Abstract
Purpose
To compare the ability of two optical biometers to acquire the axial length (AL) measurement in cataractous eyes.
Methods
This prospective, comparative, single-center study comprised 105 eyes (63 patients). AL was acquired by the composite mean value of 20 measurements (composite-20 IM) and five measurements (composite-5 IM) (IOLMaster® 500 version 7.1 software), and the standard mean value of the first five measurements (standard-5 LS, Lenstar LS 900®). Anterior chamber depth (ACD) and average keratometry (K) readings were acquired.
Results
AL was acquired in 83.8%, 92.4%, and 84.8% of eyes for the composite-5 IM, composite-20 IM, and standard-5 LS, respectively. Standard-5 LS AL measurements were significantly shorter (P<0.001). IOLMaster® 500-acquired ACD (corneal epithelium to lens) measurements were significantly shorter (P<0.001). IOLMaster® 500 average K measurements were significantly steeper (P<0.001).
Conclusion
The composite-20 IM had the highest AL acquisition success rate of the three versions evaluated. AL, ACD, and average Ks were statistically different between the two biometers, although the differences were clinically insignificant.
Keywords: cataract, biometry, IOL power calculation
Introduction
Axial length (AL) measurement is a crucial aspect in predicting correct intraocular lens (IOL) power. The advent of new premium implant technologies has increased patient expectations for exceptional postoperative vision, in turn decreasing the acceptable margin for error in IOL power calculation. Achieving successful outcomes postoperatively, then, requires accurate preoperative measurement of AL.
Typically, AL measurement is undertaken with optical biometry.1 The IOLMaster® optical biometry system (Carl Zeiss Meditec AG, Jena, Germany) uses partial coherence interferometry (PCI) and has been studied in the use of calculating IOL powers.2–5 This technology has been shown to have excellent intraobserver and interobserver reliability, with extremely high precision and performance accuracy.2,3,6–16 The Lenstar LS 900® (Haag-Streit AG, Koeniz, Switzerland) is a second optical biometer that uses optical low-coherence reflectometry. This technology has also been shown to provide reliable and accurate measurements similar to PCI.1,17–19 In clinical practice, it is fairly common to use these devices interchangeably with the presumption that similar results will be obtained for all types of cataract grades.
Although dense cataracts may limit the ability of optical biometers to measure the AL, software and hardware upgrades have decreased acquisition failure substantially.18,20 Buckhurst et al18 reported AL was not measurable in 9% to 10% of eyes using either the Lenstar® or the IOLMaster® version 5 software in cataract patients. However, Hill et al20 reported a 96.3% overall success in acquiring AL with the IOLMaster® using version 5 software, with the failures attributed to dense cataracts, specifically with posterior subcapsular (PSC) cataracts.
The purpose of this study was to compare the AL measurement acquisition rates of the IOLMaster® 500 (using version 7.1 software) with both the composite mean value of 20 measurements (composite-20 IM) and five measurements (composite-5 IM) readings to the AL measurement acquisition rate of the Lenstar LS 900® (using the standard mean value of the first five measurements [standard-5 LS]) in eyes with cataracts and to educate clinicians about the potential differences in reliability and repeatability between the two when measuring identical cataract densities. Cataracts were classified and graded according to morphology and density.
Patients and methods
This study comprised 105 eyes of 63 consecutive patients scheduled for cataract extraction in one or both eyes at The Eye Center of Columbus, Columbus, OH, USA. The study conformed to the Declaration of Helsinki and was approved by the Research Consultants Review Committee Institutional Review Board (Austin, TX, USA). Patients were prospectively recruited and signed consent forms. Inclusion criteria required that patients be scheduled for cataract extraction in one or both eyes.
Patients were excluded from the study if they had a physical inability to position at the slit lamp biomicroscope or biometry device (ie, head tremor), inability to open the eyelid widely enough so all measurements could be performed, inability to fixate due to ocular disease (eg, macular degeneration, amblyopia), corneal or media opacities other than cataract that may cause acquisition failure (eg, corneal scar, vitreous condensation), or an active ocular infection or inflammation of the eye.
Preoperative biometric measurements were obtained in all patients using two measurement algorithms with the IOLMaster® 500 and one algorithm with the Lenstar®. In addition to AL, anterior chamber depth (ACD) and keratometry (K) readings were obtained. The IOLMaster® keratometer projects six spots of light onto the cornea in a hexagonal pattern with a diameter of 2.5 mm. The Lenstar® measures Ks at 2.30 mm and 1.65 mm (most manual keratometers measure 3.0 mm diameter). Cataracts were graded and classified using the Lens Opacities Classification III scoring system (LOCS III)21 by a single LOCS III-certified examiner (AE). A complete ophthalmic examination was performed on all patients, including the measurement of uncorrected distance visual acuity and corrected distance visual acuity, best corrected visual acuity (BCVA), and slit lamp and fundus examination.
Biometric measurements
Both the IOLMaster® 500 and the Lenstar® measure AL, anterior corneal radii, ACD, and the corneal diameter (CD) in the human eye. Additionally, the Lenstar® measures pupil size and corneal, lens, and retinal thickness; the LS 900 is the only optical biometer that measures lens thickness (LT). New formulas, such as the Hoffer H, Hoffer H-5, Holladay 2, and Olsen, use LT as one of the variables for calculating IOL power; LT has been suggested as particularly useful in short eyes. Surgeons using these formulas and the IOLMaster® 500 need to measure LT in a separate step with immersion ultrasound biometry or estimate it by patient age.1,18
The IOLMaster® 500 and Lenstar LS 900® differ in how AL is acquired. Whereas the Lenstar® compiles an average of all recorded measurements, the IOLMaster® 500 applies noise reduction filtering to each individual scan, attenuating the variable noise in each measurement while calculating the composite signal. The IOLMaster® 500 offers an AL calculation algorithm that utilizes a composite of 20 scans and the Lenstar® measures five individual scans.
For this study, AL measurements reported using the Lenstar® algorithm were referred to as “standard,” and AL measurements reported using the IOLMaster® 500 software algorithm were referred to as “composite.” The following describes the measurements recorded for each study eye:
Standard mean value of the first five measurements (standard-5 LS) refers to the average AL measurement calculated by the Lenstar® after the operator performed five measurements with the device.
Composite mean value of the first five measurements (composite-5 IM) refers to the composite AL measurement calculated after taking five measurements with the IOLMaster® 500 with version 7.1 software.
Composite mean value of 20 measurements (composite-20 IM) refers to the composite AL measurement calculated after taking 20 measurements with the IOLMaster® 500 with version 7.1 software.
In steps 2 and 3, only one set of readings was performed with the IOLMaster® 500; the technician recorded the first five readings and continued with the remainder for the composite-20.
The sequence of measurement acquisition with the devices alternated between study eyes. Two examiners performed all the tests according to the manufacturers’ device recommendations. The examiners also recorded the time required by each device to acquire the measurements. The Accutome A-Scan Synergy ultrasound unit was used to measure AL in the study eyes that were not measurable with the IOLMaster® composite-20 IM. If either biometer could not acquire AL, we did an immersion scan.
Statistical analysis
The AL measurement acquisition rates were calculated for each of the three measurement methods and compared using the McNemar Test. For each measurement method, the AL availability was stratified according to nuclear color, nuclear opalescence, cortical grade, and PSC opacity, which were determined using the LOCS III grading system. The Fisher exact test was performed to compare the AL availabilities across different cataract grades of nuclear color, nuclear opalescence, cortical grade, and PSC. The mean, standard deviation, and the range of the AL, ACD, and average K measurements for both instruments were summarized and compared using the paired t-test.
All statistical analyses were performed using SAS software (version 9.1.3, SAS Institute, Inc., Cary, NC, USA).
Results
The mean age of the patients was 71.1±0.5 years (range, 38–90 years). The preoperative BCVA was 20/40 or better in 74% of eyes and 20/200 or worse in 11%. The mean spherical equivalent in all eyes was −0.15±2.63 D (range, −7.00 D to +6.50 D). The average time required to take all the measurements on the Lenstar LS 900® was 5.88±0.19 minutes (95% confidence interval [CI], 5.50–6.25) compared to 4.50±0.16 minutes (95% CI, 4.18–4.81) with the IOLMaster® 500 (P<0.001). All patients in the study had more than one type of cataract (mixed cataracts).
There was no statistically significant difference in the success rate of acquiring AL measurements between the standard-5 LS and composite-5 IM methods (84.8% versus 83.8%, respectively; P=0.782). However, the composite-20 IM method was statistically significantly more successful at acquiring AL measurements (92.4%) compared with the standard-5 LS (P=0.011) and composite-5 IM methods (P=0.003).
Table 1 shows the AL measurement availability for each of the three measurement methods stratified by cataract type and grade. Of the three measurement methods, the composite-20 IM method had the most consistent success for AL measurements across different cataract grades. The standard-5 LS and composite-5 IM methods were able to acquire AL measurements successfully in 30% of eyes with a cataract nuclear color graded >5.0, whereas the composite-20 IM was able to acquire AL measurements successfully in 60% of eyes with a cataract nuclear color graded >5.0. These data were not powered to detect a statistical significance due to the small number of patients with cataracts with a nuclear color >5.0 on the LOCS III scale (n=10).
Table 1.
Axial length acquisition
| Cataract type and grade (LOCS III) | Lenstar® (standard-5 LS) n/N (%) | IOLMaster® (composite-5 IM) n/N (%) | IOLMaster® (composite-20 IM) n/N (%) |
|---|---|---|---|
| PSC | |||
| ≤4.2 | 75/77 (97.2) | 71/77 (92.2) | 77/77 (100) |
| >4.2 | 13/27 (48.3) | 16/27 (59.2) | 19/27 (70.4) |
| Cortical cataract | |||
| ≤4.2 | 83/92 (90.2) | 84/92 (91.3) | 89/92 (96.7) |
| >4.2 | 5/12 (41.6) | 3/12 (25) | 7/12 (58.3) |
| Nuclear color | |||
| ≤5.0 | 85/94 (90.4) | 84/94 (89.4) | 90/94 (95.7) |
| >5.0 | 3/10 (30) | 3/10 (30) | 6/10 (60) |
| Nuclear opalescence | |||
| ≤5.0 | 83/93 (89.3) | 83/93 (89.3) | 89/93 (95.7) |
| >5.0 | 5/11 (45.5) | 4/11 (36.4) | 7/11 (63.6) |
Notes: Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany).
Abbreviations: LOCS, Lens Opacities Classification III scoring system; PSC, posterior subcapsular cataract; n, number of patients where the device was able to successfully obtain an axial length; N, total number of patients in each of the groups.
Similarly, the composite-5 IM had the lowest success for AL measurement for cortical grades >4.2 (Table 1). The composite-5 IM was successful in 25% of cataracts with cortical grades >4.2, compared with 41.6% and 58.3% with the standard-5 LS and composite-20 IM, respectively. The standard-5 LS had the lowest overall success for AL measurement in PSC cataracts. In the densest PSC cataracts (grades 4.2 to 5.9), the standard-5 LS and composite-5 IM were successful in 48.2% and 59.2% of cases, respectively, compared with 70.4% in the composite-20 IM method.
The ability to successfully acquire AL varied by device and acquisition algorithm. Overall, AL could not be measured in seven of 105 eyes with either device (Table 2). The IOLMaster® 500 was successful in acquiring AL in nine eyes that the Lenstar® (standard-5 LS) was not capable of measuring successfully. The standard-5 LS was able to acquire AL in one eye that could not be acquired with either the composite-5 IM or the composite-20 IM. Immersion ultrasound measurement in this eye yielded a 0.51 mm longer AL compared with the standard-5 LS measurement. The patient received an implant based on the immersion scan and had an emmetropic result.
Table 2.
Acquisition failures, Lenstar® and IOLMaster®
| Subject number | Eye | Age | Nuclear color* | Nuclear opalescence* | Cortical* | PSC* | IOL Master® (composite-5 IM) (AL, mm) | IOL Master® (composite-20 IM) (AL, mm) | Lenstar® (standard-5 LS) (AL, mm) | Immersion ultrasound (AL, mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Both IOLMaster® 500 and Lenstar® failed | ||||||||||
| 2 | OS | 65 | 5.6 | 6.2 | 5.9 | 5.9 | 22.62 | |||
| 3 | OD | 84 | 6.2 | 5.9 | 3.9 | 5.6 | 24.77 | |||
| 8 | OD | 81 | 3.5 | 2.9 | 5.3 | 4.3 | 24.61 | |||
| 8 | OS | 81 | 3.4 | 2.9 | 4.9 | 5.9 | 24.26 | |||
| 11 | OD | 80 | 4.9 | 4.6 | 3.2 | 5.9 | 24.94 | |||
| 16 | OS | 61 | 6.9 | 6.8 | 5.9 | 5.9 | 23.39 | |||
| 45 | OD | 65 | 6.9 | 6.9 | 5.9 | 5.9 | 23.05 | |||
| IOLMaster® 500 failed but Lenstar® succeeded | ||||||||||
| 23 | OD | 63 | 3.4 | 4.4 | 0.7 | 5.3 | 21.47 | 21.98 | ||
| IOLMaster® 500 succeeded but Lenstar® failed | ||||||||||
| 7 | OS | 79 | 3.6 | 2.9 | 4.8 | 5.9 | 23.84 | 23.82 | ||
| 17 | OS | 83 | 3.7 | 3.6 | 2.8 | 3.9 | 23.11 | |||
| 22 | OD | 80 | 3.8 | 4.3 | 3.9 | 5.9 | 22.24 | 22.22 | ||
| 38 | OD | 84 | 5.9 | 5.8 | 2.7 | 4.2 | 23.31 | 23.27 | ||
| 48 | OD | 39 | 2.2 | 1.9 | 1.5 | 4.9 | 22.63 | 22.60 | ||
| 56 | OS | 65 | 3.2 | 2.4 | 1.8 | 5.3 | 26.67 | |||
| 62 | OD | 78 | 5.1 | 4.3 | 2.9 | 4.9 | 24.63 | 24.55 | ||
| 65 | OS | 80 | 6.6 | 6.9 | 5.9 | 5.9 | 22.25 | |||
| 67 | OD | 44 | 2.3 | 2.1 | 2.1 | 5.5 | 23.32 | 23.32 | ||
| IOLMaster® 500 composite-20 and Lenstar® succeeded but IOLMaster® 500 composite-5 failed | ||||||||||
| 14 | OS | 74 | 4.1 | 3.9 | 0.4 | 2.9 | 23.25 | 23.25 | ||
| 26 | OD | 81 | 3.4 | 3.4 | 5.2 | 0.3 | 23.35 | 23.38 | ||
| 26 | OS | 81 | 3.4 | 3.2 | 5.3 | 0.1 | 23.13 | 23.14 | ||
| 31 | OD | 81 | 3.3 | 3.2 | 2.6 | 0.9 | 24.21 | 24.13 | ||
| 38 | OS | 84 | 5.7 | 5.1 | 2.8 | 3.8 | 23.20 | 23.17 | ||
| 43 | OD | 62 | 5.2 | 5.7 | 5.9 | 5.9 | 22.03 | 22.01 | ||
Notes:
Based on LOCS III classification scheme. Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany).
Abbreviations: AL, axial length; LOCS, Lens Opacities Classification III scoring system; OD, right eye; OS, left eye; PSC, posterior subcapsular cataract.
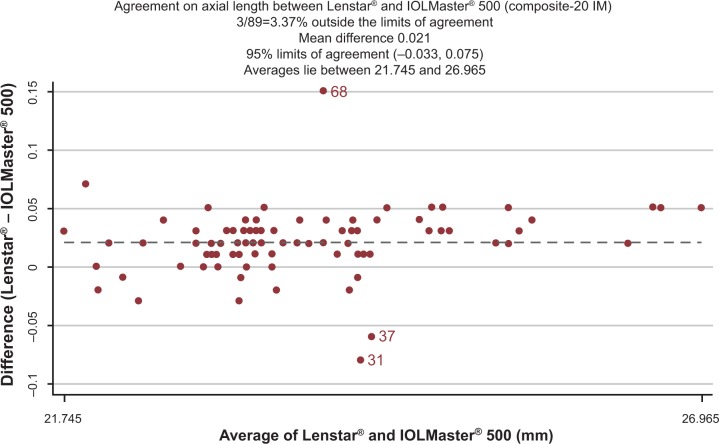
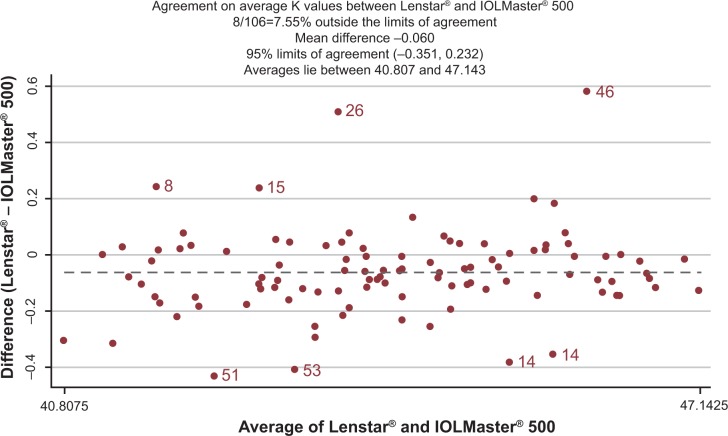
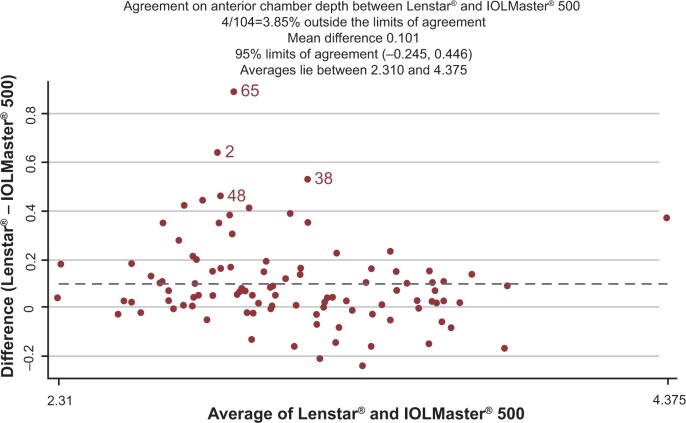
The difference in AL with both devices is illustrated in Figure 1. The measurements acquired by the standard-5 LS method were statistically significantly longer (P=<0.001) compared with the composite-5 IM and composite-20 IM. These differences are not likely to be clinically significant. The difference in K readings acquired with the two devices is illustrated in Figure 2. The differences in ACD with the two devices are illustrated in Figure 3. These differences are not likely to be clinically significant. There was no difference between the two composite methods (P=0.871).
Figure 1.
Axial length difference between IOLMaster® (composite-20 IM) and Lenstar®.
Notes: Points outside limits labelled by subject id. Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany). The dotted line signifies the mean difference in axial length (0.021).
Abbreviation: id, identification number.
Figure 2.
Corneal curvature (average K) difference between IOLMaster® and Lenstar®.
Notes: Points outside limits labelled by subject id. Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany). The dotted line signifies the mean difference in average K values (−0.060).
Abbreviation: id, identification number.
Figure 3.
Anterior chamber depth difference between IOLMaster® and Lenstar®.
Notes: Points outside limits labelled by subject id. Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany). The dotted line signifies the mean difference in anterior chamber depth (0.101).
Abbreviation: id, identification number.
Table 3 shows the differences between the two biometers in mean ACD and average K data. Although ACD values are slightly longer and average K measurements were slightly flatter with the Lenstar® compared to the IOLMaster® 500 (P<0.001), the difference is not likely to be clinically relevant, but the variation may be. Table 4 shows the differences in AL measurement, when both eyes could be assessed by both devices.
Table 3.
Comparison of parameter measurements
| Parameter | IOLMaster® 500 | Lenstar® | Difference Lenstar® – IOLMaster® 500 |
|---|---|---|---|
| ACD (mm) | |||
| Mean ± SD | 3.05±0.39 | 3.15±0.36 | 0.10±0.18 |
| Range | 2.23 to 4.19 | 2.33 to 4.56 | −0.24 to 0.89 |
| P-value | <0.001 | ||
| Average K | |||
| Mean ± SD | 44.12±1.54 | 44.06±1.56 | −0.06±0.15 |
| Range | 40.96 to 47.21 | 40.66 to 47.08 | −0.43 to 0.58 |
| P-value | <0.001 | ||
Notes: Lenstar® standard-5, Haag-Streit AG (Koeniz, Switzerland); IOLMaster® 500, Carl Zeiss Meditec AG (Jena, Germany).
Abbreviations: ACD, anterior chamber depth; average K, corneal curvature; SD, standard deviation.
Table 4.
Comparison of axial length measurements
| Difference* | Difference (Lenstar® standard-5 LS – IOLMaster® composite-5 IM) | Difference (Lenstar® standard-5 LS – IOLMaster® composite-20 IM) | Difference (IOLMaster®composite-5 IM – IOLMaster® composite-20 IM) |
|---|---|---|---|
| AL (mm) | |||
| Mean ± SD | 0.03±0.03 | 0.02±0.03 | −0.0±0.03 |
| Range | −0.06 to 0.19 | −0.08 to 0.15 | −0.20 to 0.08 |
| P-value paired-t comparing means | <0.001 | <0.001 | 0.840 |
Notes:
Based on eyes with measurements from both methods. Lenstar® standard-5, Haag-Streit (Koeniz, Switzerland); IOLMaster® composite-5; IOLMaster® composite-20, Carl Zeiss Meditec AG (Jena, Germany).
Abbreviations: AL, axial length; SD, standard deviation.
Discussion
Previous studies have shown that the AL cannot be measured with the IOLMaster® in 4% to 18% of eyes with a dense cataract.7,11,14,22,23 Although density grading and morphology is unavailable in many of these studies, the presence of PSC opacities has been shown to significantly affect the ability to measure AL.20,22,23 Dense nuclear sclerotic and PSC cataracts are commonly reported causes of acquisition failure,7,11,20,22,23 which is consistent with the findings in the current study. However, as we took AL measurements at the center of the cataract, we found the IOLMaster® 500 was more reliable and repeatable with better penetration than the Lenstar® in those patients with dense cataracts.
Hill et al20 reported success in acquiring the AL in 92.6% of eyes using the composite-5 method and a success rate of 96.3% using the composite-20 method using the IOLMaster® 5.0 with version 5 software. The presence of PSC cataracts impacted the ability of all acquisition methods to acquire AL, and the effect was most apparent with denser PSC cataracts. AL acquisition success rates were slightly lower in our study (83.8% and 92.4% for the composite-5 IM and composite-20 IM methods, respectively) compared with Hill et al.20 This disparity may be attributable to methodological differences (ie, sample size, cataract grades). Whereas Hill et al performed AL measurements around the areas of the cataract perceived to be the least dense, our study protocol called for AL measurement at the center of the cataract regardless of density.
Buckhurst et al18 reported a 9% to 10% acquisition failure rate in cataractous eyes with both the Lenstar® and the IOLMaster® using version 5 software, and attributed AL acquisition failures to dense media opacities (classification and grading of cataract according to morphology and density was not performed). Our study results add to the literature as we found the composite-5 IM method was significantly affected by nuclear color and cortical grades, while the standard-5 LS method was significantly affected by PSC and cortical grades.
In our study, neither biometer was able to measure an AL in seven of the 105 eyes. Immersion ultrasound was used to obtain an AL in eight eyes, seven in which AL was unattainable with either the IOLMaster® 500 or Lenstar® and in one eye that the IOLMaster® 500 failed to measure the AL but the Lenstar® succeeded. In this last eye, immersion ultrasound yielded an AL measurement that was 0.51 mm longer than the Lenstar®-derived AL measurement. In this eye, the Lenstar®-derived AL measurement would have resulted in a 2.00 D myopic surprise postoperatively; by using the immersion ultrasound measurement, we achieved a manifest refraction of plano at the 1 month postoperative visit. The error in calculation by the Lenstar® may be attributed to its acquisition algorithm of averaging results of multiple scans to calculate AL. By design, the Lenstar® includes outlier data in its algorithms that may result in postoperative refractive surprises. We recommend further analysis to confirm this suggestion.
Comparatively, the IOLMaster® 500 derives its ALs from a composite acquisition algorithm, which increases the probability of a precise measurement with additional scans. Across all cataract grades, the composite-20 IM achieved a greater ability to acquire AL than the composite-5 IM. In the nine eyes that could not be measured with the Lenstar® but that could be measured with the IOLMaster® 500, at 1 month postoperatively all eyes were within ±0.50 D of the intended refractive target, suggesting the accuracy of the IOLMaster® 500.
The standard-5 LS method produced AL measurements that were 0.03 mm and 0.02 mm longer than the composite-5 IM and composite-20 IM methods, respectively. Hoffer et al1 and Buckhurst et al18 also reported slightly longer (+0.026 mm and +0.01 mm, respectively) AL measurements, while Rabsilber et al17 found that the Lenstar® and the IOLMaster® produced similar AL measurement lengths in cataractous eyes. The reason for these discrepancies is unclear; however, the mean differences are very low and likely clinically insignificant.
Our findings support previous studies that also reported higher ACD values determined with the Lenstar® compared with the IOLMaster® 500.1,17,18 The variation in technology used may account for this. Lenstar® uses laser interferometry to determine all axial dimensions of the eye, including ACD, whereas the IOLMaster® 500 uses lateral slit illumination and video image analysis to evaluate the distance between the cornea and the anterior surface of the crystalline lens,17 which may explain these differences. The difference in ACD was 0.10±0.18 (range, −0.24 to 0.89), which could indicate that different results may be obtained using a formula that uses the ACD as one parameter (ie, Haigis or Olsen).
The Lenstar® produced slightly flatter K readings compared with the IOLMaster® 500 in our study (−0.06±0.15 D), which again supports Hoffer et al.1 The differences in K readings (see Table 3) may indicate that different IOL calculation results could be obtained.
We noted that the total measurement time was faster with the IOLMaster® 500 (average, 4.50 minutes) compared to the Lenstar® (average, 5.88 minutes), even though the IOLMaster® 500 requires extra time to obtain K, ACD, and CD readings. Despite the fact that the Lenstar® device measures all parameters in a single process, it took 25% longer (a difference of 1.38 minutes) to acquire measurements than the IOLMaster® 500 did (P<0.001). Moreover, the shorter acquisition time with the IOLMaster® 500 may translate into improved clinical efficiency and greater workflow.
This study is not without limitations. Our study population comprised mixed cataracts as defined by the LOCS III classification scheme, reflective of the real-world clinical setting, but may be a potential limitation to the study. Ideally, detecting associations between one specific class of cataracts and the ability of a biometric assessment of AL would entail enrollment of eyes with only the desired morphologic characteristics. Eyes also can be subdivided according to the desired morphologic characteristics (ie, PSC <4.2) with all other variables (eg, nuclear color, nuclear opalescence, and cortical grade <0.1) to isolate the effect of the desired cataract characteristic. Our sample size was also not large enough to set up a regression model in which potentially confounding data could be better controlled.
In conclusion, ACD and average K readings were statistically different between the two optical biometers, although these differences were not clinically significant in terms of refractive outcomes. AL measurements using the IOLMaster® 500 yielded higher acquisition success rates compared with the Lenstar LS 900®. In this study, the IOLMaster® 500 was more likely to acquire the AL through denser cataracts, requiring fewer immersion studies. Greater accuracy and efficiency in determining the AL should translate to more reproducible measurements, improved workflow and, therefore, improved clinical outcomes.
Acknowledgments
Equipment was provided by each manufacturer free of charge. AE has received speaker fees from Carl Zeiss Meditec Inc., Dublin, CA, USA, but has no financial or proprietary interest in any material or method mentioned. Michelle Dalton, ELS, provided editorial assistance.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Hoffer KJ, Shammas HJ, Savini G. Comparison of 2 laser instruments for measuring axial length. J Cataract Refract Surg. 2010;36(4):644–648. doi: 10.1016/j.jcrs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Olsen T. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 1992;18(2):125–129. doi: 10.1016/s0886-3350(13)80917-0. [DOI] [PubMed] [Google Scholar]
- 3.Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126(4):524–534. doi: 10.1016/s0002-9394(98)00113-5. [DOI] [PubMed] [Google Scholar]
- 4.Kiss B, Findl O, Menapace R, et al. Biometry of cataractous eyes using partial coherence interferometry: clinical feasibility study of a commercial prototype I. J Cataract Refract Surg. 2002;28(2):224–229. doi: 10.1016/s0886-3350(01)01272-x. [DOI] [PubMed] [Google Scholar]
- 5.Kiss B, Findl O, Menapace R, et al. Refractive outcome of cataract surgery using partial coherence interferometry and ultrasound biometry: clinical feasibility study of a commercial prototype II. J Cataract Refract Surg. 2002;28(2):230–234. doi: 10.1016/s0886-3350(01)01274-3. [DOI] [PubMed] [Google Scholar]
- 6.Connors R, Boseman P, Olson RJ. Accuracy and reproducibility of biometry using partial coherence interferometry. J Cataract Refract Surg. 2002;28(2):235–238. doi: 10.1016/s0886-3350(01)01179-8. [DOI] [PubMed] [Google Scholar]
- 7.Rajan MS, Keilhorn I, Bell JA. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye (Lond) 2002;16(5):552–556. doi: 10.1038/sj.eye.6700157. [DOI] [PubMed] [Google Scholar]
- 8.Eleftheriadis H. IOLMaster biometry: refractive results of 100 consecutive cases. Br J Ophthalmol. 2003;87(8):960–963. doi: 10.1136/bjo.87.8.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen T. Improved accuracy of intraocular lens power calculation with the Zeiss IOLMaster. Acta Ophthalmol Scand. 2007;85(1):84–87. doi: 10.1111/j.1600-0420.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 10.Vogel A, Dick HB, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27(12):1961–1968. doi: 10.1016/s0886-3350(01)01214-7. [DOI] [PubMed] [Google Scholar]
- 11.Németh J, Fekete O, Pesztenlehrer N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg. 2003;29(1):85–88. doi: 10.1016/s0886-3350(02)01500-6. [DOI] [PubMed] [Google Scholar]
- 12.Lam AK, Chan R, Pang PC. The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster. Ophthalmic Physiol Opt. 2001;21(6):477–483. doi: 10.1046/j.1475-1313.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 13.Findl O, Kriechbaum K, Sacu S, et al. Influence of operator experience on the performance of ultrasound biometry compared to optical biometry before cataract surgery. J Cataract Refract Surg. 2003;29(10):1950–1955. doi: 10.1016/s0886-3350(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 14.Siahmed K, Muraine M, Brasseur G. Optic biometry in intraocular lense calculation for cataract surgery. Comparison with usual methods. J Fr Ophtalmol. 2001;24(9):922–926. French. [PubMed] [Google Scholar]
- 15.Packer M, Fine IH, Hoffman RS, Coffman PG, Brown LK. Immersion A-scan compared with partial coherence interferometry: outcomes analysis. J Cataract Refract Surg. 2002;28(2):239–242. doi: 10.1016/s0886-3350(01)01259-7. [DOI] [PubMed] [Google Scholar]
- 16.Shammas HJ, Chan S. Precision of biometry, keratometry, and refractive measurements with a partial coherence interferometry-keratometry device. J Cataract Refract Surg. 2010;36(9):1474–1478. doi: 10.1016/j.jcrs.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Rabsilber TM, Jepsen C, Auffarth GU, Holzer MP. Intraocular lens power calculation: clinical comparison of 2 optical biometry devices. J Cataract Refract Surg. 2010;36(2):230–234. doi: 10.1016/j.jcrs.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Buckhurst PJ, Wolffsohn JS, Shah S, Naroo SA, Davies LN, Berrow EJ. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol. 2009;93(7):949–953. doi: 10.1136/bjo.2008.156554. [DOI] [PubMed] [Google Scholar]
- 19.Holzer MP, Mamusa M, Auffarth GU. Accuracy of a new partial coherence interferometry analyser for biometric measurements. Br J Ophthalmol. 2009;93(6):807–810. doi: 10.1136/bjo.2008.152736. [DOI] [PubMed] [Google Scholar]
- 20.Hill W, Angeles R, Otani T. Evaluation of a new IOLMaster algorithm to measure axial length. J Cataract Refract Surg. 2008;34(6):920–924. doi: 10.1016/j.jcrs.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Chylack LT, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 22.Tehrani M, Krummenauer F, Blom E, Dick HB. Evaluation of the practicality of optical biometry and applanation ultrasound in 253 eyes. J Cataract Refract Surg. 2003;29(4):741–746. doi: 10.1016/s0886-3350(02)01740-6. [DOI] [PubMed] [Google Scholar]
- 23.Freeman G, Pesudovs K. The impact of cataract severity on measurement acquisition with the IOLMaster. Acta Ophthalmol Scand. 2005;83(4):439–442. doi: 10.1111/j.1600-0420.2005.00473.x. [DOI] [PubMed] [Google Scholar]