Abstract
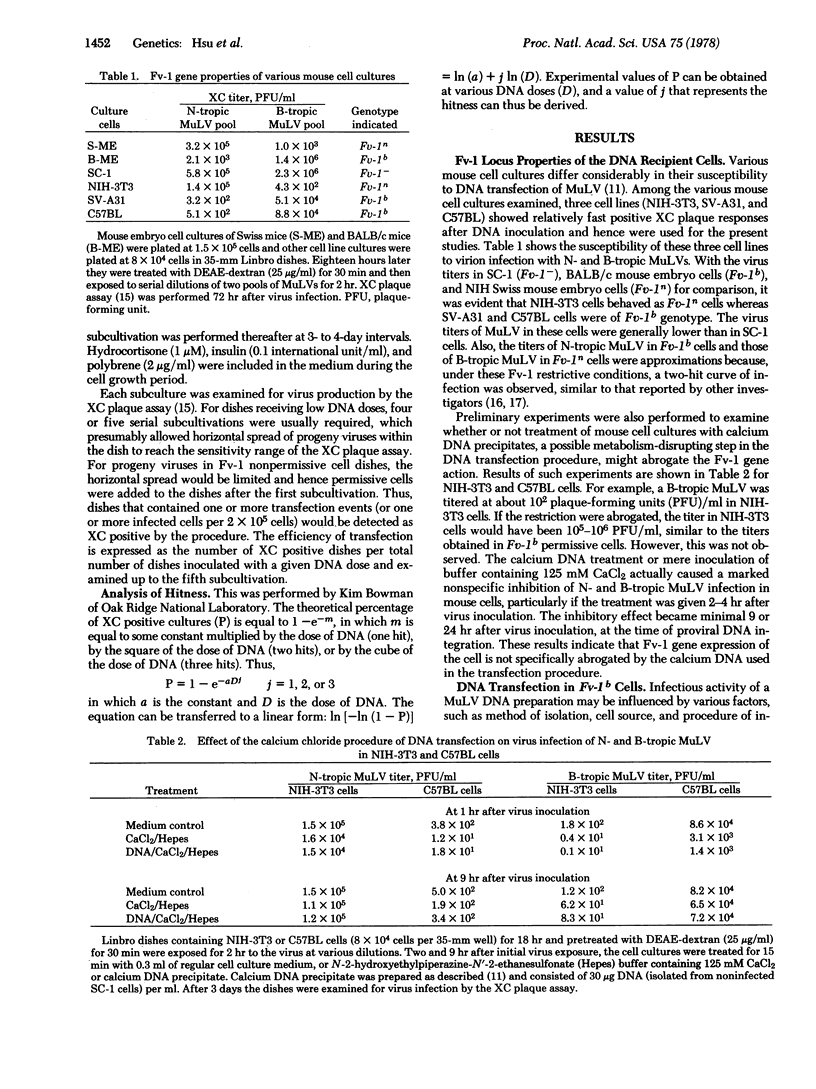
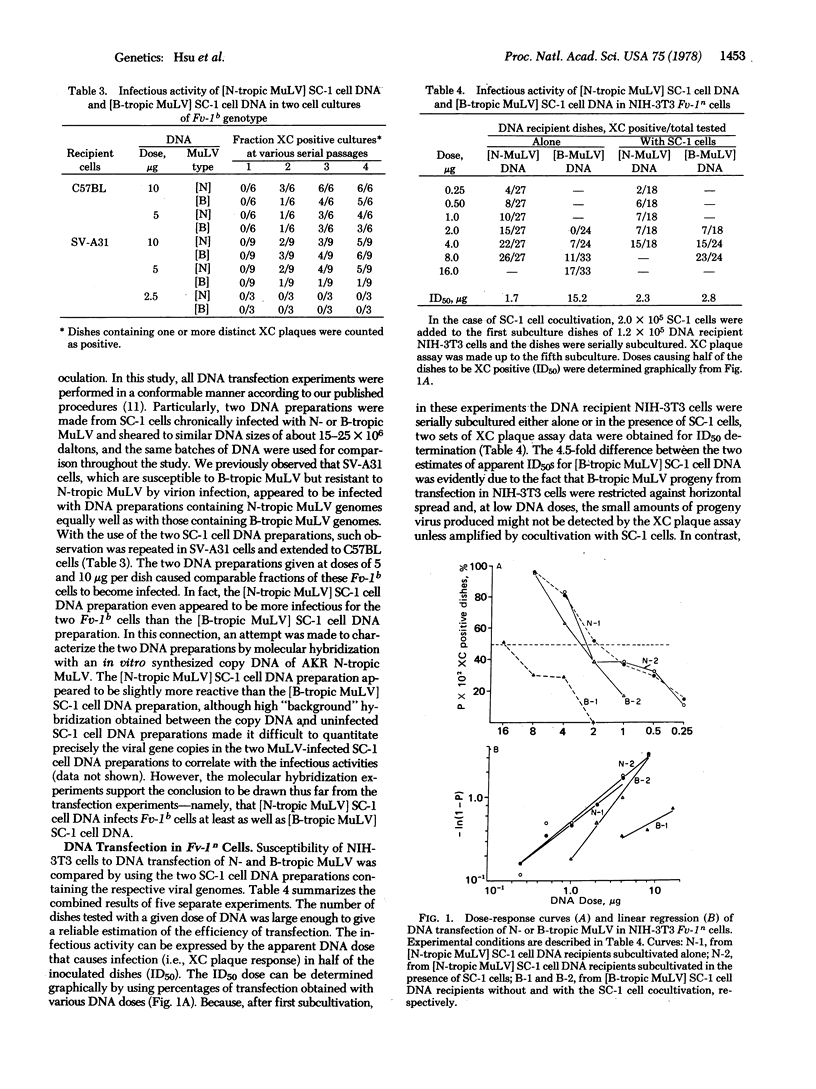
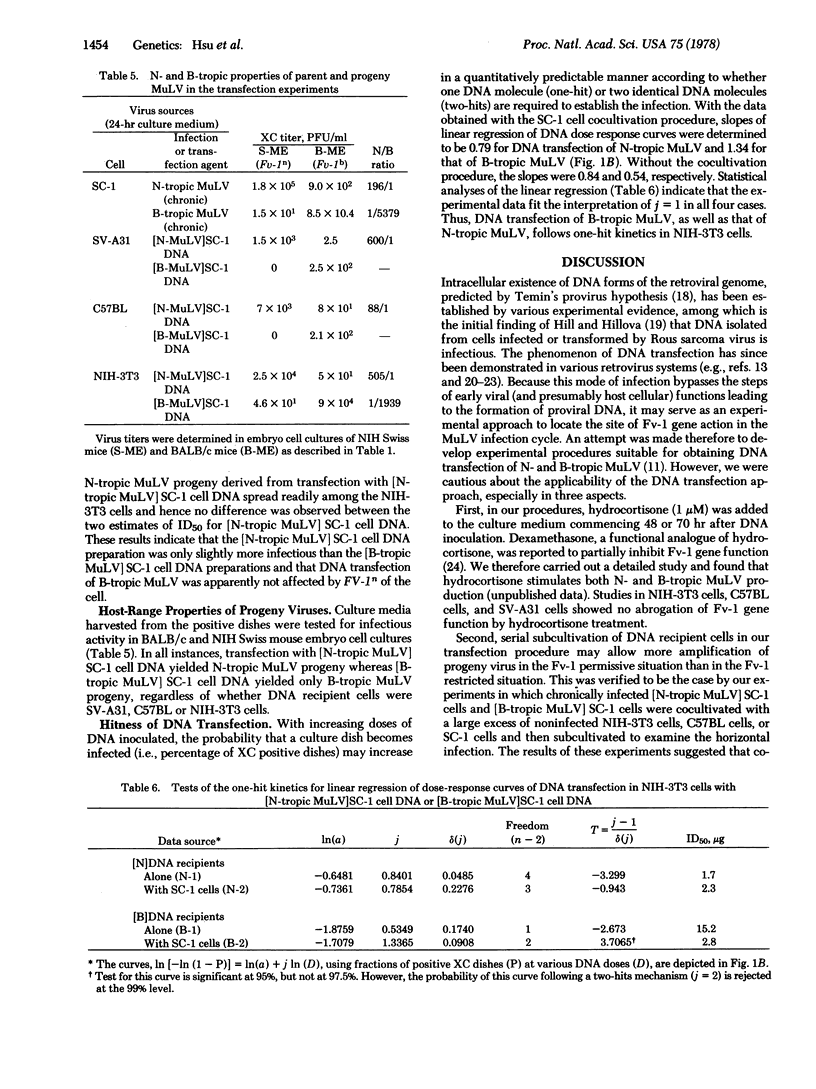
Whole-cell DNA preparations isolated from SC-1 cells chronically infected with N- or B-tropic murine leukemia viruses (MuLV) were tested for infectious activity in an Fv-1n (NIH-3T3) and two Fv-Ib (C57BL/6 and SV-A31) cell cultures. Efficiency of transfection for all DNAs was better in the NIH-3T3 cells than in C57BL/6 or SV-A31 cells; and an [N-tropic MuLV]SC-1 cell DNA preparation was slightly more infectious than a [B-tropic MuLV]SC-1 cell DNA preparation in all three cell cultures, regardless of their Fv-1 genotypes. Progeny viruses from the transfection showed N- or B-tropism corresponding to that of the parent viruses produced by the infected SC-1 cells that were used for the DNA preparation. DNA dose-response studies in NIH-3T3 cells revealed a one-hit mechanism for both the [B-tropic MuLV]SC-1 cell DNA and the [N-tropic MuLV]SC-1 cell DNA preparation. These results demonstrate that, in contrast to virion infection, transfection of N- and B-tropic MuLV with DNA preparations from chronically infected cells is not affected by the Fv-1 gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. Genetic control of susceptibility to Friend leukemia virus in mice: studies with the spleen focus assay method. Natl Cancer Inst Monogr. 1966 Sep;22:619–629. [PubMed] [Google Scholar]
- Blackstein M. E., Kochman M. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: effect of dexamethasone on the expression of the Fv-1 gene in the congenic strains SIM and SIM.R. Virology. 1976 Oct 1;74(1):252–255. doi: 10.1016/0042-6822(76)90150-1. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology. 1975 Jun;65(2):320–332. doi: 10.1016/0042-6822(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Grundner G., Wiener F., Klein E., Klein G., Harris H. The influence of the partner cell on the production of L virus and the expression of viral surface antigen in hybrid cells. J Exp Med. 1973 May 1;137(5):1240–1255. doi: 10.1084/jem.137.5.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Hillová J. Production virale dans les fibroblastes de poule traités par l'acide désoxyribonucléique de cellulex XC de rat transformées par le virus de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 14;272(24):3094–3097. [PubMed] [Google Scholar]
- Hsu I. C., Yang W. K. DNA transfection of ecotropic murine leukemia viruses in mouse cell cultures. Cancer Res. 1977 Jun;37(6):1709–1714. [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on synthesis of N-tropic and B-tropic murine leukemia viral RNA. Cell. 1976 Jan;7(1):33–39. doi: 10.1016/0092-8674(76)90252-x. [DOI] [PubMed] [Google Scholar]
- Karpas A., Milstein C. Recovery of the genome of murine sarcoma virus (MSV) after infection of cells with nuclear DNA from MSV transformed non-virus producing cells. Eur J Cancer. 1973 Apr;9(4):295–299. doi: 10.1016/0014-2964(73)90097-2. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Nicolson M. O., Hariri F., Krempin H. M., McAllister R. M., Gilden R. V. Infectious proviral DNA in human cells infected with transformation-defective type C viruses. Virology. 1976 Apr;70(2):301–312. doi: 10.1016/0042-6822(76)90273-7. [DOI] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Kaplan H. S. Conversion of restrictive mouse cells to permissiveness during sequential and mixed double infection by murine leukemia viruses. Virology. 1976 Oct 1;74(1):140–153. doi: 10.1016/0042-6822(76)90136-7. [DOI] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. V. Introduction of a gene responsible for susceptibility in the genetic complement of resistant mice. J Virol. 1969 Jun;3(6):543–548. doi: 10.1128/jvi.3.6.543-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Ray U., Soeiro R., Fields B. N. Host restriction of Friend Leukemia virus coat protein synthesis. J Virol. 1976 Apr;18(1):370–373. doi: 10.1128/jvi.18.1.370-373.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Humphrey J. B., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. 3. Assignment of the Fv-1 locus to linkage group 8 of the mouse. J Exp Med. 1973 Mar 1;137(3):850–853. doi: 10.1084/jem.137.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Gianni A. M., Rozenblatt S., Weinberg R. A. Infectious viral DNA of murine leukemia virus. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4910–4913. doi: 10.1073/pnas.72.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Fields B. N., Soeiro R. Host restriction of friend leukemia virus; fate of input virion RNA. Cell. 1974 Aug;2(4):271–277. doi: 10.1016/0092-8674(74)90021-x. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O. Detection of chicken sarcoma virus after transfection of chicken fibroblasts with DNA isolated from mammalian cells transformed with Rous Virus. Folia Biol (Praha) 1972;18(2):149–153. [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Myer F. E., McGrath L. Effect of the Fv-1 gene on leukemia virus in mouse cell heterokaryons. Int J Cancer. 1974 Oct 15;14(4):504–513. doi: 10.1002/ijc.2910140410. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Schluter B., Yang W., Brown A. Reciprocal inhibition of mouse leukemia virus infection by Fv-1 allele cell extracts. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4241–4245. doi: 10.1073/pnas.71.10.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]



