Abstract
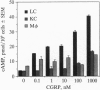
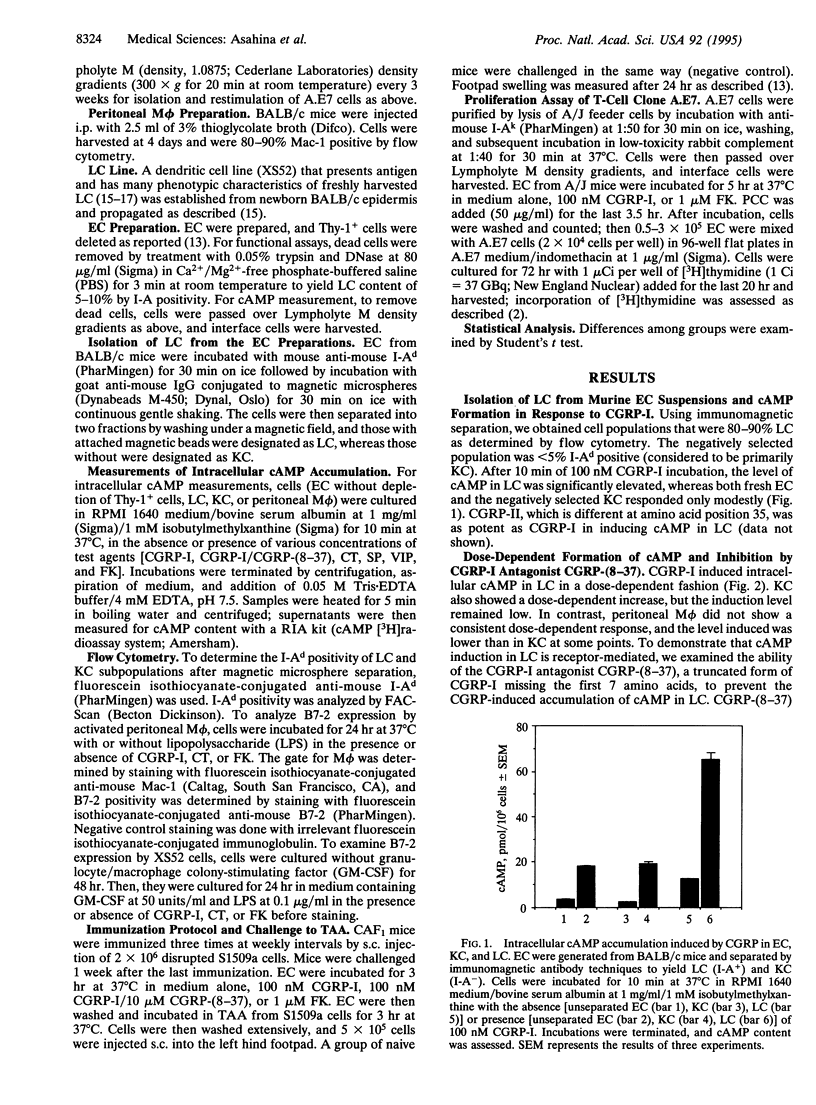
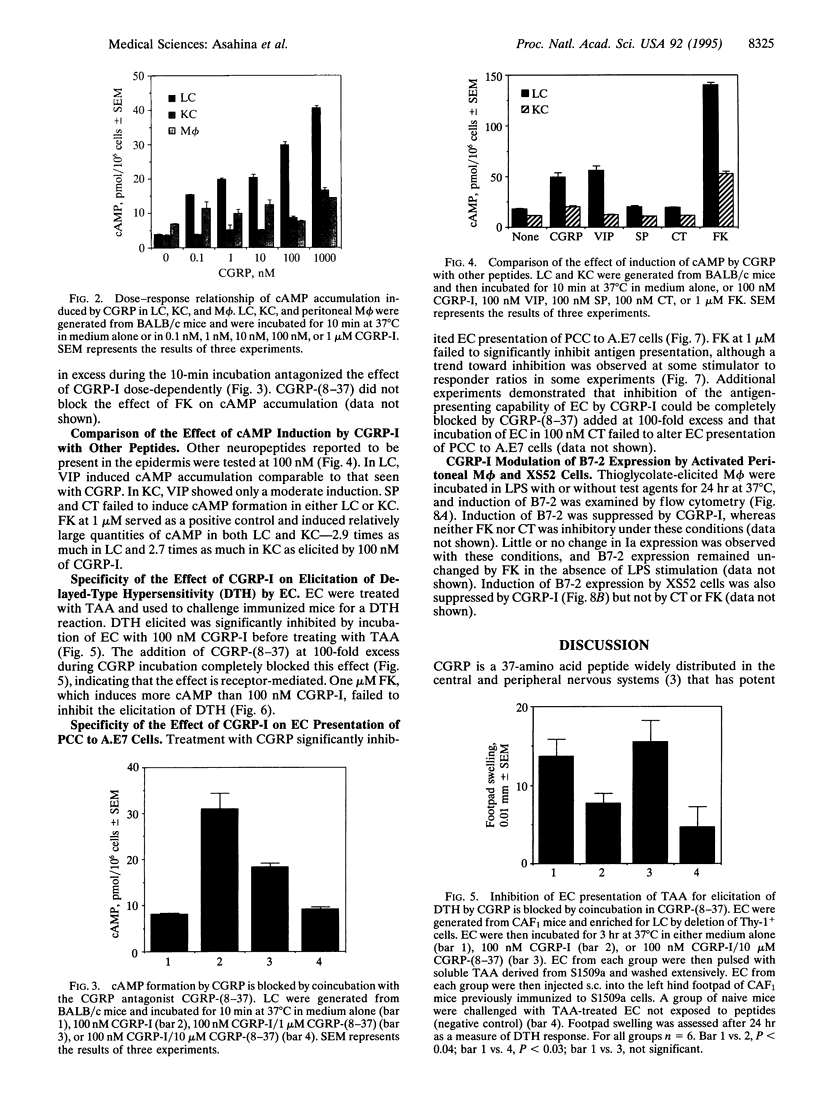
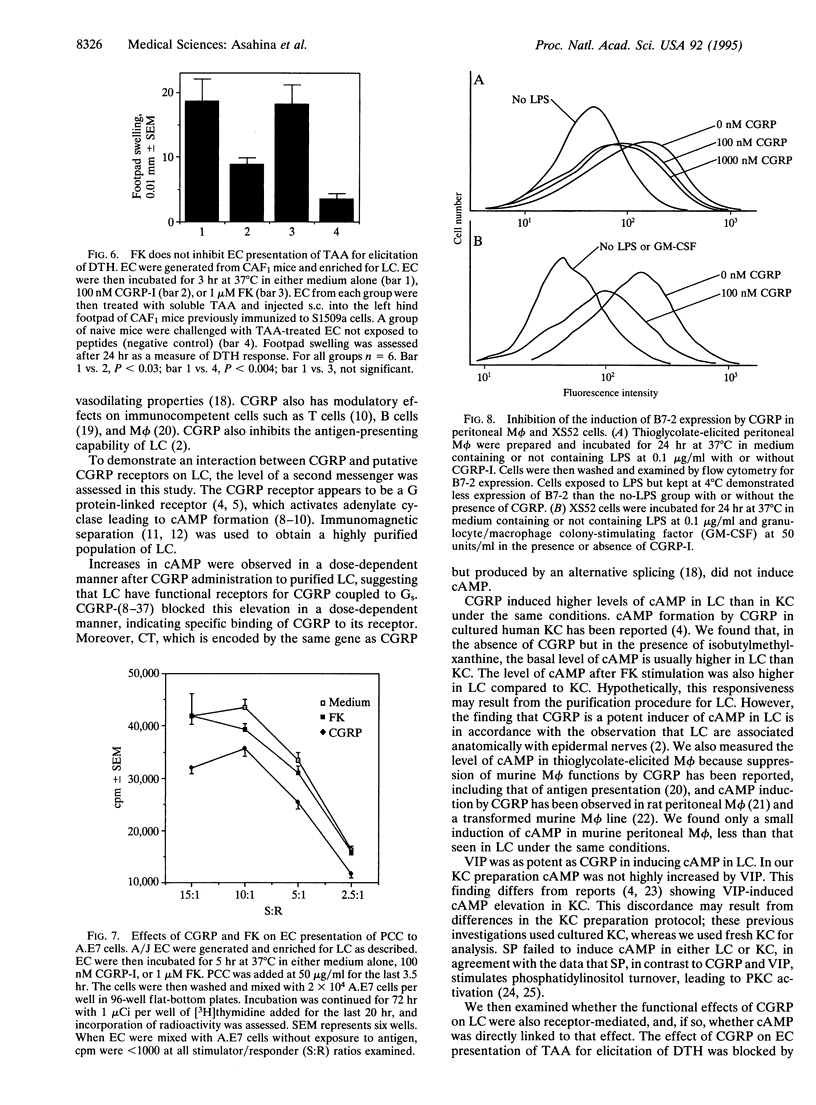
Epidermal Langerhans cells (LC) are associated anatomically with epidermal nerves, and a product of these nerves, calcitonin gene-related peptide (CGRP), inhibits the antigen-presenting capacity of LC and macrophages. As the CGRP receptor appears to be coupled to Gs alpha protein, which in turn activates adenylate cyclase, the ability of CGRP to induce cAMP in LC was examined and correlated with functional effects. LC were isolated from murine epidermal cells using antibodies on magnetic microspheres. Exposure to CGRP induced a significant increase in cAMP content, which could be inhibited by coculture with a truncated form of CGRP [CGRP-(8-37)] that is a specific competitive inhibitor of CGRP. Substance P and calcitonin failed to induce cAMP in LC. Although culture in CGRP reduced the ability of murine epidermal cells enriched for LC content to present pigeon cytochrome c to a responsive clone or to present antigen for elicitation of delayed-type hypersensitivity in immune mice, culture in forskolin had little or no effect on antigen presentation despite increased cAMP content of LC as much or more than that induced by CGRP. The effect of CGRP on antigen presentation in these systems could be blocked with CGRP-(8-37). CGRP inhibited the induction of B7-2 by lipopolysaccharide on peritoneal macrophages and a LC line, whereas calcitonin did not. CGRP induces specific accumulation of cAMP in LC and inhibits LC antigen-presenting function by a receptor-mediated event. However, the induction of cAMP by itself does not account for inhibition of antigen presentation. Suppression of the expression of B7-2 may be one mechanism by which CGRP inhibits antigen presentation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abello J., Kaiserlian D., Cuber J. C., Revillard J. P., Chayvialle J. A. Characterization of calcitonin gene-related peptide receptors and adenylate cyclase response in the murine macrophage cell line P388 D1. Neuropeptides. 1991 May;19(1):43–49. doi: 10.1016/0143-4179(91)90072-q. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Chedeville A., Mirossay L., Chastre E., Hurbain-Kosmath I., Lopez M., Gespach C. Interaction of VIP, PACAP and related peptides in normal and leukemic human monocytes and macrophages. FEBS Lett. 1993 Mar 15;319(1-2):171–176. doi: 10.1016/0014-5793(93)80061-x. [DOI] [PubMed] [Google Scholar]
- Crook R. B., Yabu J. M. Calcitonin gene-related peptide stimulates intracellular cAMP via a protein kinase C-controlled mechanism in human ocular ciliary epithelial cells. Biochem Biophys Res Commun. 1992 Oct 30;188(2):662–670. doi: 10.1016/0006-291x(92)91107-2. [DOI] [PubMed] [Google Scholar]
- Epstein S. P., Baer R. L., Belsito D. V. Effect of triggering epidermal Fc gamma receptors on the interleukin-2- and interleukin-6-induced upregulation of Ia antigen expression by murine epidermal Langerhans cells: the role of prostaglandins and cAMP. J Invest Dermatol. 1991 Sep;97(3):461–472. doi: 10.1111/1523-1747.ep12481477. [DOI] [PubMed] [Google Scholar]
- Fiscus R. R., Hao H., Wang X., Arden W. A., Diana J. N. Nitroglycerin (exogenous nitric oxide) substitutes for endothelium-derived nitric oxide in potentiating vasorelaxations and cyclic AMP elevations induced by calcitonin gene-related peptide (CGRP) in rat aorta. Neuropeptides. 1994 Feb;26(2):133–144. doi: 10.1016/0143-4179(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Frohman E. M., Vayuvegula B., Gupta S., van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S., Bruvers S., Gallo R. L., Knisely T. L., Nazareno R., Granstein R. D. Tumor antigen presentation by murine epidermal cells. J Immunol. 1991 May 15;146(10):3656–3661. [PubMed] [Google Scholar]
- Haegerstrand A., Jonzon B., Dalsgaard C. J., Nilsson J. Vasoactive intestinal polypeptide stimulates cell proliferation and adenylate cyclase activity of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5993–5996. doi: 10.1073/pnas.86.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D., Schmitt D. A., Fabre M., Cazenave J. P. A method for the rapid isolation of human epidermal Langerhans cells using immunomagnetic microspheres. J Invest Dermatol. 1988 Sep;91(3):274–279. doi: 10.1111/1523-1747.ep12470445. [DOI] [PubMed] [Google Scholar]
- Hosoi J., Murphy G. F., Egan C. L., Lerner E. A., Grabbe S., Asahina A., Granstein R. D. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993 May 13;363(6425):159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Tanaka H., Fukaya T., Ohkawara A. Substance P induces intracellular calcium increase and translocation of protein kinase C in epidermis. Br J Dermatol. 1992 Dec;127(6):595–599. doi: 10.1111/j.1365-2133.1992.tb14872.x. [DOI] [PubMed] [Google Scholar]
- Lauder J. M. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993 Jun;16(6):233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Calcitonin gene-related peptide and cyclic AMP stimulate phosphoinositide turnover in skeletal muscle cells. Interaction between two second messenger systems. J Biol Chem. 1989 Feb 15;264(5):2683–2689. [PubMed] [Google Scholar]
- Lewis D. L., Lechleiter J. D., Kim D., Nanavati C., Clapham D. E. Intracellular regulation of ion channels in cell membranes. Mayo Clin Proc. 1990 Aug;65(8):1127–1143. doi: 10.1016/s0025-6196(12)62726-8. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Humphreys S., Rangnekar V., Ciallella J. Modulation of B lymphocyte differentiation by calcitonin gene-related peptide (CGRP). II. Inhibition of LPS-induced kappa light chain expression by CGRP. Cell Immunol. 1993 Sep;150(2):405–416. doi: 10.1006/cimm.1993.1208. [DOI] [PubMed] [Google Scholar]
- Morris J., Alaibac M., Jia M. H., Chu T. Purification of functional active epidermal Langerhans cells: a simple and efficient new technique. J Invest Dermatol. 1992 Aug;99(2):237–240. doi: 10.1111/1523-1747.ep12650459. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Chen Z. M., Schwartz R. H., Gorman D. M., Kennedy M. K. Subset of CD4+ T cell clones expressing IL-3 receptor alpha-chains uses IL-3 as a cofactor in autocrine growth. J Immunol. 1994 Oct 1;153(7):3014–3027. [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Najar H. M., Ruhl S., Bru-Capdeville A. C., Peters J. H. Adenosine and its derivatives control human monocyte differentiation into highly accessory cells versus macrophages. J Leukoc Biol. 1990 May;47(5):429–439. doi: 10.1002/jlb.47.5.429. [DOI] [PubMed] [Google Scholar]
- Nong Y. H., Titus R. G., Ribeiro J. M., Remold H. G. Peptides encoded by the calcitonin gene inhibit macrophage function. J Immunol. 1989 Jul 1;143(1):45–49. [PubMed] [Google Scholar]
- Oksenberg D., Oksenberg J. R., Sakai K., Peroutka S. J., Steinman L. Cyclic adenosine 3',5'-monophosphate metabolism in activated T-cell clones. Immunology. 1989 Aug;67(4):484–488. [PMC free article] [PubMed] [Google Scholar]
- Powers G. D., Faherty D. A., Connaughton S. E., Biondi D. A., Godfrey D. I., Gault A., Chen C. Y., Nabavi N. Expression and functional analysis of murine B7 delineated by a novel monoclonal antibody. Cell Immunol. 1994 Feb;153(2):298–311. doi: 10.1006/cimm.1994.1030. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994 Feb 15;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabier M. J., Farber E. M., Wilkinson D. I. Neuropeptides modulate leukotriene B4 mitogenicity toward cultured human keratinocytes. J Invest Dermatol. 1993 Feb;100(2):132–136. doi: 10.1111/1523-1747.ep12462780. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Bergstresser P. R. Langerhans cells: antigen presenting cells of the epidermis. Immunobiology. 1984 Dec;168(3-5):285–300. doi: 10.1016/S0171-2985(84)80117-5. [DOI] [PubMed] [Google Scholar]
- Szabo G., Kodys K., Miller-Graziano C. L. Dibutyryl-cAMP modulation of receptor expression and antigen presentation capacity in monocyte subpopulations. Int J Immunopharmacol. 1994 Feb;16(2):151–162. doi: 10.1016/0192-0561(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi S., Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol. 1993 Nov;101(5):646–651. doi: 10.1111/1523-1747.ep12371670. [DOI] [PubMed] [Google Scholar]
- Takashima A., Edelbaum D., Kitajima T., Shadduck R. K., Gilmore G. L., Xu S., Taylor R. S., Bergstresser P. R., Ariizumi K. Colony-stimulating factor-1 secreted by fibroblasts promotes the growth of dendritic cell lines (XS series) derived from murine epidermis. J Immunol. 1995 May 15;154(10):5128–5135. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Vignery A., Wang F., Ganz M. B. Macrophages express functional receptors for calcitonin-gene-related peptide. J Cell Physiol. 1991 Nov;149(2):301–306. doi: 10.1002/jcp.1041490217. [DOI] [PubMed] [Google Scholar]
- Wang F., Millet I., Bottomly K., Vignery A. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. J Biol Chem. 1992 Oct 15;267(29):21052–21057. [PubMed] [Google Scholar]
- Weihe E., Hartschuh W. Multiple peptides in cutaneous nerves: regulators under physiological conditions and a pathogenetic role in skin disease? Semin Dermatol. 1988 Dec;7(4):284–300. [PubMed] [Google Scholar]
- Xu S., Ariizumi K., Caceres-Dittmar G., Edelbaum D., Hashimoto K., Bergstresser P. R., Takashima A. Successive generation of antigen-presenting, dendritic cell lines from murine epidermis. J Immunol. 1995 Mar 15;154(6):2697–2705. [PubMed] [Google Scholar]
- Xu S., Ariizumi K., Edelbaum D., Bergstresser P. R., Takashima A. Cytokine-dependent regulation of growth and maturation in murine epidermal dendritic cell lines. Eur J Immunol. 1995 Apr;25(4):1018–1024. doi: 10.1002/eji.1830250424. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- Zona C., Farini D., Palma E., Eusebi F. Modulation of voltage-activated channels by calcitonin gene-related peptide in cultured rat neurones. J Physiol. 1991 Feb;433:631–643. doi: 10.1113/jphysiol.1991.sp018447. [DOI] [PMC free article] [PubMed] [Google Scholar]