Abstract
We have successfully used a DNA·cDNA molecular hybridization assay to directly determine the presence or absence of human β globin gene sequences in 20 human—mouse somatic cell hybrids, each of which contained a different subset of human chromosomes. The assay is specific for the individual human globin genes and will detect the presence of a globin gene if the relevant chromosome is present in only 10% of the cells of a hybrid population. The content of human chromosomes in each hybrid clone was characterized by Giemsa 11 staining, Giemsa trypsin-Hoechst 33258 staining, and by the use of 22 independent isozyme markers for 17 different human chromosomes. All human chromosomes were present in one or more cell lines devoid of the human β globin gene except for 6, 8, 9, 11, and 13. Among these latter chromosomes, only chromosome 11 was present in the six hybrid clones that contained the human β globin gene. In fact, chromosome 11 was the only human chromosome that was present in all of the six hybrid clones found to be positive for the human β globin gene. Two sister clones, 157-BNPT-1 and 157-BNPT-4, had similar subsets of human chromosomes except that 11 was present only in 157-BNPT-4. 157-BNPT-4 contained the human β globin gene while 157-BNPT-1 did not. DNA from three hybrid lines was also annealed to purified human γ globin cDNA; two lines positive for human β globin gene sequences also contained human γ globin gene sequences while one line was negative for both β and γ gene sequences. On the basis of these results, the human β and γ globin genes have been assigned to human chromosome 11.
Keywords: gene mapping, cloning, cDNA, fibroblasts, molecular hybridization
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. J., Geist C. E., Steggles A. W., Barker J. E., Nienhuis A. W. Hemoglobin switching in sheep and goats. Preparation and characterization of complementary DNAs specific for the alpha-, beta-, and gamma-globin messenger RNAs of sheep. J Biol Chem. 1977 Mar 25;252(6):1908–1916. [PubMed] [Google Scholar]
- Bishop J. O., Jones K. W. Chromosomal localization of human haemoglobin structural genes. Nature. 1972 Nov 17;240(5377):149–150. doi: 10.1038/240149a0. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Madan K., Pearson P. L. Staining of some specific regions of human chromosomes, particularly the secondary constriction of No. 9. Nat New Biol. 1972 Jul 26;238(82):122–124. doi: 10.1038/newbio238122a0. [DOI] [PubMed] [Google Scholar]
- Boone C., Chen T. R., Ruddle F. H. Assignment of three human genes to chromosomes (LDH-A to 11, TK to 17, and IDH to 20) and evidence for translocation between human and mouse chromosomes in somatic cell hybrids (thymidine kinase-lactate dehydrogenase A-isocitrate dehydrogenase-C-11, E-17, and F-20 chromosomes). Proc Natl Acad Sci U S A. 1972 Feb;69(2):510–514. doi: 10.1073/pnas.69.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker H., Nijenhuis L. E. Possible linkage between a gene causing reinclusion of molar I and blood group P. Cytogenet Cell Genet. 1975;14(3-6):255–256. doi: 10.1159/000130356. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Studies on nucleic acid reassociation kinetics: empirical equations describing DNA reassociation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):415–419. doi: 10.1073/pnas.73.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. W., Tishler P. V., Atkins L., Sengupta S. K., Modest E. J., Forget B. G. Gene mapping by fluorescent in situ hybridization. Cell Biol Int Rep. 1977 May;1(3):255–262. doi: 10.1016/0309-1651(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Gilles H. M. Hereditary persistence of foetal haemoglobin associated with a gamma beta fusion variant, haemoglobin Kenya. Nat New Biol. 1973 Dec 12;246(154):184–186. doi: 10.1038/newbio246184a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Nienhuis A. Study of markers of human erythroid differentiation in hybrid cells. In Vitro. 1976 Nov;12(11):734–742. doi: 10.1007/BF02835448. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Nienhuis A., Turner P., Velez R., Anderson W. F., Ruddle F., Lawrence J., Creagan R., Kucherlapati R. Localization of the human alpha-globin structural gene to chromosome 16 in somatic cell hybrids by molecular hybridization assay. Cell. 1977 Sep;12(1):205–218. doi: 10.1016/0092-8674(77)90198-2. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Velez R., Burk R. D., Minna J., Anderson W. F., Nienhuis A. Extinction of globin gene expression in human fibroblast x mouse erythroleukemia cell hybrids. Somatic Cell Genet. 1976 Jul;2(4):373–384. doi: 10.1007/BF01538841. [DOI] [PubMed] [Google Scholar]
- Faber H. E., Kucherlapati R. S., Poulik M. D., Ruddle F. H., Smithies O. beta2-microglobulin locus on human chromosome 15. Somatic Cell Genet. 1976 Mar;2(2):141–153. doi: 10.1007/BF01542627. [DOI] [PubMed] [Google Scholar]
- Friend K. K., Chen S., Ruddle F. H. Differential staining of interspecific chromosomes in somatic cell hybrids by alkaline Giemsa stain. Somatic Cell Genet. 1976 Mar;2(2):183–188. doi: 10.1007/BF01542631. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Turner P., Benz E. J., Jr Relative stability of alpha- and beta-globin messenger RNAs in homozygous beta+ thalassemia. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3960–3964. doi: 10.1073/pnas.74.9.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
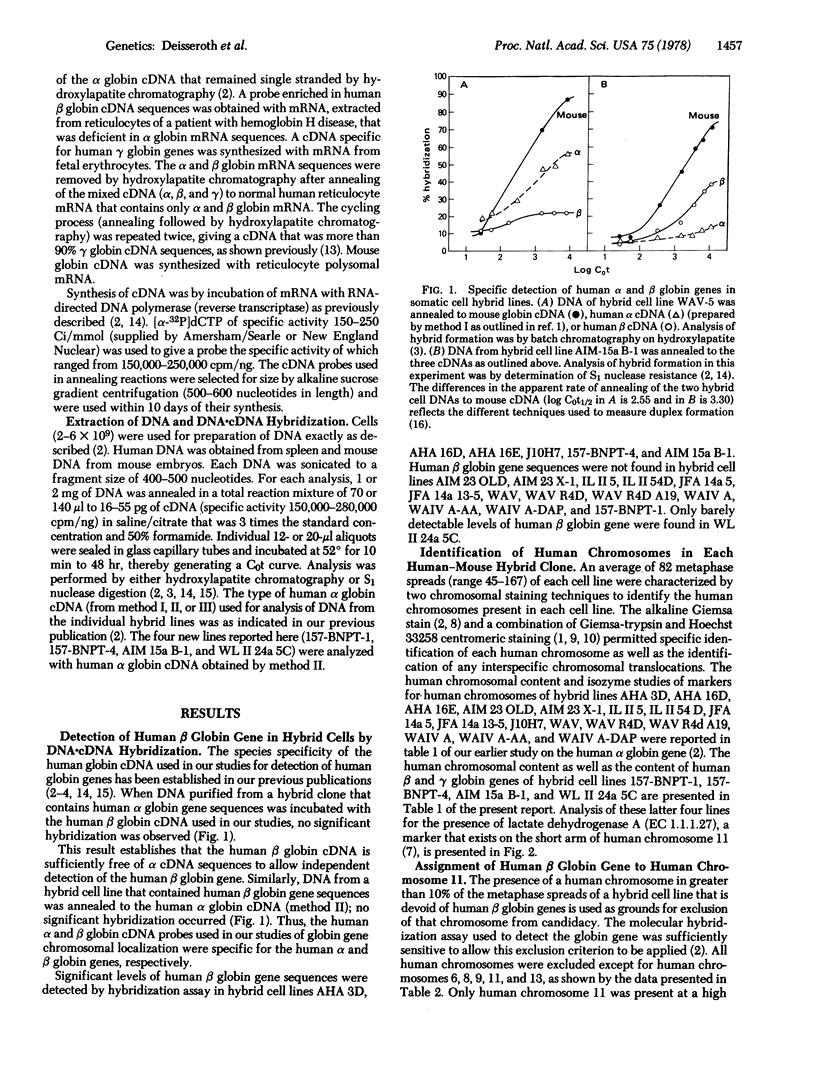
- Price P. M., Conover J. H., Hirschhorn K. Chromosomal localization of human haemoglobin structural genes. Nature. 1972 Jun 9;237(5354):340–342. doi: 10.1038/237340a0. [DOI] [PubMed] [Google Scholar]
- Price P. M., Hirschhorn K. In situ hybridization of chromosome loci. Fed Proc. 1975 Dec;34(13):2227–2232. [PubMed] [Google Scholar]