conversion, the specific activity (units/mg of protein) of glutamine synthetase [L-glutamate:ammonia ligase (ADP-forming), EC 6.3.1.2] increases more than 100-fold. The specific activities of hexokinase (ATP:D-hexose 6-phosphotransferase, EC 2.7.1.1) and glucose-6-P dehydrogenase (D-glucose-6-phosphate:NADP+ 1-oxidoreductase, EC 1.1.1.49) also increase but less dramatically (1.5- to 3-fold). In contrast, confluent cells maintained in the absence of insulin for the same time (12-20 days after confluence) display only minimal increases in the activity of these enzymes. Maintenance of confluent cells in culture medium lacking added L-glutamine has little, if any, effect on glutamine synthetase activity in either control or insulin-treated cultures. Treatment of confluent 3T3-L1 cultures with hydrocortisone (1 μg/ml) for 3 days prior to harvesting results in an increase in glutamine synthetase specific activity of 12-fold for control cultures maintained for 13 days in the absence of insulin and 1.4-fold for adipocyte cultures maintained for 13 days in the presence of insulin (10 μg/ml). Treatment of 3T3-L1 control cells and adipocytes with dibutyryl cyclic AMP (1 mM) plus theophylline (1 mM) decreases the glutamine synthetase specific activity and almost completely reverses the insulin- and hydrocortisone-mediated increases in enzyme activity. In contrast, treatment with dibutyryl cyclic AMP plus theophylline has relatively little effect on the specific activities of hexokinase or glucose-6-P dehydrogenase or on the protein content of the cultures. These data indicate that glutamine synthetase activity is hormonally regulated in 3T3-L1 cells.
Keywords: 3T3-L1 adipocytes, hexokinase, glucose-6-P dehydrogenase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arad G., Freikopf A., Kulka R. G. Glutamine-stimulated modification and degradation of glutamine synthetase in hepatoma tissue culture cells. Cell. 1976 May;8(1):95–101. doi: 10.1016/0092-8674(76)90190-2. [DOI] [PubMed] [Google Scholar]
- Chader G. J. Hormonal effects on the neural retina: induction of glutamine synthetase by cyclic-3',5'-AMP. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1102–1105. doi: 10.1016/0006-291x(71)90575-4. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977 Sep 9;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- Foster L. B., Dunn R. T. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin Chem. 1974 Mar;20(3):365–368. [PubMed] [Google Scholar]
- Freikopf A., Kulka R. G. Specificity of the glutamine-binding site involved in the reguation of glutamine-synthetase activity in hepatoma tissue-culture cells. Eur J Biochem. 1975 Aug 15;56(2):483–492. doi: 10.1111/j.1432-1033.1975.tb02254.x. [DOI] [PubMed] [Google Scholar]
- Geisler R. W., Hansen R. J. Effects of insulin on the adaptation of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in rat adipose tissue. Biochim Biophys Acta. 1972 Aug 18;279(1):139–145. doi: 10.1016/0304-4165(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hansen R. J., Pilkis S. J., Krahl M. E. Effect of insulin on the synthesis in vitro of hexokinase in rat epididymal adipose tissue. Endocrinology. 1970 Jan;86(1):57–65. doi: 10.1210/endo-86-1-57. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hoffmann S. S., Kolodny G. M. Insulin receptors in 3T3 fibroblasts. Relationship to growth phase, transformation and differentiation into new cell types. Exp Cell Res. 1977 Jul;107(2):293–299. doi: 10.1016/0014-4827(77)90352-4. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka R. G., Cohen H. Regulation of glutamine synthetase activity of hepatoma tissue culture cells by glutamine and dexamethasone. J Biol Chem. 1973 Oct 10;248(19):6738–6743. [PubMed] [Google Scholar]
- Kulka R. G., Tokins G. M., Crook R. B. Clonal differences in glutamine synthetase activity of hepatoma cells. Effects of glutamine and dexamethasone. J Cell Biol. 1972 Jul;54(1):175–179. doi: 10.1083/jcb.54.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977 Mar 25;252(6):2158–2160. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Meyuhas O., Reshef L., Ballard F. J., Hanson R. W. The effect of insulin and glucocorticoids on the synthesis and degradation of phosphoenolpyruvate carboxykinase (GTP) in rat adipose tissue cultured in vitro. Biochem J. 1976 Jul 15;158(1):9–16. doi: 10.1042/bj1580009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Milman G., Portnoff L. S., Tiemeier D. C. Immunochemical evidence for glutamine-mediated degradation of glutamine synthetase in cultured Chinese hamster cells. J Biol Chem. 1975 Feb 25;250(4):1393–1399. [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Leu F. Y., Meister A. Rat liver glutamine synthetase. Preparation, properties, and mechanism of inhibition by carbamyl phosphate. J Biol Chem. 1972 Sep 10;247(17):5312–5321. [PubMed] [Google Scholar]
- Tiemeier D. C., Milman G. Regulation of glutamine synthetase in cultured Chinese hamster cells. Induction and repression by glutamine. J Biol Chem. 1972 Sep 25;247(18):5722–5727. [PubMed] [Google Scholar]
- Van R. L., Bayliss C. E., Roncari D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976 Sep;58(3):699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]


